Abstract
Bordetella pertussis is the causative agent of pertussis (whooping cough). Despite high vaccination coverage, pertussis remains a significant disease in many countries. Besides vaccination, transient carriage of Bordetella spp. or other cross-reacting organisms adds to the immunity against pertussis. However, the various immunological mechanisms conferring protection remain largely unknown. In this study, paired serum samples from 464 healthy Norwegian military recruits were collected, the first at enrolment and the second about 8 months later. The prevalence of pertussis during military service was examined by comparing the paired serum samples for immunoglobulin G (IgG) antibodies against pertussis toxin (PT) by enzyme-linked immunosorbent assay (ELISA). Seventy-eight percent of the recruits had low levels of IgG antibodies against PT in both samples. Conversely, 8.4% of the recruits demonstrated high anti-PT IgG levels in the first sample, indicative of recent pertussis prior to enrolment. One recruit experienced seroconversion, indicating pertussis during service. A subset of 248 serum samples with low, medium, and high anti-PT IgG titers were analyzed by a different ELISA kit for IgG and IgA antibodies against PT and filamentous hemagglutinin (FHA) and for opsonophagocytic activity (OPA), for induction of C3b deposition products, and for IgG binding with live B. pertussis as the antigen. We observed high correlations between OPA and IgG against live bacteria (r = 0.83), between OPA and IgG anti-FHA (r = 0.79), between OPA and anti-PT IgG (r = 0.68), and between OPA and C3b binding (r = 0.70) (P < 0.0001 for all). Anti-PT IgA did not correlate closely with the other assays.
Immunization against pertussis (whooping cough) has been part of the child vaccination programs in many countries for several decades. Despite high vaccine coverage, pertussis represents a significant contribution to disease in many age groups (40). Although disease risk and severity are highest in nonimmunized children, vaccine-induced protection wanes over the years and an increased incidence of pertussis in adolescent and adults represents both an important disease burden and a reservoir for spreading the disease to nonimmunized children.
The bacterium Bordetella pertussis causes localized infection of the respiratory mucosa without systemic spreading and induces systemic T- and B-cell immune responses (26). Much of the pathology of pertussis can be explained by virulence factors produced by the bacteria during the early colonization process, e.g., pertussis toxin (PT), adenylate cyclase toxin (ACT), dermonecrotic toxin, and tracheal cytotoxin (15). PT exists as both cell-bound and secreted molecules. Cell-bound PT is, together with adhesins like filamentous hemagglutinin (FHA), pertactin, and fimbriae, an important factor for growth and colonization of the upper respiratory tract by B. pertussis. Although it is still unclear how the protective immune response against pertussis works, antibodies against the various toxins and colonization factors have proved protective (15). The new acellular pertussis vaccines containing inactivated PT and various combinations of bacterial adhesins are documented to give immunity against pertussis (9, 41). In particular, antibodies against PT, pertactin, and fimbriae have been shown to correlate with immunity (34). Antibodies to toxins and adhesins probably neutralize toxins directly and inhibit bacteria from binding to host cells, respectively (37). However, other antibody-mediated mechanisms like opsonophagocytosis may also play important roles in protection (16). Indeed, polymorphonuclear neutrophils (PMNs) are a distinct cell type of the acutely inflamed mucosa (14) harboring complement receptors (CRs) and Fc receptors to execute opsonophagocytosis. Different methods for measuring opsonophagocytosis against B. pertussis have been used, with rather inconsistent results (16, 19, 27, 31-33, 42). Some studies found no correlation between immunity against pertussis and opsonophagocytic activity (OPA) or direct complement-mediated killing (42, 44, 45), whereas others found that antibody specificity is important for inducing phagocytosis (11, 19, 43). Once phagocytosed, however, B. pertussis bacteria are readily killed by PMNs (16, 27).
The number of pertussis notifications in Norway has been increasing since 1997 in all age groups, with an incidence of 170 cases per 100,000 population in 2004. The highest incidence rate was recorded in infants under 6 months of age (392 cases per 100,000) (http://www.msis.no/). The acellular pertussis vaccine replaced the whole-cell vaccine in 1998.
In this study, we have analyzed the serological immune response against B. pertussis in paired serum samples from healthy young military recruits by using different immunological assays. The study had two goals. One was to record the incidence of pertussis among first-time military recruits; the other was to compare various serological tests for detection of antibodies against pertussis.
