Abstract
Vibrio vulnificus is part of the natural estuarine microflora and accumulates in shellfish through filter feeding. It is responsible for the majority of seafood-associated fatalities in the United States mainly through consumption of raw oysters. Previously we have shown that a V. vulnificus mutant unable to express PilD, the type IV prepilin peptidase, does not express pili on the surface of the bacterium and is defective in adherence to human epithelial cells (R. N. Paranjpye, J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom, Infect. Immun. 66:5659-5668, 1998). A mutant unable to express one of the type IV pilins, PilA, is also defective in adherence to epithelial cells as well as biofilm formation on abiotic surfaces (R. N. Paranjpye and M. S. Strom, Infect. Immun. 73:1411-1422, 2005). In this study we report that the loss of PilD or PilA significantly reduces the ability of V. vulnificus to persist in Crassostrea virginica over a 66-h interval, strongly suggesting that pili expressed by this bacterium play a role in colonization or persistence in oysters.
Vibrio vulnificus is a normal inhabitant of brackish water environments particularly in subtropical and tropical climates. The bacterium is recognized as the most invasive and rapidly lethal Vibrio species and is the cause of the majority of deaths that result from consumption of raw contaminated shellfish (19, 20). Susceptible individuals include those who are immunocompromised or suffer from cirrhosis or hemochromatosis (17, 18, 37). It is also a significant source of infections in otherwise healthy individuals through wounds or skin lesions that come in contact with contaminated water, fish, and shellfish (4, 36, 37).
Mollusks that are filter feeders, such as oysters, accumulate indigenous and nonindigenous bacteria from the surrounding water. Methods frequently used to reduce the concentrations of these microorganisms to acceptable and safe levels in oysters include relaying, where shellfish are transferred to clean shellfish-growing waters, and depuration, where shellfish are held in tanks with flowthrough water for 48 to 72 h (30, 31, 38). While these purification methods are effective in eliminating nonindigenous bacteria, such as fecal coliforms, indigenous flora, such as vibrios, remain associated with the oysters. As such, Vibrio spp. appear to selectively colonize, persist, and may even multiply in oysters between the time they are harvested and consumed (8, 11, 14, 16, 21, 39). Presently, the precise mechanism involved in their persistence is not understood. Several studies have examined the interactions of vibrios with components of the bivalve hemolymph, since microbial destruction typically results from the combined action of bivalve hemocyte and humoral defenses (2, 6, 12). Harris-Young et al. demonstrated that the presence of the V. vulnificus and Vibrio parahaemolyticus exopolysaccharide inhibits the hemocyte phagocytic response (15). Other studies show that hemolymph serum from mussels promotes the interactions between Vibrio cholerae El Tor strains expressing the type IV pilin mannose-sensitive hemagglutinin (MSHA) and hemocytes in vitro (40). A similar effect was also observed with fimbriated Escherichia coli, suggesting that hemolymph-soluble factors are involved in d-mannose-sensitive interactions and might have a broad spectrum of specificity (3). However, in spite of the common mechanisms of interactions between surface components and hemocytes, V. cholerae strains are less efficient in triggering signaling pathways in hemocytes than E. coli strains are, which may account for their increased persistence in bivalve hosts (2, 28).
Several factors, including flagella, pili, nonfimbrial surface proteins, and surface polysaccharides, likely contribute to the adherence of V. vulnificus to surfaces in its natural environment as well as during opportunistic infections. The role of type IV pili in biofilm formation on both abiotic and biotic surfaces has been established in several gram-negative pathogens (7, 13, 22, 29, 34). These pili are also important virulence determinants because of their role in adherence and colonization of mammalian host tissue (22, 25, 35). In addition, it has been demonstrated that one type IV pilus, the MSHA pilus of V. cholerae, plays a role in colonization of zooplankton in the marine environment (7). In previous studies we have shown that the PilA type IV pilin of V. vulnificus is involved in adherence to human epithelial (HEp-2) cells as well as in biofilm formation on abiotic surfaces (24), while the absence of PilD, the type IV prepilin peptidase, resulted in loss of all surface pili and a significantly greater defect in adherence to human epithelial cells (23). The V. vulnificus genome also encodes a second type IV pilin homologous to the V. cholerae MSHA, but it carries only a single prepilin peptidase gene (5). Therefore, the loss of all surface pili on the pilD mutant suggests that PilD processes both type IV pilins of V. vulnificus (23). The present study was designed to evaluate the potential role of PilA and PilD of V. vulnificus in colonization by the bacterium in oysters by comparing the uptake and persistence of a wild-type V. vulnificus strain to those of the pilA and pilD mutant strains.
