Abstract
We determined the 16S rRNA gene sequences of three crustacean “Rickettsiella armadillidii” strains. Rickettsiella bacteria overall appear to form a monophyletic group that diverged from Coxiella bacteria ∼350 million years ago. Therefore, the genus Rickettsiella as a whole (not just Rickettsiella grylli) should be classified among the Gammaproteobacteria instead of the Alphaproteobacteria.
Members of the genus Rickettsiella are intracellular bacterial pathogens of arthropods (13). They are found in a wide range of hosts including insects, crustaceans, and arachnids, and they exhibit a worldwide geographic distribution (11-13). In naturally infected hosts, the Rickettsiella-mediated disease affects both larvae and adults and develops very slowly (13). In its crustacean hosts, “Rickettsiella armadillidii” induces death, preceded by loss of weight and a white coloration of intersegmentary membranes, and the host general cavity is filled with an iridescent white liquid (12). Rickettsiella bacteria can potentially be very contagious, since they are capable of surviving in soil for years before contaminating new hosts (13).
On the basis of ultrastructural observations, Rickettsiella bacteria have been classified among the Alphaproteobacteria, within the order Rickettsiales, the family Rickettsiaceae, and the tribe Wolbachieae (13). However, the 16S rRNA gene sequence of R. grylli isolated from the cricket suggested that this strain is a Gammaproteobacterium related to the genus Coxiella (10). Therefore, if the genus Rickettsiella is monophyletic (i.e., all Rickettsiella species share an exclusive, common ancestor), then the taxonomic position of the genus Rickettsiella as a whole needs to be reassessed. Otherwise, if only R. grylli has been misclassified among the Gammaproteobacteria, the genus Rickettsiella is polyphyletic (i.e., different Rickettsiella species have different evolutionary origins). To obtain new insight into the evolution of Rickettsiella bacteria, we characterized Rickettsiella molecular genetic variation by analyzing the 16S rRNA gene sequences of three strains of the crustacean pathogen “R. armadillidii” (12) along with a data set of Rickettsiella-like 16S rRNA gene sequences encompassing their entire known host spectrum, gathered through database searches.
Wild-caught individuals belonging to the three isopod crustacean species Armadillidium vulgare (from Camarade, Ariège, France), Helleria brevicornis (from Pietracorbara, Corsica, France), and Philoscia muscorum (from Santiago de Compostela, Galicia, Spain) were studied. The animals displayed the characteristic external symptoms of an infection by “R. armadillidii,” as described above (12). The diagnosis was further confirmed during the dissection of the samples and by electron microscopy. We determined the nucleotide sequences of the 16S rRNA genes of the three “R. armadillidii” strains from the aforementioned isopods by using standard methods of DNA extraction, PCR amplification, and sequencing (2, 9). We performed PCR amplification with primers 27f and 973r and sequencing with primers 27f, 530f, 685r, and 973r (6, 9).
Taxonomic considerations.
The three sequences exhibited only 0.3% divergence, based on observed nucleotide substitutions. This is more than 10 times lower than the 3.5% average divergence estimated among the three “R. armadillidii” strains and R. grylli. Therefore, on the basis of currently available data, R. grylli falls outside of the range of variation of “R. armadillidii.” This result backs up the previous suggestion that “R. armadillidii” and R. grylli may represent two distinct species (5), although they are considered synonymous species in the currently accepted nomenclature (13).
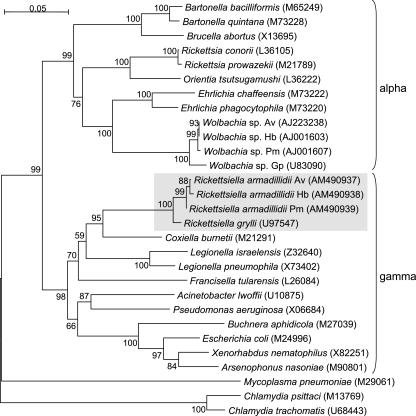
To test the monophyletic or polyphyletic status of the genus Rickettsiella, we performed a phylogenetic analysis including both “R. armadillidii” and R. grylli strains, together with 25 Alphaproteobacteria and Gammaproteobacteria representatives (Fig. 1). The two important results that emerged from this analysis were that (i) the four Rickettsiella strains formed a monophyletic group supported by a bootstrap score of 100% and (ii) all Rickettsiella strains were most closely related to Coxiella bacteria (bootstrap score of 95%), within the Gammaproteobacteria. Therefore, our results suggest that members of the Rickettsiella genus are monophyletic and thus that the entire genus Rickettsiella (not just R. grylli [10]) should be classified among the Gammaproteobacteria rather than among the Alphaproteobacteria.
