Abstract
Among the new microbiological criteria that have been incorporated in EU Regulation 2073/2005, of particular interest are those concerning Listeria monocytogenes in ready-to eat (RTE) foods, because for certain food categories, they no longer require zero tolerance but rather specify a maximum allowable concentration of 100 CFU/g or ml. This study presents a probabilistic modeling approach for evaluating the compliance of RTE sliced meat products with the new safety criteria for L. monocytogenes. The approach was based on the combined use of (i) growth/no growth boundary models, (ii) kinetic growth models, (iii) product characteristics data (pH, aw, shelf life) collected from 160 meat products from the Hellenic retail market, and (iv) storage temperature data recorded from 50 retail stores in Greece. This study shows that probabilistic analysis of the above components using Monte Carlo simulation, which takes into account the variability of factors affecting microbial growth, can lead to a realistic estimation of the behavior of L. monocytogenes throughout the food supply chain, and the quantitative output generated can be further used by food managers as a decision-making tool regarding the design or modification of a product's formulation or its “use-by” date in order to ensure its compliance with the new safety criteria. The study also argues that compliance of RTE foods with the new safety criteria should not be considered a parameter with a discrete and binary outcome because it depends on factors such as product characteristics, storage temperature, and initial contamination level, which display considerable variability even among different packages of the same RTE product. Rather, compliance should be expressed and therefore regulated in a more probabilistic fashion.
Listeria monocytogenes is a gram-positive nonsporulating pathogenic bacterium with widespread presence in nature, affecting a wide range of domestic and wild animals and humans (5, 12, 21). In the vast majority of human cases, infection is the result of consumption of contaminated food (15, 19). Although the infectious dose remains unknown and is most likely host dependent, the resulting invasive disease, listeriosis, is a serious illness with a high fatality rate (14). Owing to its complex and versatile physiological adaptation mechanisms, L. monocytogenes can persist and often proliferate in contaminated foods under a wide range of antimicrobial conditions, such as low water activity, low pH, and low temperature (8).
On 1 January 2006, Commission Regulation (EC) 2073/2005 became effective for all European Union (EU) states (4). Annex I of Regulation 2073 lists the microbiological criteria for foodstuffs, which are classified into food safety criteria and process hygiene criteria. According to the new EU regulation, food safety criteria are those that “define the acceptability of a product or a batch of foodstuff applicable to products placed on the market.” Of particular interest in the food safety criteria—compared to previously existing legislation—are the legislative amendments regarding L. monocytogenes in ready-to-eat (RTE) foods. Thus, for the first time, RTE foods are legislatively distinguished according to three major factors. First, RTE foods are distinguished based on the target population for which they are intended, i.e., whether they are intended for consumption by infants or by people with special medical conditions versus other target human subpopulations. RTE foods for infants or for special medical purposes are still required to be free of L. monocytogenes (absence in 25 g in a 10-unit sampling plan). Second, RTE foods other than those intended for infants or special medical purposes are then subdivided into those that are able to support the growth of L. monocytogenes and into those that are not. Products “with pH ≤ 4.4 or aw ≤ 0.92, products with pH ≤ 5.0 and aw ≤ 0.94 and products with a shelf-life of less than five days” are automatically considered to belong to the category of RTE foods that are unable to support the growth of L. monocytogenes. The regulation also states that “other categories of products can also belong this category, subject to scientific justification.” Last, the food safety criteria for L. monocytogenes are adjusted according to their temporal stage in the food chain. Thus, for RTE foods that are able to support the growth of L. monocytogenes, the new regulation demands the absence of the pathogen (in 25 g) “before the food has left the immediate control of the food business operator, who has produced it,” but allows up to 100 CFU/g in “products placed on the market during their shelf-life.” The 100-CFU/g limit also applies throughout the shelf life of marketed RTE foods unable to support L. monocytogenes growth.
At first glance, the new safety criteria for L. monocytogenes might appear more lenient towards food manufacturers than the previous ones; however, this is not necessarily the case. Rather, the new regulation can be viewed as more pragmatic, albeit not comprehensive (see Discussion), and certainly generates novel responsibilities for food manufacturers. For RTE foods that are able to support the growth of L. monocytogenes, Regulation 2073 specifies that the 100-CFU/g criterion “applies if the manufacturer is able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit of 100 CFU/g throughout the shelf-life” and the “absence in 25 g” criterion applies only when the manufacturer is “not able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit of 100 CFU/ml throughout the shelf-life.” It is therefore the responsibility of the manufacturer to engage in research and generate product-specific data in order to provide scientific proof that the food product meets the above requirements.
The purpose of this work was to illustrate the usefulness of predictive modeling as a tool for assessing the compliance of RTE foods with the new safety criteria for L. monocytogenes. For this purpose we used a stochastic modeling approach based on published data on the prevalence of the pathogen in RTE deli meats together with data on product characteristics from 160 deli meat samples (such as pH and water activity, which affect the behavior of food-borne pathogens in foods) and data on the temperature distribution of refrigerators in retail stores in Greece.
MATERIALS AND METHODS
Sampling.
The method for sampling RTE deli meats was described previously (1). Briefly, samples consisted of sliced RTE deli meat products that were packaged under vacuum or modified atmosphere and stored under refrigeration in retail settings with a shelf life of 2 or more months. Each distinct product (i.e., a specified product of a certain manufacturer) was sampled and examined at least twice, taking care that different samples of the same product belonged to different lots. The samples were collected within a 4-month period from 13 retail stores in and around the city of Thessaloniki, representing all major supermarket stores in Greece.
Determination of product characteristics (pH, aw, and shelf life).
The aw of all RTE meat samples was determined at 25°C using an Aqualab series 3 water activity determination device (Decagon Devices, Inc., Pullman, WA). pH was determined at 22°C in 25-g food portions emulsified in sterile double-distilled water (in a 1:1 ratio) using a pH meter (pH 211 Microprocessor; Hanna Instruments BV, Ijsselstein, The Netherlands). Product shelf life was calculated as the difference between the expiration and production dates specified on the label. However, the shelf lives of some products could not be calculated as no production information was recorded on the label.
Temperature monitoring in retail refrigerators.