Crassostrea virginica oysters used in this study were harvested from Totten Inlet, Washington, and obtained from a local grower (Taylor Shellfish Farms, Shelton, WA). Oysters used in the study were of a uniform size, between 3 and 4 in., and the average weight of oyster meat plus liquor was between 17 and 25 g. All experiments were conducted between the months of November and May to avoid the spawning season. Upon receipt, oysters were scrubbed with a brush and transferred to 20-gallon tanks containing artificial seawater (ASW; Instant Ocean, Aquarium Systems, OH) at a salinity between 15 and 17 ppt. Oysters were allowed to acclimate for 4 days without feeding. The water temperature in the tanks was gradually increased from 12°C to 18°C over 4 days. Each tank was equipped with an air stone. After acclimation, oysters were transferred into tanks with fresh ASW at 18°C.
The bacterial strains included nalidixic acid-resistant isolates of a V. vulnificus clinical strain (C7184), and the isogenic pilA (C7184AΩ) and pilD (C7184DΩ) mutant strains (23, 24). Nalidixic acid resistance allowed differentiation of the inoculated bacteria from the natural background flora, including other vibrios potentially present in the commercial oysters used in this study. The spectinomycin/streptomycin resistance marker in the omega fragment (27) in V. vulnificus pilA and pilD mutants also facilitated their differentiation from the background flora.
Strains were grown overnight, with shaking, at 30°C in TCG broth (10, 24), with the appropriate antibiotics at the following concentrations: streptomycin, 25 μg/ml; nalidixic acid, 5 μg/ml. Overnight cultures were centrifuged at 6,000 × g for 20 min. After the supernatant was discarded, the pellet was washed once in sterile ASW (15 to 17 ppt) and resuspended in the same diluent. For each experiment, the inocula were adjusted to the same optical density (optical density at 600 nm of 2.0), so that the concentrations were ∼1 × 109 CFU per ml. The actual concentrations of the inocula were determined by plating serial 10-fold dilutions on LB agar with the appropriate antibiotic. For each challenge, the prepared inoculum was added to the ASW to a final concentration between 1 × 106 and 1 × 107 CFU/ml. A suspension of instant alga concentrate (Tetraselmis 3600 Premium Fresh; Reed Mariculture, Inc., San Jose, CA) was also added at the concentration of 1% of algae (dry wt)/1,000 g of oyster meat to stimulate feeding in the oysters.
Initial experiments designed to compare uptake of each of the V. vulnificus strains were performed in duplicate. For each experiment, eight oysters were distributed in each of three tanks. A separate tank containing four oysters was used for uninoculated controls for detection of any background vibrios. The initial concentration of the inoculum in the tanks was approximately 1 × 106 CFU/ml. After addition of the inocula, oysters were allowed to feed for 10 h before analysis. Each oyster was treated as a separate sample. Prior to shucking, the surface of the oyster shell was rinsed with 70% ethanol. Oysters were shucked with sterile knives, and the shucked meat along with all the liquid was transferred to sterile bags. An aliquot of 1× phosphate-buffered saline equal to the weight of the oyster plus liquid was added to each bag, and the mixture was homogenized in a blender (Stomacher, Seward, United Kingdom) for 1 min.
