Abstract
In the event of another influenza virus pandemic, strategies for effective mass vaccination will urgently be needed. We used a novel transdermal patch delivery technology, known as the PassPort system, to vaccinate mice with recombinant H5 hemagglutinin with or without immunomodulators. This needle-free form of vaccine delivery induced robust serum antibody responses that were augmented by different immunomodulators that stimulated the innate immune system and protected mice against lethal challenge with a highly pathogenic avian H5N1 influenza virus.
The world has experienced three influenza pandemics during the 20th century, including the devastating Spanish influenza pandemic of 1918-1919, in which approximately 40 million to 50 million people died worldwide. Highly pathogenic avian H5N1 influenza A viruses now pose a new pandemic threat to public health as they expand their geographical distribution in domestic and wild birds (17) throughout Asia and parts of the Middle East, Europe, and Africa. In addition, they exhibit substantial genetic and antigenic diversity (26) and an expanded mammalian host range (11).
Since late 2003, the World Health Organization has reported over 300 laboratory-confirmed cases of human H5N1 virus infection that resulted in over 180 deaths, primarily due to the direct transmission of the virus from infected birds to humans (25). If H5N1 viruses acquire the capacity for sustained human-to-human transmission, a pandemic may be inevitable. Vaccination against influenza virus is the most important public health measure that helps to protect against the annual morbidity and mortality associated with seasonal influenza virus outbreaks. However, the protection conferred by the currently licensed influenza virus vaccines, which offer protection against three circulating human strains, namely, H1N1, H3N2, and B, will not provide protection against H5N1 strains, as the protection induced by annual vaccines is subtype specific. Development of a vaccine against highly pathogenic H5N1 strains poses a number of challenges, such as containment, evaluation of its immunogenicity, the ability to meet the demand, and assurance of an uninterrupted supply of embryonated eggs. The use of vaccines produced by egg-independent technologies, such as tissue culture-derived vaccines and DNA vaccines, may provide a safer approach, but these methods of production are still in the experimental stages (1, 9, 14). Hence, attempts to develop a vaccine against pandemic avian influenza viruses were largely directed toward a recombinant hemagglutinin (rHA)-based approach or an inactivated subunit vaccine. These vaccines were derived from a surrogate nonpathogenic H5N3 virus or inactivated vaccines based on H5N1 reassortant vaccine strains that were generated by plasmid-based reverse genetics technology and that bore the N1 and genetically attenuated H5 glycoproteins and internal genes from A/Puerto Rico/8/34, a strain that grows at a high yield in embryonated eggs. However, when these vaccines were evaluated in humans, they all performed far below expectations, even at high antigen doses (5, 18, 23, 24), perhaps due to the poor immunogenicity of the avian HA.
In the event of a pandemic, effective mass immunization worldwide will require the development of not only more immunogenic and dose-sparing vaccines but also improved vaccine delivery technologies (19). Since a majority of pathogens enter the body through the skin or mucosal surfaces, the skin and mucosa have well-developed pathogen sensors and defenses, including an extensive network of resident professional antigen-presenting cells (3, 13, 22). The primary antigen-presenting cell type found in the epidermis, the Langerhans cell (LC), is a bone marrow-derived dendritic cell. After antigen capture, the activated LCs migrate to the draining lymph node, where they orchestrate potent systemic immune responses. Thus, a route of delivery through the skin offers a potential alternative to the traditional routes through injection for the development of more effective vaccines against pandemic strains. The stratum corneum, the outermost layer of skin, is an effective barrier to the penetration of fluids, large molecules, particles, and microbes. Hence, to deliver the antigen, disruption of this barrier is an absolute requirement. A variety of techniques (such as disruption of the stratum corneum by intradermal injection, by taper stripping, or with sand paper and the use of topical application, ultrasound, microneedles, hydration, and the gene gun) have been shown to deliver antigen through the skin and to generate immune responses with various degrees of success (10, 15, 16, 21). Glenn et al. (6) extended their seminal findings on the transcutaneous delivery of vaccines with adjuvants in BALB/c mice to humans with several phase I human clinical trials (7, 8). In the case of influenza virus, it has been shown that sparing of the vaccine dose can be achieved by the intradermal delivery of a seasonal influenza virus vaccine (2, 12). The translational nature of the studies described above substantiate the usefulness of the mouse model, despite the architectural differences, such as the location of melanocytes, between human skin and mouse skin.