The temperature in 50 display cabinet refrigerators for deli meat products was monitored in six supermarkets located in five cities in Greece (Athens, Thessaloniki, Larissa, Patra, and Iraklio). The temperature was recorded using electronic data loggers (Cox Tracer; Cox Technologies Inc., Belmont, NC). Data loggers were placed on the middle shelf of the refrigerators, and temperature measurements were taken every 10 min for 1 week. Data were extracted to Microsoft Excel using Cox Tracer software for Windows (version 1.62.06; Cox Technologies Inc.), and the mean temperature for each refrigerator was calculated. Temperature data were then fitted to various distributions using @Risk software (version 4.5; Palisade Corporation, Newfield, NY).
Probabilistic modeling approach. (i) Evaluation of the ability of RTE meat products to support the growth of L. monocytogenes.
The ability of the tested RTE meat products to support the growth of L. monocytogenes was evaluated using the growth/no growth interface model published by Koutsoumanis and Sofos (10):
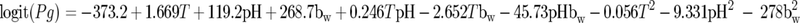 |
(1) |
where logit(Pg) is an abbreviation of ln[Pg/(1 − Pg)], Pg is the probability of growth (in the range of 0 to 1), T is the temperature, and bw is the square root of 1 − aw. The measured pH and aw values for each product as well as the temperature distribution of retail refrigerators were introduced into the model, and the distribution of the probability of growth of the pathogen was estimated based on a Monte Carlo simulation technique (30,000 iterations) using @Risk software. The concentration of NaNO2 was not taken into account, since its quantitative effect on growth initiation is not known (there are no available growth/no growth interface models that include the effect of NaNO2). The percentage of packages of each product which are able to support growth of the pathogen during storage in retail settings was calculated by treating the data on the probability of growth derived from the Monte Carlo simulation as a binomial random variable with the parameter Pg:
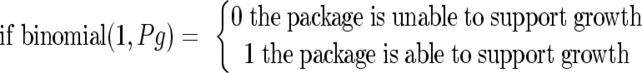 |
(2) |
where Pg is the probability of growth derived from equation 1.
(ii) Evaluation of the L. monocytogenes concentration in RTE meat products at the end of the shelf life.
The concentration of L. monocytogenes in RTE meat products at the end of the shelf life was estimated using a combination of a growth/no growth model and a kinetic model. The exponential growth rate (μ) and the lag phase were calculated from the models of Buchanan and Phillips (2):
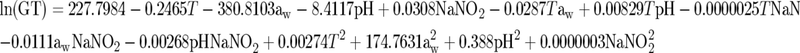 |
(3) |
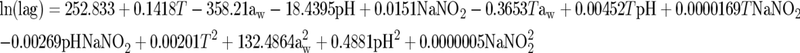 |
(4) |
where GT is the generation time [GT = log(2)/μ] in hours. The exponential growth rate (μ) and the lag phase were calculated using equations 3 and 4 based on the values of pH and aw that were measured for each of the products tested and the distribution of temperature in the retail setting. In addition, a mean concentration of 50 ppm NaNO2 was assumed for RTE meat products based on previous examinations (data not shown). Growth of the pathogen was calculated using a modification of the three-phase linear model (3):
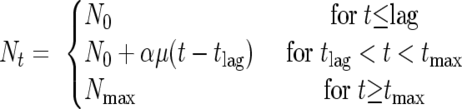 |
(5) |
where Nt is the log of the population density at time t [log(CFU/g)], N0 is the log of the initial population density [log(CFU/g)], Nmax is the log of the maximum population density [log(CFU/g)], t is the elapsed time (h), tlag is the time when the lag phase ends (h), tmax is the time when the maximum population density is reached (h), μ is the exponential growth rate [log(CFU/g)/h], and α is the output of the binomial(1,Pg) distribution, where Pg is the probability of growth derived from equation 1. Based on the above modification, equation 5 predicts no growth of the pathogen when α is 0, whereas when α is 1, growth is predicted, with both μ and lag phase being calculated from equations 3 and 4, respectively. The initial contamination level of L. monocytogenes was assumed to follow a normal distribution, normal(−9, 3.5) log(CFU/g) (6), truncated to −2.3 log(CFU/g) based on an average package weight of 200 g. The maximum population density was assumed to be constant. with a mean value of 10 log(CFU/g) (2). For products with a known shelf life, the distribution of the concentration of L. monocytogenes at the end of the shelf life was calculated based on the above modeling procedure using a Monte Carlo simulation technique (30,000 iterations) with @Risk software.
RESULTS AND DISCUSSION
The new regulation is essentially directing the food industry towards the adoption of alternative approaches to food safety assurance, such as the use of quantitative microbiology. Indeed, the use of quantitative microbiology tools such as the growth/no growth interface and kinetics models in combination with a systematic application procedure can built an effective modeling approach not only for evaluating the compliance of RTE foods with the new safety criteria but also for identifying the appropriate corrective actions for meeting these criteria (11). The present work highlights the need for a modeling approach with a probabilistic character and discusses its components in detail through a case study of RTE sliced meat products.
Evaluation of the ability of RTE meat products to support the growth of L. monocytogenes.
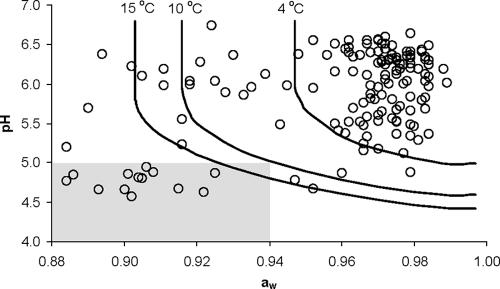
The pH and aw values of each tested product are shown in Fig. 1. According to the regulation criteria, only 8.2% of these products belong to the category of being unable to support L. monocytogenes growth. This indicates that for the majority of the RTE meat products that are available in the market, the food industry should evaluate their ability to support growth of L. monocytogenes.
FIG. 1.
pH and aw values of sliced RTE meat products and growth/no growth boundaries (50% probability level) of L. monocytogenes at 4, 10, and 15°C predicted by the model of Koutsoumanis and Sofos (10). Products to the right of a growth boundary do not support growth of L. monocytogenes at the specified storage temperature. The shaded area indicates products that are automatically considered unable to support growth of L. monocytogenes according to EC Regulation 2073/2005.