FIG. 1.
Neighbor-joining tree of bacterial strains based on 16S rRNA gene sequences. The tree was built by using the software MEGA version 3.1, based on the Kimura two-parameter substitution model. Bootstrap values (based on 1,000 replicates) are shown on branches (percentages). Rickettsiella strains are in a gray box, demonstrating their closer relationship to the Gammaproteobacteria (gamma cluster) than to the Alphaproteobacteria (alpha cluster). GenBank accession numbers for all sequences are indicated after species or strain names. Wolbachia and Rickettsiella strains were isolated from A. vulgare (Av), H. brevicornis (Hb), P. muscorum (Pm), and Gryllus pennsylvanicus (Gp).
Genetic diversity and evolution.
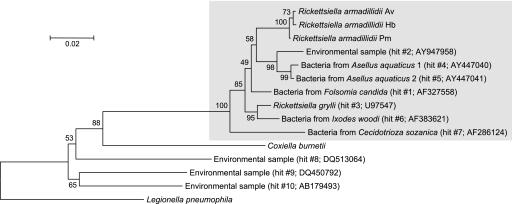
We queried GenBank with the 16S rRNA gene sequence of “R. armadillidii” from A. vulgare through BLASTN searches and selected the 10 best hits for further analyses. A phylogenetic analysis revealed that hits 8 to 10 are not derived from Rickettsiella bacteria, since they branch off in the tree at a position that is more basal than the Coxiella-Rickettsiella split (Fig. 2). By contrast, hits 1 to 7, together with “R. armadillidii” strains, form a monophyletic group, suggesting that all of these sequences are derived from Rickettsiella bacteria. Quantitatively, these conclusions are also supported by the fact that “R. armadillidii” strains and Coxiella burnetii exhibit an average divergence of 13.9% versus 13.5% between “R. armadillidii” strains and hits 8 to 10. In comparison, hits 1 to 7 show an average divergence of only 4.0% with “R. armadillidii” strains, similar to the 3.5% divergence observed between “R. armadillidii” and R. grylli. Interestingly, the 10 Rickettsiella strains for which 16S rRNA gene sequence data are available (Fig. 2) encompass the whole range of arthropod organisms known to be natural hosts of Rickettsiella bacteria, including insects, crustaceans, and arachnids (13). This observation provides further support for the suggestion that the Rickettsiella genus, as a whole, has been misclassified among the Alphaproteobacteria.
FIG. 2.
Neighbor-joining tree of bacterial strains based on 16S rRNA gene sequences. The tree was built as described in the legend to Fig. 1. Bootstrap values (based on 1,000 replicates) are shown on branches (percentages). The monophyletic group of Rickettsiella strains is in a gray box. Strains followed by a hit number correspond to the 10 best hits resulting from a BLASTN search using the 16S rRNA gene sequence of “R. armadillidii” isolated from A. vulgare (Av) as a query against GenBank (hit accession numbers are shown). GenBank accession numbers for other sequences and Rickettsiella strain names are as in Fig. 1. Host systematics for the 10 best hits are as follows: Insecta, hits 1, 3, and 7; Crustacea, hits 4 and 5; Acari, hit 6; unknown, hits 2, 8, 9, and 10.