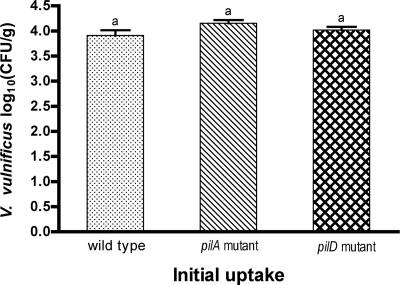
VVA agar (9) was used as a selective medium for isolation of V. vulnificus with the addition of nalidixic acid or streptomycin as appropriate. Growth of the wild-type strain C7184 and mutant strains C7184AΩ and C7184DΩ was initially compared on the selective VVA agar to a nonselective medium, Trypticase soy agar (TSA; Difco), to ensure that VVA was not inhibitory to the strains used. Background flora in the oysters was assessed by determination of total aerobic plate counts on TSA. Serial 10-fold dilutions of the homogenate (100-μl aliquots) were plated on VVA medium and incubated at 37°C for 24 h. Similar aliquots of homogenates of two samples from each treatment group were also plated on TSA and incubated at 25°C for 3 days for determination of total aerobes. Control samples were plated on both VVA agar and TSA. In order to determine whether there was any difference in initial uptake of the wild type and pilA and pilD mutant strains, the concentration of V. vulnificus in the oysters was determined immediately after the 10-h feeding period. As shown in Fig. 1, there was no significant difference between the strains. Vibrios were not detected in any of the control samples.
FIG. 1.
Comparison of the uptake of V. vulnificus wild type (C7184), pilA mutant (C7184AΩ), and pilD mutant (C7184DΩ) in C. virginica. The means plus standard errors (error bars) are shown. These values are not significantly different at a P value of ≤0.05, as indicated by the same letter above each bar.
Subsequent experiments were designed to determine the persistence of V. vulnificus in oysters. For these experiments, 30 oysters were distributed in each of three tanks. Again, a separate tank containing 12 oysters was used for uninoculated controls for detection of background vibrios. After the initial uptake period of 10 h, oysters were transferred to fresh ASW (15 to 17 ppt) and allowed to depurate for 18 to 66 h. Ten oysters from each of the treatment groups were removed for sampling at intervals of 18, 42, and 66 h from each of the tanks. Four untreated oysters were also removed at the same intervals for control samples. Oysters were transferred to the laboratory in sterile containers and analyzed as described above for the uptake experiments.
Experiments were repeated three times, and the results represent the analyses of 30 oysters at each interval. In each experiment, the actual counts were normalized to that of the wild-type strain. Statistical analyses were performed using Statview (32). Three-way analysis of variance (treatment, time, and experiment number) was performed after the data were transformed to remove heteroscedasticity (X′ = log (X + 1) (1). Posthoc comparisons were made using Fisher's protected least significant difference test. Values where P was <0.05 were considered significant.
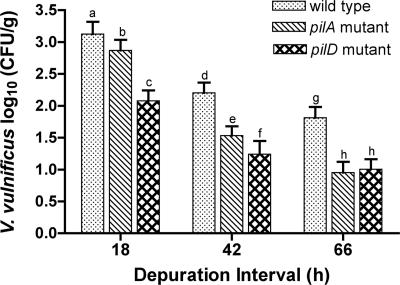
After 18 h, the mean concentration of V. vulnificus recovered from oysters challenged with the wild-type strain was 1.8 times higher than that recovered from oysters challenged with the pilA mutant strain and 11.2 times higher than that challenged with the pilD mutant strain (Fig. 2). After 42 h, the mean concentration of V. vulnificus recovered from oysters challenged with the wild-type strain was 4.7 times higher than that for pilA mutant-challenged oysters and 9.2 times higher than that for pilD mutant-challenged oysters. The increased retention of the wild-type strain compared to both mutant strains remained consistent throughout the depuration interval, and after 66 h, the mean recovery of wild-type V. vulnificus was 7.3 times higher than that for the pilA mutant and 6.5 times higher than that for the pilD mutant. In addition, V. vulnificus was recovered from only 60% of the oysters originally challenged with pilA or pilD mutants after 66 h (data not shown). In contrast, wild-type V. vulnificus was recovered from 84% of oysters after 66 h, emphasizing the decreased persistence of V. vulnificus with defective expression of type IV pili in oysters. Again, vibrios were not detected in any of the control samples.
FIG. 2.