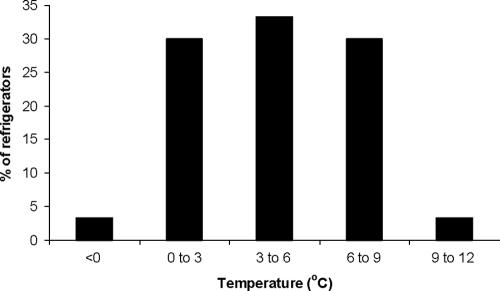
The characteristics of the tested meat products (pH and aw) were compared with the pH and aw limits for growth predicted by equation 1 at 4, 10, and 15°C (Fig. 1). The results showed that 121 of 160 products (75.6%) are predicted to be able to support growth at 4°C. Increasing storage temperature, however, leads to a shift in the growth limits. As a result, the percent of the tested meat products that are predicted to be able to support growth at 10 and 15°C increased to 85.0% and 89.4%, respectively (Fig. 1). For example, this means that, depending on their pH and aw, some products are unable to support growth at 4°C but are able to do so at 10°C. However, Regulation 2073/2005 does not include a clear guideline regarding the temperature at which the industry should evaluate the ability of its products to support the growth of L. monocytogenes. The only reference to temperature in the regulation is in the general requirements in Article 3, where it is stated that “Food business operators shall ensure that the food safety criteria applicable throughout the shelf life of the products can be met under reasonably foreseeable conditions of distribution, storage and use.” In the present study, in order to evaluate the “reasonably foreseeable conditions” of storage of RTE meat products, we recorded the temperature in 50 retail refrigerators for deli meats. The results showed that temperature can vary significantly among retail refrigerators (Fig. 2). Temperature data were fitted to various distributions; a normal distribution with a mean value of 4.42°C and a standard deviation of 2.63°C provided the best fit based on the χ2 test.
FIG. 2.
Mean temperatures in display cabinet refrigerators in the Greek retail market.
The high variability observed in the storage temperature of RTE foods leads to the conclusion that a probabilistic approach would be more appropriate for evaluating both the ability of products to support growth of L. monocytogenes and the total growth of the pathogen during the products' shelf life. Indeed, when Fig. 1 and 2 are combined, it becomes evident that, for many RTE meat products, the ability of a product unit (retail package) to support the growth of L. monocytogenes as well as the total growth of the pathogen during the unit's shelf life is strongly dependent on the temperature of the refrigerator that the package will be stored in. Thus, more realistic estimations can be obtained by taking the variability of storage temperature into account.
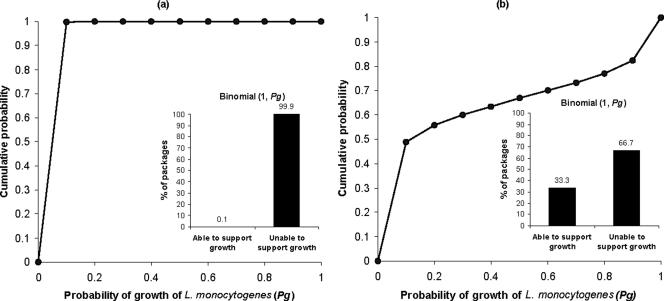
Using the probabilistic approach proposed in the present study, both the distribution of the probability of growth of L. monocytogenes in a given product and the percent of the product's packages in the market that are able to support growth of the pathogen can be estimated. The cumulative distributions of the probability of growth of L. monocytogenes in two representative products as predicted by the model are shown in Fig. 3. For bresaola (pH = 6.75 and aw = 0.924), only 0.1% of the packages are predicted to support growth of the pathogen (Fig. 3a). For a pork shoulder product (pH = 5.49 and aw = 0.943), however, it is predicted that 33.3% of the packages will be able to support the growth of L. monocytogenes (Fig. 3b). The question arising for the latter product is whether it should be categorized in the group of RTE foods that are able to support the growth of L. monocytogenes or to the group that are unable to support the growth of the pathogen. Interestingly, as in the case of the pork shoulder product, for most of the RTE products available in the market the answer to the above question is not clear. As shown in Table 1, for only 27 of the 160 RTE meat products tested in the present study (16.9%) was the percent of packages that are able to support the growth of L. monocytogenes zero. The above results indicate the need for guidelines on categorizing the products in a more probabilistic way. Although it is not easy to include such guidelines in a regulation, some recommendation on the “level of agreement” of a product to each category is required.
FIG. 3.
Cumulative distribution of the probability of growth of L. monocytogenes in bresaola (product 2 in Table 1) (a) and pork shoulder (product 87 in Table 1) (b) and percent of packages that are able or unable to support growth of the pathogen during storage in retail settings.
TABLE 1.