In the present study we evaluated a novel needle-free transdermal patch delivery technology, the PassPort system. Transdermal immunization by use of the PassPort system creates 80 micropores within a 1-cm2 area with a disposable filament attached to an applicator applying an electrical current. This area is covered with a disposable liquid reservoir patch containing the relevant vaccine formulation (4). For transdermal immunization, the abdomens of mice were shaved (∼2 cm2), with care taken not to breach the integrity of the skin, 48 h prior to application of the patch. Using the PassPort system, we immunized three groups of female BALB/c mice (10 mice per group; ages, 10 to 12 weeks; Charles River Laboratories, Wilmington, MA) with 3 μg of baculovirus-expressed H5 rHA protein from the A/Hong Kong/156/97 (H5N1; HK/156) virus (Protein Sciences Corporation, Meriden, CT) either alone or with one of two adjuvants: 25 μg of CpG oligodeoxynucleotide (CpG ODN; TCCATGACGTTCCTGATCGT), a Toll-like receptor 9 (TLR9) ligand obtained from GENSET Corp. (La Jolla, CA), or 30 μg of R-848 (resiquimod hydrochloride), a TLR7 ligand purchased from GL Synthesis (Worcester, MA). The fourth group of mice was immunized transdermally with 3 μg of bovine serum albumin (BSA) as a negative control. A fifth group was immunized by intraperitoneal (i.p.) injection of 106 50% egg infective doses (EID50s) of A/DK/Singapore-Q/F119-3/97 (Dk/Sing), an avian influenza H5N3 virus with low pathogenicity, as a positive control. Patches from the transdermally immunized groups were removed after overnight application. After two booster immunizations given at 4-week intervals, sera were collected 3 weeks after the final immunization to assess the development of HK/156 virus-specific hemagglutination-inhibition (HI) antibody by using horse red blood cells, as described previously (20). As shown in Table 1, transdermal immunization with H5HA elicited HI titers that were significant (P < 0.05) compared to the titers obtained for the negative control group immunized transdermally with BSA. Compared to results for the H5HA group, the use of CpG ODN, but not R-848, significantly increased the HI titers (P < 0.05) to levels comparable to those in animals immunized by i.p. injection of live virus.
TABLE 1.
TLR9 ligand (CpG ODN) but not TLR7 ligand (R-848) enhances the immunogenicity of transdermally delivered H5 HA
| Group | Prechallenge HI titer (GM)a | Survival (%) with challenge with:
|
|
|---|---|---|---|
| 10 LD50s | 50 LD50s | ||
| BSA | 10 | 0 | 0 |
| H5HA | 78 | 100 | 0 |
| H5HA + CpG ODN | 258 | 100 | 83 |
| H5HA + R-848 | 29 | 100 | 40 |
| H5N3 virus i.p. | 236 | 100 | 100 |
GM, geometric mean. The HI titers in the group that received H5HA plus CpG were significantly higher than those in the group that received H5HA only (P < 0.05) or the group that received H5HA plus R-848 (P = 0.001), as determined by Tukey's multiple-comparison test.
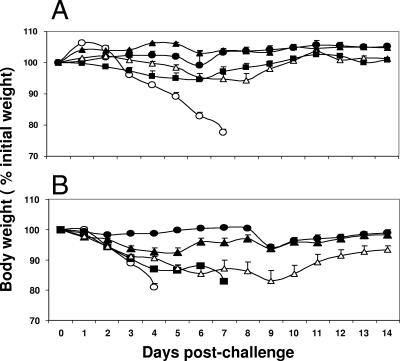
The level of protective immunity induced by vaccination was determined by challenging five mice in each group intranasally with 10 times (Fig. 1A) or 50 times (Fig. 1B) the 50% lethal dose (LD50) of A/Hong Kong/483/97 (HK/483) virus, an H5N1 virus that is antigenically similar to the HK/156 virus and that is highly pathogenic in mammals. The mice were monitored for clinical signs and changes in body weight daily for 14 days postchallenge. The mean percent loss in body weight was determined daily as an indicator of morbidity after challenge. Mice vaccinated transdermally with H5HA plus CpG or i.p. with live Dk/Sing virus either did not lose weight (Fig. 1A) or lost on average ≤6% of their body weight (Fig. 1B) and survived the lethal H5N1 infection, whereas mice vaccinated transdermally with BSA lost an average of >20% of their body weight and succumbed to the H5N1 infection by day 8 postinfection.
FIG. 1.
Transdermal immunization with H5HA plus CpG ODN (a TLR9 ligand) as the adjuvant completely protects mice against morbidity following challenge with a lethal H5N1 virus. Groups of BALB/c mice were immunized transdermally with 3 μg of H5HA protein alone (▪) or with the H5HA protein with 25 μg of CpG ODN (▴) or 30 μg of R-848 (Δ). Control animals were immunized transdermally with 3 μg of BSA (○) or i.p. with 106 EID50s of A/DK/Singapore-Q/F119-3/97 influenza virus (•). After two booster immunizations with the same formulation delivered at intervals of 4 weeks, five mice per group were challenged intranasally with 10 LD50s (A) or 50 LD50s (B) of HK/483 virus in 50 μl PBS while they were under light anesthesia. Individual mice were monitored for clinical signs and changes in body weight every day for 14 days. Error bars represent the standard errors of the means.