The average divergence between the 10 Rickettsiella strains and C. burnetii was estimated to be 14.1%. Assuming a substitution rate of the eubacterial 16S rRNA gene of 1% per 50 million years (My) (7), the origin of Rickettsiella bacteria is estimated to have occurred ∼350 My ago. On the other hand, the divergence among the 10 Rickettsiella strains is 3.6% ± 1.6%, suggesting that diversification within the genus Rickettsiella started 90 ± 40 My ago. These 10 Rickettsiella strains are found in crustaceans, insects, and acari (Fig. 2), which probably diverged back in the Precambrian era (3) over 500 My ago. If this is so, the presence of ∼100 My-old Rickettsiella bacteria in such a wide range of arthropod hosts makes the occurrence of horizontal transmission of these bacteria necessary. This is not unexpected, since Rickettsiella bacteria are known to be able to survive in the soil outside of their arthropod hosts for years (13).
Concluding remarks.
The crustacean populations studied here are also known to harbor intracellular Wolbachia pipientis bacteria (2, 4, 8) (Fig. 1). This intracellular arena (1) could therefore represent a remarkable environment in which to promote evolutionary novelty by horizontal gene transfer among unrelated bacteria. Beyond this evolutionary interest, Rickettsiella spp. are pathogenic bacteria whose introduction into laboratory insectaries or other animal collections might be devastating and needs to be prevented (13). Therefore, our results not only offer new insight into the evolution of these bacteria but also provide the basis for a genetic test to easily recognize the presence of Rickettsiella in suspect samples.
Nucleotide sequence accession numbers.
The three “R. armadillidii” sequences generated in this study have been deposited in GenBank under accession numbers AM490937 to AM490939.
Acknowledgments
This research was supported by funds from the Centre National de la Recherche Scientifique (CNRS) and the French Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Bordenstein, S. R., and J. J. Wernegreen. 2004. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol. Biol. Evol. 21:1981-1991. [DOI] [PubMed] [Google Scholar]
- 2.Bouchon, D., T. Rigaud, and P. Juchault. 1998. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc. Biol. Sci. 265:1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusca, R. C., and G. J. Brusca. 1990. Invertebrates. Sinauer Associates, Inc., Sunderland, MA.
- 4.Cordaux, R., A. Michel-Salzat, and D. Bouchon. 2001. Wolbachia infection in crustaceans: novel hosts and potential routes for horizontal transmission. J. Evol. Biol. 14:237-243. [Google Scholar]
- 5.Frutos, R., B. A. Federici, B. Revet, and M. Bergoin. 1994. Taxonomic studies of Rickettsiella, Rickettsia, and Chlamydia using genomic DNA. J. Invertebr. Pathol. 63:294-300. [DOI] [PubMed] [Google Scholar]
- 6.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Wiley & Sons, Inc., New York, NY.
- 7.Ochman, H., and A. C. Wilson. 1987. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26:74-86. [DOI] [PubMed] [Google Scholar]
- 8.Rousset, F., D. Bouchon, B. Pintureau, P. Juchault, and M. Solignac. 1992. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. Biol. Sci. 250:91-98. [DOI] [PubMed] [Google Scholar]
- 9.Rousset, F., D. Vautrin, and M. Solignac. 1992. Molecular identification of Wolbachia, the agent of cytoplasmic incompatibility in Drosophila simulans, and variability in relation with host mitochondrial types. Proc. Biol. Sci. 247:163-168. [DOI] [PubMed] [Google Scholar]
- 10.Roux, V., M. Bergoin, N. Lamaze, and D. Raoult. 1997. Reassessment of the taxonomic position of Rickettsiella grylli. Int. J. Syst. Bacteriol. 47:1255-1257. [DOI] [PubMed] [Google Scholar]
- 11.Vago, C., and R. Martoja. 1963. Rickettsiosis in the Gryllidae (Orthoptera). C. R. Acad. Sci. 256:1045-1047. [PubMed] [Google Scholar]
- 12.Vago, C., G. Meynadier, P. Juchault, J. J. Legrand, A. Amargier, and J. L. Duthoit. 1970. A rickettsial disease of isopod crustaceans. C. R. Acad. Sci. D 271:2061-2063. [PubMed] [Google Scholar]
- 13.Weiss, E., G. A. Dasch, and K.-P. Chang. 1984. Genus VIII. Rickettsiella Philip 1956, 267AL, p. 713-717. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]