Characteristics and contamination predictions for sliced RTE meat products in the Hellenic retail market
| Product
|
n | pH | aw | Shelf life (days) | Predicted % of packages
|
|||
|---|---|---|---|---|---|---|---|---|
| No. | Name | Manufacturer | Able to support growth | With >100 CFU/g at the end of shelf life (contaminated) | ||||
| 1 | Bresaola | V | 1 | 6.37 | 0.930 | 98 | 6.4 | 9.5 |
| 2 | Bresaola | V | 2 | 6.75 | 0.924 | 98 | 0.1 | 3.3 |
| 3 | Chicken breast | XV | 1 | 5.98 | 0.968 | 36 | 86.0 | 82.4 |
| 4 | Chicken breast | XV | 2 | 5.57 | 0.974 | 36 | 85.9 | 66.0 |
| 5 | Chicken breast | XV | 3 | 5.52 | 0.965 | 36 | 74.8 | 41.5 |
| 6 | Chicken breast | XV | 4 | 5.76 | 0.966 | 36 | 81.7 | 67.6 |
| 7 | Coppa | IV | 1 | 6.28 | 0.921 | —a | 2.1 | — |
| 8 | Coppa | V | 1 | 6.04 | 0.925 | 97 | 7.7 | 11.0 |
| 9 | Coppa | V | 2 | 6.11 | 0.905 | 98 | 0.1 | 3.3 |
| 10 | Ham (cooked) | IV | 1 | 6.37 | 0.965 | — | 76.6 | — |
| 11 | Ham (cooked) | IV | 2 | 6.04 | 0.983 | — | 95.9 | — |
| 12 | Ham (cooked) | IV | 3 | 5.52 | 0.975 | — | 85.6 | — |
| 13 | Ham (cooked) | IV | 4 | 6.10 | 0.984 | — | 95.8 | — |
| 14 | Ham (cooked) | IV | 5 | 6.19 | 0.988 | — | 97.4 | — |
| 15 | Ham (cooked) | VII | 1 | 5.29 | 0.981 | 59 | 79.8 | 77.6 |
| 16 | Ham (cooked) | VII | 2 | 5.53 | 0.970 | — | 81.5 | — |
| 17 | Ham (cooked) | VII | 3 | 6.35 | 0.983 | 93 | 95.1 | 94.9 |
| 18 | Ham (cooked) | VII | 4 | 6.13 | 0.983 | — | 95.5 | — |
| 19 | Ham (cooked) | VII | 5 | 6.56 | 0.962 | 60 | 60.8 | 62.8 |
| 20 | Ham (cooked) | VII | 6 | 6.47 | 0.962 | — | 65.2 | — |
| 21 | Ham (cooked) | VII | 7 | 5.82 | 0.968 | — | 85.7 | — |
| 22 | Ham (cooked) | IX | 1 | 6.25 | 0.972 | 88 | 87.9 | 88.5 |
| 23 | Ham (cooked) | IX | 2 | 6.21 | 0.979 | 88 | 93.4 | 94.0 |
| 24 | Ham (cooked) | IX | 3 | 5.66 | 0.979 | 88 | 91.5 | 91.7 |
| 25 | Ham (cooked) | X | 1 | 6.32 | 0.973 | — | 87.8 | — |
| 26 | Ham (cooked) | X | 2 | 6.04 | 0.978 | — | 94.0 | — |
| 27 | Ham (cooked) | XIV | 1 | 5.35 | 0.979 | — | 81.3 | — |
| 28 | Ham (cooked) | XIV | 2 | 5.84 | 0.979 | — | 93.3 | — |
| 29 | Ham (cooked) | XVII | 1 | 6.42 | 0.979 | — | 91.5 | — |
| 30 | Ham (cooked) | XVII | 2 | 6.36 | 0.978 | — | 91.8 | — |
| 31 | Ham (cooked) | XVII | 3 | 6.25 | 0.984 | — | 96.0 | — |
| 32 | Ham (cooked) | XVII | 4 | 6.42 | 0.970 | — | 83.0 | — |
| 33 | Ham (cooked) | XXI | 1 | 6.30 | 0.975 | — | 89.5 | — |
| 34 | Ham (cooked) | XXI | 2 | 5.25 | 0.966 | — | 58.2 | — |
| 35 | Ham (cooked) | XXII | 1 | 6.25 | 0.977 | — | 92.3 | — |
| 36 | Ham (cooked) | XXII | 2 | 6.02 | 0.989 | — | 97.5 | — |
| 37 | Ham (fermented) | IV | 1 | 5.96 | 0.935 | — | 25.6 | — |
| 38 | Ham (fermented) | IV | 2 | 6.00 | 0.918 | — | 2.8 | — |
| 39 | Ham (fermented) | VIII | 1 | 6.13 | 0.939 | — | 29.6 | — |
| 40 | Ham (fermented) | VIII | 2 | 5.95 | 0.952 | — | 62.4 | — |
| 41 | Ham (fermented) | XIII | 1 | 6.18 | 0.855 | — | 0.0 | — |
| 42 | Ham (fermented) | XIII | 2 | 5.98 | 0.911 | — | 0.6 | — |
| 43 | Mortadella | II | 1 | 6.12 | 0.967 | 88 | 84.8 | 84.4 |
| 44 | Mortadella | III | 1 | 5.70 | 0.963 | — | 78.0 | — |
| 45 | Mortadella | IV | 1 | 6.37 | 0.974 | — | 88.5 | — |
| 46 | Mortadella | IV | 2 | 6.52 | 0.972 | — | 81.5 | — |
| 47 | Mortadella | VII | 1 | 6.38 | 0.948 | — | 39.2 | — |
| 48 | Mortadella | VIII | 1 | 6.42 | 0.978 | — | 91.0 | — |
| 49 | Mortadella | VIII | 2 | 6.34 | 0.975 | — | 89.7 | — |
| 50 | Mortadella | XVII | 1 | 6.56 | 0.952 | — | 35.8 | — |
| 51 | Mortadella | XVII | 2 | 6.45 | 0.961 | — | 66.1 | — |
| 52 | Mortadella | XXI | 1 | 4.88 | 0.979 | — | 29.0 | — |
| 53 | Mortadella | XXI | 2 | 6.26 | 0.976 | — | 91.2 | — |
| 54 | Mortadella | XXII | 1 | 5.80 | 0.982 | — | 94.5 | — |
| 55 | Mortadella | XXII | 2 | 5.83 | 0.983 | — | 95.2 | — |
| 56 | Parizer (bologna) | I | 1 | 6.30 | 0.971 | — | 86.0 | — |
| 57 | Parizer (bologna) | II | 1 | 5.37 | 0.975 | 88 | 78.8 | 79.5 |
| 58 | Parizer (bologna) | III | 1 | 5.34 | 0.967 | 60 | 67.5 | 58.7 |
| 59 | Parizer (bologna) | III | 2 | 6.65 | 0.979 | 60 | 86.4 | 86.8 |
| 60 | Parizer (bologna) | VII | 1 | 5.37 | 0.984 | 59 | 86.3 | 85.6 |
| 61 | Parizer (bologna) | VII | 2 | 5.68 | 0.975 | 66 | 89.5 | 89.8 |