Comparison of the persistence of V. vulnificus wild type (C7184), pilA mutant (C7184AΩ), and pilD mutant (C7184DΩ) in C. virginica. The means plus standard errors (error bars) are shown. Values with different letters within each time interval are significantly different at a P value of ≤0.05.
We have previously demonstrated that the pilA and pilD mutations are not polar and can be complemented with the wild-type genes in trans, demonstrating that the introduced mutations do not affect expression of upstream or downstream genes (23, 24). While this is sufficient proof that any measured phenotype of C7184AΩ (pilA mutant) and C7184DΩ (pilD mutant) is due solely to lack of PilA or PilD, we attempted to examine the complemented mutant strains, C7184AΩ(pRP383) and C7184DΩ(pRPD1) (23, 24), in the oyster uptake and persistence experiments. This proved to be problematic. Stable maintenance of pRP383 or pRPD1 requires continuous selection with chloramphenicol, without which the pMMB67EH.cam vector is quickly lost (unpublished results). Although attempts were made to carry out the uptake experiment with the complemented strains in the presence of 10 μg/ml chloramphenicol, it was difficult to maintain a constant concentration of active antibiotic over the time required, and recovery of chloramphenicol-resistant strains was inconsistent. Second, adequate expression of PilA and PilD in the complemented strains requires induction of the tac promoter with isopropyl thio-β-d-galactoside (IPTG) (23, 24), which could not be included in the challenge tanks.
To assess the effects of introduced V. vulnificus and depuration treatment on the natural microflora of the oysters, the number of total aerobes was determined after the initial uptake period and at each depuration interval. Mean counts ranged from 3.7 × 104 CFU/g to 2.3 × 106 CFU/g with no correlation between challenged or control oysters, demonstrating that introduction of V. vulnificus had no impact on the natural microflora of the oysters (Table 1).
TABLE 1.
Total aerobe plate counts in oysters
| Inoculated strain | Total aerobe plate count (CFU/g)a
|
|||
|---|---|---|---|---|
| After initial uptake | After depuration
|
|||
| 18 h | 42 h | 66 h | ||
| C7184 | 1.2 × 105 ± 1.7 × 105 | 4.0 × 105 ± 3.8 × 105 | 5.5 × 105 ± 1.9 × 105 | 1.0 × 105 ± 7.1 × 104 |
| C7184AΩ | 1.4 × 106 ± 1.9 × 106 | 4.8 × 105 ± 3.9 × 105 | 2.3 × 106 ± 3.5 × 105 | 4.5 × 104 ± 6.5 × 104 |
| C7184DΩ | 3.2 × 105 ± 1.5 × 105 | 4.1 × 105 ± 4.1 × 105 | 4.7 × 105 ± 4.6 × 105 | 3.7 × 104 ± 3.7 × 104 |
| None (control) | 3.7 × 105 ± 3.2 × 105 | 3.4 × 105 ± 1.9 × 105 | 3.0 × 105 ± 5.7 × 104 | 5.1 × 104 ± 4.2 × 104 |
Each value represents the mean ± 1 standard deviation.
In summary, this study demonstrates that the pilA-encoded type IV pilus contributes to the persistence of V. vulnificus in oysters. In addition, data showing that the persistence of the V. vulnificus pilD mutant is significantly less than that of the pilA mutant suggest that both type IV pili (PilA and MSHA) expressed by this bacterium are likely involved in its ability to preferentially accumulate in oysters. While the additional absence of secreted exoenzymes in the pilD strain (23) may also contribute to the observed decrease in bacterial retention, taken together, the results indicate a role for bacterial pilus adhesins in colonization of bivalves for the first time. As shown with the type IV pili of Pseudomonas aeruginosa (33) and pathogenic Neisseria (26), the V. vulnificus pili may specifically bind to carbohydrate-containing receptors on oyster cells. The identification of specific adhesins is essential for the development of competitive inhibitors of host cell-receptor binding. Such strategies may ultimately lead to a method to specifically remove V. vulnificus from oysters through a postharvest treatment process. As a first step in the development of such a method, we are currently assessing the feasibility of using peptides specific to the adhesin epitope (13) to reduce PilA type IV pilus-mediated adherence of V. vulnificus to biotic surfaces and ultimately identify a possible receptor(s) or binding site of these pili in oysters.