Our results demonstrate that needle-free transdermal patch delivery induces protective humoral immune responses against lethal challenge with a highly pathogenic avian H5N1 influenza virus. Furthermore, addition of an adjuvant such as CpG ODN, but not R-848, enhanced the immunogenicity of the transdermally delivered vaccine against a pandemic influenza virus. Our findings suggest that the immunogenicity of current prepandemic influenza virus vaccines may be improved substantially by the use of novel adjuvants and the needle-free transdermal method of immunization. Furthermore, the disposable metal filament that facilitates the microporation on the skin addresses and eliminates the potential transmission of blood-borne pathogens. This delivery method has the potential for rapid needle-free immunization of large populations against pandemic influenza virus.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Bardiya, N., and J. H. Bae. 2005. Influenza vaccines: recent advances in production technologies. Appl. Microbiol. Biotechnol. 67299-305. [DOI] [PubMed] [Google Scholar]
- 2.Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 3512286-2294. [DOI] [PubMed] [Google Scholar]
- 3.Bergstresser, P. R., G. B. Toews, J. N. Gilliam, and J. W. Streilein. 1980. Unusual numbers and distribution of Langerhans cells in skin with unique immunologic properties. J. Investig. Dermatol. 74312-314. [DOI] [PubMed] [Google Scholar]
- 4.Bramson, J., K. Dayball, C. Evelegh, Y. H. Wan, D. Page, and A. Smith. 2003. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 10251-260. [DOI] [PubMed] [Google Scholar]
- 5.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 3671657-1664. [DOI] [PubMed] [Google Scholar]
- 6.Glenn, G. M., M. Rao, G. R. Matyas, and C. R. Arving. 1998. Skin immunization made possible by cholera toxin. Nature 391851. [DOI] [PubMed] [Google Scholar]
- 7.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 61403-1406. [DOI] [PubMed] [Google Scholar]
- 8.Guerena-Burgueno, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 701874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halperin, S. A., B. Smith, T. Mabrouk, M. Germain, P. Trepanier, T. Hassell, J. Treanor, R. Gauthier, and E. L. Mills. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 201240-1247. [DOI] [PubMed] [Google Scholar]
- 10.Hammond, S. A., M. Guebre-Xabier, J. Yu, and G. M. Glenn. 2001. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit. Rev. Ther. Drug Carrier Syst. 18503-526. [PubMed] [Google Scholar]
- 11.Keawcharoen, J., K. Oraveerakul, T. Kuiken, R. A. Fouchier, A. Amonsin, S. Payungporn, S. Noppornpanth, S. Wattanodorn, A. Theambooniers, R. Tantilertcharoen, R. Pattanarangsan, N. Arya, P. Ratanakorn, D. M. Osterhaus, and Y. Poovorawan. 2004. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 102189-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 3512295-2301. [DOI] [PubMed] [Google Scholar]
- 13.Kupper, T. S. 1990. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J. Clin. Investig. 861783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabrouk, T., and R. W. Ellis. 2002. Influenza vaccine technologies and the use of the cell-culture process (cell-culture influenza vaccine). Dev. Biol. (Basel) 110125-134. [PubMed] [Google Scholar]
- 15.Matriano, J. A., M. Cormier, J. Johnson, W. A. Young, M. Buttery, K. Nyam, and P. E. Daddona. 2002. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 1963-70. [DOI] [PubMed] [Google Scholar]
- 16.Mikszta, J. A., V. J. Sullivan, C. Dean, A. M. Waterston, J. B. Alarcon, J. P. Dekker III, J. M. Brittingham, J. Huang, C. R. Hwang, M. Ferriter, G. Jiang, K. Mar, K. U. Saikh, B. G. Stiles, C. J. Roy, R. G. Ulrich, and N. G. Harvey. 2005. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 191278-288. [DOI] [PubMed] [Google Scholar]
- 17.Monto, A. S. 2005. The threat of an avian influenza pandemic. N. Engl. J. Med. 352323-325. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 3571937-1943. [DOI] [PubMed] [Google Scholar]
- 19.Sambhara, S., and G. A. Poland. 2006. Avian influenza vaccines: what's all the flap? Lancet 3671636-1638. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson, I., J. M. Wood, K. G. Nicholson, A. Charlett, and M. C. Zambon. 2004. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 10391-95. [DOI] [PubMed] [Google Scholar]
- 21.Tezel, A., S. Paliwal, Z. Shen, and S. Mitragotri. 2005. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 233800-3807. [DOI] [PubMed] [Google Scholar]
- 22.Toews, G. B., P. R. Bergstresser, and J. W. Streilein. 1980. Langerhans cells: sentinels of skin associated lymphoid tissue. J. Investig. Dermatol. 7578-82. [DOI] [PubMed] [Google Scholar]
- 23.Treanor, J. J., B. E. Wilkinson, F. Masseoud, J. Hu-Primmer, R. Battaglia, D. O'Brien, M. Wolff, G. Rabinovich, W. Blackwelder, and J. M. Katz. 2001. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 191732-1737. [DOI] [PubMed] [Google Scholar]
- 24.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 3541343-1351. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2007. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_04_11/en/index.html. Accessed 4 May 2007.
- 26.World Health Organization Global Influenza Program Surveillance Network. 2005. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 111515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]