| 62 | Parizer (bologna) | VII | 3 | 6.45 | 0.972 | — | 84.8 | — |
| 63 | Parizer (bologna) | VII | 4 | 6.51 | 0.958 | 60 | 55.4 | 56.8 |
| 64 | Parizer (bologna) | VII | 5 | 6.37 | 0.956 | 60 | 58.0 | 59.5 |
| 65 | Parizer (bologna) | VII | 6 | 5.52 | 0.962 | 45 | 70.9 | 50.7 |
| 66 | Parizer (bologna) | XXI | 1 | 5.81 | 0.969 | — | 85.6 | — |
| 67 | Parizer (bologna) | XXI | 2 | 6.52 | 0.967 | — | 72.7 | — |
| 68 | Parizer (bologna) | XXII | 1 | 5.59 | 0.967 | — | 79.2 | — |
| 69 | Parizer (bologna) | XXII | 2 | 5.18 | 0.970 | — | 57.0 | — |
| 70 | Pastirma | XVIII | 1 | 5.86 | 0.933 | 117 | 22.9 | 24.4 |
| 71 | Pastirma | XVIII | 2 | 5.88 | 0.866 | 118 | 0.0 | 2.8 |
| 72 | Pork loin | II | 1 | 5.50 | 0.958 | 88 | 63.7 | 64.2 |
| 73 | Pork loin | III | 1 | 5.69 | 0.983 | — | 94.1 | — |
| 74 | Pork loin | VII | 1 | 6.01 | 0.973 | — | 89.7 | — |
| 75 | Pork loin | IX | 1 | 6.40 | 0.962 | 88 | 70.0 | 70.4 |
| 76 | Pork loin | IX | 2 | 6.17 | 0.975 | 88 | 91.4 | 91.7 |
| 77 | Pork loin | IX | 3 | 5.72 | 0.976 | 88 | 90.5 | 90.6 |
| 78 | Pork loin | XVII | 1 | 5.41 | 0.959 | — | 59.3 | — |
| 79 | Pork loin | XVII | 2 | 6.01 | 0.973 | — | 90.8 | — |
| 80 | Pork loin | XXII | 1 | 5.97 | 0.984 | — | 95.9 | — |
| 81 | Pork loin | XXII | 2 | 5.96 | 0.970 | — | 88.3 | — |
| 82 | Pork shoulder | III | 1 | 5.49 | 0.979 | 60 | 87.6 | — |
| 83 | Pork shoulder | III | 2 | 6.57 | 0.970 | 60 | 76.6 | 86.6 |
| 84 | Pork shoulder | III | 3 | 6.04 | 0.978 | — | 93.6 | 77.8 |
| 85 | Pork shoulder | VII | 1 | 6.56 | 0.970 | — | 77.1 | — |
| 86 | Pork shoulder | VII | 2 | 6.49 | 0.974 | — | 85.7 | — |
| 87 | Pork shoulder | XII | 1 | 5.49 | 0.943 | 113 | 33.3 | 35.3 |
| 88 | Pork shoulder | XIV | 1 | 6.52 | 0.971 | — | 79.4 | — |
| 89 | Pork shoulder | XIV | 2 | 6.60 | 0.972 | — | 77.8 | — |
| 90 | Pork shoulder | XVII | 1 | 6.33 | 0.974 | — | 88.8 | — |
| 91 | Pork shoulder | XIX | 1 | 5.13 | 0.977 | 59 | 61.3 | 54.3 |
| 92 | Pork shoulder | XIX | 2 | 5.47 | 0.980 | 59 | 87.3 | 87.4 |
| 93 | Pork shoulder | XIX | 3 | 5.81 | 0.974 | 59 | 90.9 | 90.5 |
| 94 | Pork shoulder | XXI | 1 | 5.16 | 0.966 | — | 48.8 | — |
| 95 | Pork shoulder | XXII | 1 | 6.49 | 0.984 | — | 94.2 | — |
| 96 | Pork shoulder | XXII | 2 | 6.12 | 0.967 | — | 84.7 | — |
| 97 | Prosciutto | V | 1 | 6.04 | 0.918 | 98 | 2.5 | 5.8 |
| 98 | Prosciutto | V | 2 | 6.19 | 0.911 | 98 | 0.3 | 3.6 |
| 99 | Prosciutto | XII | 1 | 5.90 | 0.928 | 115 | 13.6 | 16.0 |
| 100 | Prosciutto | XII | 2 | 5.55 | 0.916 | 115 | 1.8 | 4.2 |
| 101 | Prosciutto | XVI | 1 | 5.99 | 0.945 | — | 47.3 | — |
| 102 | Prosciutto | XVI | 2 | 5.70 | 0.890 | — | 0.0 | — |
| 103 | Salami | III | 1 | 4.48 | 0.850 | — | 0.0 | — |
| 104 | Salami | IV | 1 | 4.66 | 0.893 | — | 0.0 | — |
| 105 | Salami | V | 1 | 6.23 | 0.902 | 98 | 0.0 | 2.8 |
| 106 | Salami | V | 2 | 6.38 | 0.894 | 97 | 0.0 | 2.8 |
| 107 | Salami | VI | 1 | 5.09 | 0.879 | — | 0.0 | — |
| 108 | Salami | VIII | 1 | 4.88 | 0.908 | — | 0.0 | — |
| 109 | Salami | VIII | 2 | 4.80 | 0.905 | — | 0.0 | — |
| 110 | Salami | XII | 1 | 4.66 | 0.900 | 117 | 0.0 | 2.8 |
| 111 | Salami | XII | 2 | 4.77 | 0.884 | 114 | 0.0 | 2.8 |
| 112 | Salami | XIV | 1 | 4.67 | 0.952 | — | 0.1 | — |
| 113 | Salami | XIV | 2 | 4.78 | 0.947 | — | 0.4 | — |
| 114 | Salami | XIV | 3 | 4.87 | 0.960 | — | 7.4 | — |
| 115 | Salami | XVI | 1 | 5.23 | 0.916 | — | 0.2 | — |
| 116 | Salami | XVI | 2 | 5.31 | 0.855 | — | 0.0 | — |
| 117 | Salami | XVII | 1 | 4.87 | 0.925 | — | 0.0 | — |
| 118 | Salami | XVII | 2 | 4.63 | 0.922 | — | 0.0 | — |
| 119 | Salami | XVII | 3 | 4.82 | 0.904 | — | 0.0 | — |
| 120 | Salami | XIX | 1 | 4.52 | 0.829 | 58 | 0.0 | 2.8 |
| 121 | Salami | XIX | 2 | 4.60 | 0.830 | 59 | 0.0 | 2.8 |
| 122 | Salami | XX | 1 | 6.26 | 0.837 | 151 | 0.0 | 2.8 |
| 123 | Salami | XXI | 1 | 4.95 | 0.906 | — | 0.0 | — |
| 124 | Salami | XXI | 2 | 4.85 | 0.886 | — | 0.0 | — |
| 125 | Salami | XXI | 3 | 4.86 | 0.901 | — | 0.0 | — |
| 126 | Salami | XXI | 4 | 5.11 | 0.861 | — | 0.0 | — |
| 127 | Salami | XXII | 1 | 5.20 | 0.884 | — | 0.0 | — |
| 128 | Salami | XXII | 2 | 5.06 | 0.878 | — | 0.0 | — |
| 129 | Salami (beer, semidry) | VIII | 1 | 6.12 | 0.972 | — | 89.3 | — |