Acknowledgments
We thank Mark Peterson for help in setting up the oyster challenge tanks and Karl Shearer for help with the statistical analysis.
This publication was supported by the West Coast Center for Oceans and Human Health (WCCOHH) as part of the NOAA Oceans and Human Health Initiative, WCCOHH publication no. 21. The WCCOHH is part of the National Marine Fisheries Service's Northwest Fisheries Science Center, Seattle, WA.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Bartlett, M. S. 1947. The use of transformations. Biometrics 3:39-52. [PubMed] [Google Scholar]
- 2.Canesi, L., G. Gallo, M. Gavioli, and C. Pruzzo. 2002. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 57:469-476. [DOI] [PubMed] [Google Scholar]
- 3.Canesi, L., C. Pruzzo, R. Tarsi, and G. Gallo. 2001. Surface interactions between Escherichia coli and hemocytes of the Mediterranean mussel Mytilus galloprovincialis Lam. leading to efficient bacterial clearance. Appl. Environ. Microbiol. 67:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2005. Vibrio illnesses after Hurricane Katrina—multiple states, August-September 2005. Morb. Mortal. Wkly. Rep. 54:928-931. [PubMed] [Google Scholar]
- 5.Chen, C. Y., K. M. Wu, Y. C. Chang, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, and S. F. Tsai. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, T. C. 1975. Functional morphology and biochemistry of molluscan phagocytes. Ann. N. Y. Acad. Sci. 266:343-379. [DOI] [PubMed] [Google Scholar]
- 7.Chiavelli, D. A., J. W. Marsh, and R. K. Taylor. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook, D. W. 1989. Indicator bacteria and Vibrionaceae multiplication in post-harvest shellstock oysters. J. Food Prot. 52:343-349. [DOI] [PubMed] [Google Scholar]
- 9.DePaola, A., Jr., and C. A. Kaysner. 2004. Chapter 9, Vibrio. In Bacteriological analytical manual. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration. AOAC International, Arlington, VA.
- 10.Ehara, M., M. Ishibashi, Y. Ichinose, M. Iwanaga, S. Shimotori, and T. Naito. 1987. Purification and partial characterization of fimbriae of Vibrio cholerae O1. Vaccine 5:283-288. [DOI] [PubMed] [Google Scholar]
- 11.Eyles, M. J., and G. R. Davey. 1988. Vibrio cholerae and enteric bacteria in oyster-producing areas of two urban estuaries in Australia. Int. J. Food Microbiol. 6:207-218. [DOI] [PubMed] [Google Scholar]
- 12.Fryer, S. E., and C. J. Bayne. 1996. Host-parasite interactions in molluscs. Prog. Mol. Subcell. Biol. 15:131-153. [PubMed] [Google Scholar]
- 13.Giltner, C. L., E. J. van Schaik, G. F. Audette, D. Kao, R. S. Hodges, D. J. Hassett, and R. T. Irvin. 2006. The Pseudomonas aeruginosa type IV pilin receptor binding domain functions as an adhesin for both biotic and abiotic surfaces. Mol. Microbiol. 59:1083-1096. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg, E. P., M. Duboise, and B. Palhof. 1982. The survival of marine vibrios in Mercenaria mercenaria, the hardshell clam. J. Food Saf. 4:113-123. [Google Scholar]
- 15.Harris-Young, L., M. L. Tamplin, W. S. Fisher, and J. W. Mason. 1993. Effects of physiochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with haemocytes of Crassostrea virginica. Appl. Environ. Microbiol. 59:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, S. H., T. L. Howell, and K. R. O'Neill. 1991. Differential elimination of indicator bacteria and pathogenic Vibrio spp. from Eastern oysters (Crassostrea virginica Gmelin, 1791) in a commercial controlled purification facility in Maine. J. Shellfish Res. 10:105-112. [Google Scholar]
- 17.Klontz, K. C., S. Lieb, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 18.Koenig, K. L., J. Mueller, and T. Rose. 1991. Vibrio vulnificus. Hazard on the half shell. West. J. Med. 155:400-403. [PMC free article] [PubMed] [Google Scholar]