| 130 | Salami (beer, semidry) | VIII | 2 | 6.13 | 0.975 | — | 91.6 | — |
| 131 | Salami (beer, semidry) | XVII | 1 | 5.38 | 0.961 | — | 60.6 | — |
| 132 | Salami (beer, semidry) | XVII | 2 | 5.65 | 0.971 | — | 85.4 | — |
| 133 | Salami (cooked) | X | 1 | 4.57 | 0.902 | — | 0.0 | — |
| 134 | Salami (cooked) | X | 2 | 4.67 | 0.915 | — | 0.0 | — |
| 135 | Salami (cooked) | XI | 1 | 6.06 | 0.972 | — | 89.9 | — |
| 136 | Salami (cooked) | XI | 2 | 6.17 | 0.975 | — | 91.3 | — |
| 137 | Salami (cooked) | XV | 1 | 6.01 | 0.977 | 36 | 93.2 | 91.5 |
| 138 | Salami (cooked) | XV | 2 | 6.16 | 0.958 | 36 | 70.7 | 70.0 |
| 139 | Tongue, smoked | XVIII | 1 | 5.48 | 0.966 | 147 | 74.3 | 74.7 |
| 140 | Turkey breast | III | 1 | 5.53 | 0.975 | — | 85.7 | — |
| 141 | Turkey breast | VII | 1 | 6.41 | 0.982 | 62 | 93.7 | 93.7 |
| 142 | Turkey breast | VII | 2 | 6.19 | 0.981 | 62 | 95.1 | 94.6 |
| 143 | Turkey breast | VII | 3 | 6.32 | 0.983 | — | 95.0 | — |
| 144 | Turkey breast | VII | 4 | 5.88 | 0.968 | 60 | 86.4 | 86.3 |
| 145 | Turkey breast | VII | 5 | 6.16 | 0.958 | — | 69.6 | — |
| 146 | Turkey breast | VII | 6 | 6.35 | 0.963 | — | 73.8 | — |
| 147 | Turkey breast | VII | 7 | 6.27 | 0.970 | — | 85.5 | — |
| 148 | Turkey breast | VII | 8 | 6.16 | 0.963 | — | 79.3 | — |
| 149 | Turkey breast | VII | 9 | 5.61 | 0.970 | 88 | 83.4 | 83.9 |
| 150 | Turkey breast | VIII | 1 | 6.20 | 0.976 | — | 92.0 | — |
| 151 | Turkey breast | VIII | 2 | 6.28 | 0.971 | — | 86.2 | — |
| 152 | Turkey breast | X | 1 | 6.26 | 0.979 | — | 93.2 | — |
| 153 | Turkey breast | X | 2 | 6.34 | 0.979 | — | 92.7 | — |
| 154 | Turkey breast | XVII | 1 | 6.44 | 0.982 | — | 93.5 | — |
| 155 | Turkey breast | XIX | 1 | 6.31 | 0.984 | 58 | 95.7 | 95.7 |
| 156 | Turkey breast | XIX | 2 | 6.31 | 0.973 | 58 | 88.0 | 88.9 |
| 157 | Turkey breast | XIX | 3 | 5.86 | 0.971 | 58 | 88.3 | 88.5 |
| 158 | Turkey breast | XXI | 1 | 6.40 | 0.968 | — | 79.9 | — |
| 159 | Turkey breast | XXII | 1 | 6.56 | 0.967 | — | 71.2 | — |
| 160 | Turkey breast | XXII | 2 | 6.37 | 0.979 | — | 91.9 | — |
—, production date not available.
Estimation of the L. monocytogenes concentration in RTE meat products at the end of the shelf life.
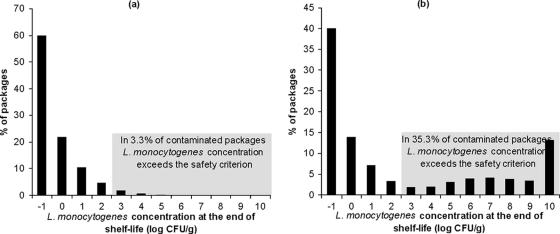
The distributions of the L. monocytogenes concentration in the packages of bresaola (shelf life = 98 days) and pork shoulder products (shelf life = 113 days) at the end of their shelf life are shown in Fig. 4. The simulation results showed that the pathogen will exceed the criterion of 100 CFU/g in 3.3% of contaminated bresaola packages at the end of the shelf life (Fig. 4a). This means that the level of compliance of this product with the safety criterion is 96.7%. For the pork shoulder product, the simulation results showed that the pathogen will exceed the criterion of 100 CFU/g in 35.3% of the packages at the end of the shelf life (Fig. 4b). The estimated concentration of the pathogen at the end of the shelf life of the latter product varies significantly, from −2.3 to 10 log(CFU/g). As it is shown in Fig. 4b, there are two groups of packages, with low and high concentrations of the pathogen. This bimodal pattern of distribution can be attributed to the variability of the storage temperature in retail settings (Fig. 2). The group of packages with L. monocytogenes concentrations less than 2 log(CFU/g) (64.5% of the total packages) are those stored at temperatures which do not allow growth of the pathogen, and thus, the L. monocytogenes concentration at the end of the shelf life is predicted to be equal to the initial level of contamination. In about 22.4% of the packages the predicted total growth of the pathogen during the shelf life ranged from 2 to 9 log(CFU/g) depending on the storage temperature, while in 13.1% of the packages the pathogen reached the assumed maximum population density [10 log(CFU/g)] at the end of the shelf life. The above results indicate that depending on the storage temperature, some packages will not allow growth of the pathogens, whereas in some other packages the pathogen can reach high levels, especially when the product has a long shelf life, as in the case of pork shoulder (113 days).
FIG. 4.
Distribution of predicted L. monocytogenes concentration in contaminated bresaola (product 2 in Table 1) (a) and pork shoulder (product 87 in Table 1) (b) packages at the end of the shelf life in the retail setting.
The level of compliance for all tested RTE meat products for which the shelf life was available is presented in Table 1. In 37 out of 56 (66.1%) products tested in this work the level of compliance was less than 50% (i.e., 66.1% of the products tested are expected to have more than 50% of their contaminated packages exceeding 100 CFU/g by the end of their shelf life), while in only 14 products (25%) the level of compliance was found to be higher than 90% (Table 1). However, 100% compliance was not observed for any of the tested products. Indeed, achieving absolute (100%) compliance with the safety criterion may not be feasible, because even for contaminated products that do not support growth of L. monocytogenes there is a finite probability that the initial contamination will exceed 100 cells/g.
Given a desired level of compliance, the proposed approach can estimate an appropriate adjustment of the product's shelf life or a modification in its formulation in order to achieve this compliance. For example, for the pork shoulder product discussed above, in order to increase the level of compliance from 64.7% (the value predicted with its current shelf life of 113 days) to 90 or 95%, the shelf life would have to be decreased to 50 or 36 days, respectively (Fig. 5). Alternatively, a 90% level of compliance could be achieved by maintaining a shelf life of 113 days but decreasing the aw of the product from 0.943 to 0.930 and increasing the concentration of NaNO2 from 50 to 100 ppm (Fig. 6). This capability of the proposed approach can also be utilized by the food industry for the development of new products. The approach can provide useful information which can serve as the basis for an appropriate product design that will assure placement of the product in the desired food category. It should be noted that it may be beneficial for the food industry to prove that a product does not support L. monocytogenes growth, since in this case the zero tolerance limit for the time period until “the food has left the immediate control of the food business operator who has produced it” does not apply.
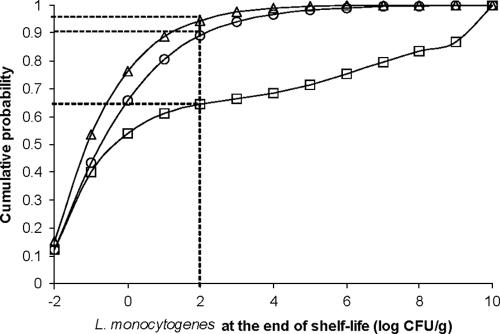
FIG. 5.
Effect of shelf life modifications on the cumulative probability distribution of the L. monocytogenes concentration in contaminated pork shoulder packages (product 87 in Table 1) at the end of shelf life. □, current shelf life of 113 days; ○, shelf life of 50 days; ▵, shelf life of 36 days. Dotted lines indicate the level corresponding to compliance with the 100-CFU/g safety criterion.
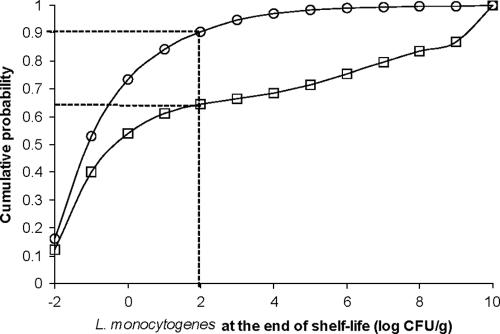
FIG. 6.
Effect of modifications of product formulation on the cumulative probability distribution of L. monocytogenes concentration in contaminated pork shoulder packages (product 87 in Table 1) at the end of the shelf life. □, current formulation (pH = 5.49, aw = 0.943, NaNO2 = 50 ppm); ○, modified formulation (pH = 5.49, aw = 0.930, NaNO2 = 100 ppm). Dotted lines indicate the level corresponding to compliance with the new safety criteria.
Conclusions.
EC Regulation 2073/2005 clearly states that the “food business operators responsible for the manufacture of a product shall conduct studies in order to investigate compliance with the criteria throughout the shelf-life.” It is expected that most operators will respond to the above requirement following the classical approach of challenge tests. Although challenge tests may provide the food industry with useful information, they present several disadvantages, which have been discussed extensively in the literature (13). A major disadvantage is that the results obtained from challenge tests are valid only for the experimental conditions that were used, and any changes to these conditions require the repetition of the test. It is well known that, even for a given product, significant variations in the food milieu can occur among different batches. This was confirmed for the products tested in the present work. For example, in a cooked ham product produced by manufacturing company VII, the pH and aw ranged from 5.29 to 6.56 and 0.962 to 0.983, respectively (Table 1). It is therefore reasonable to believe that the results of a challenge test conducted using a product batch with a pH of 6.35 and a aw value of 0.983 (product 17 in Table 1) cannot be used for evaluating the compliance with the safety criteria of another batch of the same product which has a pH of 5.82 and a aw value of 0.968 (product 21 in Table 1), because the kinetic behavior of the pathogen in the two batches is expected to be completely different. Besides the variability in the initial level of contamination of the products, this work has shown that storage temperature can also vary significantly among retail refrigerators. A probabilistic modeling approach can lead to more realistic results because it accounts for the variability in the parameters affecting the growth of the pathogen. Furthermore, it can be applied easily and rapidly for each separate batch of products and provide information for choosing an appropriate shelf life (targeted to each batch based on its characteristics) that can lead to compliance of the batch with the safety criteria.
The effective application of a probabilistic modeling procedure by the food industry requires the following components.