- 19.Morris, J. G., Jr. 1988. Vibrio vulnificus—a new monster of the deep? Ann. Intern. Med. 109:261-263. [DOI] [PubMed] [Google Scholar]
- 20.Morris, J. G., Jr., and R. E. Black. 1985. Cholera and other vibrioses in the United States. N. Engl. J. Med. 312:343-350. [DOI] [PubMed] [Google Scholar]
- 21.Murphree, R. L., and M. L. Tamplin. 1995. Uptake and retention of Vibrio cholerae O1 in the Eastern oyster, Crassostrea virginica. Appl. Environ. Microbiol. 61:3656-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nudleman, E., and D. Kaiser. 2004. Pulling together with type IV pili. J. Mol. Microbiol. Biotechnol. 7:52-62. [DOI] [PubMed] [Google Scholar]
- 23.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpye, R. N., and M. S. Strom. 2005. A Vibrio vulnificus type IV pilin contributes to biofilm formation, adherence to epithelial cells, and virulence. Infect. Immun. 73:1411-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizarro-Cerda, J., and P. Cossart. 2006. Bacterial adhesion and entry into host cells. Cell 124:715-727. [DOI] [PubMed] [Google Scholar]
- 26.Plant, L., and A. B. Jonsson. 2003. Contacting the host: insights and implications of pathogenic Neisseria cell interactions. Scand. J. Infect. Dis. 35:608-613. [DOI] [PubMed] [Google Scholar]
- 27.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 28.Pruzzo, C., G. Gallo, and L. Canesi. 2005. Persistence of vibrios in marine bivalves: the role of interactions with haemolymph components. Environ. Microbiol. 7:761-772. [DOI] [PubMed] [Google Scholar]
- 29.Reguera, G., and R. Kolter. 2005. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J. Bacteriol. 187:3551-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards, G. P. 1988. Microbial purification of shellfish: a review of depuration and relaying. J. Food Prot. 51:218-251. [DOI] [PubMed] [Google Scholar]
- 31.Roderick, G. E., and K. R. Schneider. 1994. Depuration and relaying of molluscan shellfish, p. 331-363. In C. R. Hackney and M. D. Pierson (ed.), Environmental indicators and shellfish safety. Chapman and Hall, New York, NY.
- 32.SAS Institute. 1998. Statview 5.0.1. SAS Institute, Cary, NC.
- 33.Sheth, H. B., K. K. Lee, W. Y. Wong, G. Srivastava, O. Hindsgaul, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1994. The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence beta GalNAc(1-4)beta Gal found in glycosphingolipids asialo-GM1 and asialo-GM2. Mol. Microbiol. 11:715-723. [DOI] [PubMed] [Google Scholar]
- 34.Shime-Hattori, A., T. Iida, M. Arita, K. S. Park, T. Kodama, and T. Honda. 2006. Two type IV pili of Vibrio parahaemolyticus play different roles in biofilm formation. FEMS Microbiol. Lett. 264:89-97. [DOI] [PubMed] [Google Scholar]
- 35.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47:565-596. [DOI] [PubMed] [Google Scholar]
- 36.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 37.Tacket, C. O., F. Brenner, and P. A. Blake. 1984. Clinical features and an epidemiological study of Vibrio vulnificus infections. J. Infect. Dis. 149:558-561. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration. 2003. National Shellfish Sanitation Program. Guide for the control of molluscan shellfish. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Washington, DC.
- 39.Wright, A. C., R. T. Hill, J. A. Johnson, M. C. Roghman, R. R. Colwell, and J. G. Morris, Jr. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zampini, M., L. Canesi, M. Betti, C. Ciacci, R. Tarsi, G. Gallo, and C. Pruzzo. 2003. Role for mannose-sensitive hemagglutinin in promoting interactions between Vibrio cholerae El Tor and mussel hemolymph. Appl. Environ. Microbiol. 69:5711-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]