The first component is accurate growth/no growth interface and kinetics models that include all the parameters which can affect the behavior of L. monocytogenes. Most of the published models for L. monocytogenes include the effect of pH, aw, and temperature. However, other factors in RTE foods and especially the presence and concentrations of chemical preservatives, such as nitrites, organic acids, and their salts, can significantly affect the growth limits and growth kinetics of pathogens. Furthermore the majority of available mathematical models for L. monocytogenes are based on data obtained under well-controlled laboratory settings using microbiological media. Such models do not necessarily predict microbial behavior in complex food environments, because significant factors that affect microbial growth, such as the food structure (17, 18, 22) and the interactions among microorganisms (7, 9, 16, 20), are not taken into account. For example, the models used in the present study predicted high levels of L. monocytogenes at the end of the shelf life in a number of tested products. These models, however, have been developed in laboratory media and thus do not take into account the potential inhibitory effect of the lactic acid bacteria present in RTE meat products on L. monocytogenes. The development of predictive models which are targeted to specific RTE products can yield significantly higher accuracy and lead to increased confidence in the evaluation of the compliance with the safety criteria.
The second component is a database with data on the temperature conditions that the products will be exposed to. This database must include temperature data from the products' entire chill chain, including the stages of distribution, retail storage, and domestic storage. Most food companies do not have any information regarding the temperature conditions which their products are exposed to after the products leave their immediate control. Collection of such data is now necessary for meeting the new safety criteria.
The third component is incorporation of predictive models into user-friendly software packages that provide the option of a probabilistic approach and allow users to obtain the desired information in a rapid and convenient fashion.
The application of the probabilistic approach to RTE meat products showed that compliance cannot be considered a discrete characteristic. Thus, there is a need for translating the safety criteria in probabilistic terms. Regulators should provide guidelines for categorizing different RTE products in the different groups (supporting the growth of L. monocytogenes or not) based on both the probability of growth of the pathogen in each food product and the accepted/desired level of compliance of each product to the criterion of 100 CFU/g.
Acknowledgments
This study was carried out partly with the financial support of the Commission of the European Communities, specific RTD program “Quality of Life and Management of Living Resources,” Key Action 1-Health Food and Environment, project no. QLK1-CT2002-02545. It does not necessarily reflect the Commission's views and in no way anticipates its future policy in this area.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Angelidis, A. S., and K. Koutsoumanis. 2006. Prevalence and concentration of Listeria monocytogenes in sliced ready to eat meat products in the Hellenic retail market. J. Food Prot. 69:938-942. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan, R. L., and J. G. Phillips. 2000. Updated models for the effects of temperature, initial pH, NaCl and NaNO2 on the aerobic and anaerobic growth of Listeria monocytogenes. Quant. Microbiol. 2:103-128. [Google Scholar]
- 3.Buchanan, R. L., R. C. Whiting, and W. C. Damert. 1997. When is simple good enough: a comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 14:313-326. [Google Scholar]
- 4.European Commission. 2005. Commission Regulation (EC) no. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official J. Eur. Union L 338:1-26. [Google Scholar]
- 5.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FSIS. 2003. FSIS risk assessment for Listeria monocytogenes in deli meats. Food Safety and Inspection Service, U.S. Department of Agriculture, Washington, DC.
- 7.Gram, L., and J. Melchiorsen. 1996. Interaction between fish spoilage bacteria Pseudomonas sp. and Shewanella putrefaciens in fish extracts and on fish tissue. J. Appl. Bacteriol. 80:589-595. [DOI] [PubMed] [Google Scholar]
- 8.Hill, C., P. D. Cotter, R. D. Sleator, and C. G. M. Gahan. 2002. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. Int. Dairy J. 12:273-283. [Google Scholar]
- 9.Koutsoumanis, K., and G.-J. E. Nychas. 1999. Chemical and sensory changes associated with microbial flora of Mediterranean boque (Boops boops) stored aerobically at 0, 3, 7 and 10°C. Appl. Environ. Microbiol. 65:698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutsoumanis, K., and J. N. Sofos. 2005. Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. Int. J. Food Microbiol. 104:83-91. [DOI] [PubMed] [Google Scholar]
- 11.Koutsoumanis K., A. S. Angelidis, and G.-J. E. Nychas. 2006. A probabilistic modelling approach for evaluating the compliance of RTE foods with the new safety criteria for Listeria monocytogenes, p. 572. In Abstr. FoodMicro 2006, Food Safety Food Biotechnol. Diversity Global Impact. Bologna, Italy.
- 12.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 13.McMeekin, T. A., and T. Ross. 1996. Shelf life prediction: status and future possibilities. Int. J. Food Microbiol. 33:65-83. [DOI] [PubMed] [Google Scholar]
- 14.Mead, P., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. Griffin, and R. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson, L. J., and E. H. Marth. 1990. Listeria monocytogenes—threat to a safe food supply: a review. J. Dairy Sci. 73:912-928. [DOI] [PubMed] [Google Scholar]
- 16.Pin, C., and J. Baranyi. 1998. Predictive models as means to quantify the interactions of spoilage organisms. Int. J. Food Microbiol. 41:59-72. [DOI] [PubMed] [Google Scholar]
- 17.Pin, C., J. P. Sutherland, and J. Baranyi. 1999. Validating predictive models of food spoilage organisms. J. Appl. Microbiol. 87:491-499. [DOI] [PubMed] [Google Scholar]
- 18.Robins, M. M., and P. D. G. Wilson. 1994. Food structure and microbial growth. Trends Food Sci. Technol. 5:289-293. [Google Scholar]
- 19.Schlech, W. F., III, P. M. Lavigne, R. A. Bortolussi, A. C. Allen, E. V. Haldane, A. J. Wort, A. W. Hightower, S. E. Johnson, S. H. King, E. S. Nicholls, and C. V. Broome. 1983. Epidemic listeriosis—evidence for transmission by food. N. Engl. J. Med. 308:203-206. [DOI] [PubMed] [Google Scholar]
- 20.Tsigarida, E., I. S. Boziaris, and G.-J. E. Nychas. 2003. Bacterial synergism or antagonism in a gel cassette system. Appl. Environ. Microbiol. 69:7204-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weis, J., and H. P. R. Seeliger. 1975. Incidence of Listeria monocytogenes in nature. Appl. Microbiol. 30:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson, P. D. G., T. F. Brocklehurst, S. Arino, D. Thuault, M. Jakobsen, M. Lange, J. Farkas, J. W. T. Wimpenny, and J. F. Van Impe. 2002. Modelling microbial growth in structured foods: towards a unified approach. Int. J. Food Microbiol. 73:275-289. [DOI] [PubMed] [Google Scholar]