B. pertussis harbors species-specific antigens but also cross-reacting antigens shared with other Bordetella species and possibly other bacterial species (6, 12, 13, 39). Conceivably, several of these shared antigens may give rise to antibodies important for protection. In this study, immunoglobulin G (IgG) and IgA antibodies against PT and FHA were measured by two different enzyme-linked immunosorbent assays (ELISAs), whereas total anti-B. pertussis IgG antibodies were quantified against live B. pertussis by a flow cytometry method.
The membrane-located BrkA (bordetella resistance to killing) protein may arrest antibody-induced complement activation and thus protect B. pertussis from complement-mediated lysis (5). Although direct complement-mediated bactericidal activity seems to be a less important effector function for immunity against pertussis (42, 45), complement activation may add significant contributions to opsonophagocytosis. In particular, activation of the complement protein C3 causes deposition of C3 split products (C3b) on target structures, thus serving as opsonins for phagocytic cells. We therefore measured the C3b deposition on live B. pertussis induced by the recruits' serum samples.
OPA was measured as a respiratory burst which may be regarded as a more terminal step of the phagocytic process. A respiratory burst may be more relevant for protection against pertussis than just measurement of internalization of the bacteria. It has been reported that B. pertussis may use the FHA interaction with CR3 as a docking receptor and thus enter phagocytic cells silently without triggering bactericidal effector functions like a respiratory burst (38).
The present study is, to our knowledge, the first study in which a comprehensive panel of human serum samples (n = 248) was analyzed for OPA and the results were compared to other anti-B. pertussis serological activities.
MATERIALS AND METHODS
Study population.
The study population consisted of conscripts enrolled for military service in August 2004. Both males and females were recruited into the study; however, less than 5% of the participants were females. The individual vaccination status of the participants was unknown; however, most of the subjects were likely to have received three doses of diphtheria, tetanus, and whole-cell pertussis vaccine (Trivax; Wellcome, United Kingdom) within the first year of life. The vaccine coverage in this year group is estimated to be 85 to 90%. Pertussis vaccine had not been given later to subjects in this study population. The present project is part of a larger study, IR04, in which we also studied the immune response against other vaccines (36). All participants received a booster dose of morbilli, mumps, and rubella vaccine on the day of enrolment after the blood sample was collected. Written informed consent was obtained from all of the conscripts who assented to participate in the study. The Norwegian Regional Committee of Medical Research Ethics approved the study. A biobank for the collected specimens was established and registered according to current guidelines.
Collection of human serum samples.
Paired blood specimens from 464 military recruits (median age, 19 years; range, 19 to 27 years) were collected, the first at enrolment and the second after 8 months of service. These serum samples, designated S1 and S2, respectively, were stored at −20°C until assayed.
Bacterial strain.
A nasopharyngeal clinical isolate of B. pertussis (Norwegian Institute of Public Health reference number 105/06) serotype 1, 3, from a 30-year-old woman with clinical pertussis, was used as the target strain in the OPA assay, in the C3 deposition assay, and in the measurements of IgG against live bacteria. The bacteria were grown on Bordet-Gengou agar plates for 2 days and stored at 4°C until use, usually within 1 week. More than 90% viability of the bacteria was confirmed by a LIVE/DEAD Bac/Light kit (Molecular Probes, Invitrogen, Carlsbad, CA).
Antibody analysis against specific vaccine antigens and study design.
All serum samples were screened for IgG antibodies against PT by the Serion classic ELISA (Serion Immunodiagnostica GmbH, Würzburg, Germany) according to the manufacturer's instructions. An IgG level of >80 FDA U/ml indicates recent infection, borderline IgG levels are within 20 to 30 FDA U/ml, and <20 FDA U/ml indicates negative IgG levels. On the basis of the levels of IgG against PT from the Serion assay, 124 selected serum sample pairs were analyzed by the other assays. Specific IgG and IgA antibodies against FHA and PT were analyzed by Pertusscan 2 + 2 (Euro-Diagnostica AB, Malmö, Sweden) according to the manufacturer's instructions, and OPA, IgG binding, and C3 deposition were analyzed with live B. pertussis as target cells. The Serion classic ELISA is a quantitative assay, and the results are reported in FDA units per milliliter. Pertusscan 2 + 2 is a semiquantitative assay, and the results are given as a percentage of the cutoff (the cutoff is an optical density [OD] of 0.3, which equals 100%).
OPA.
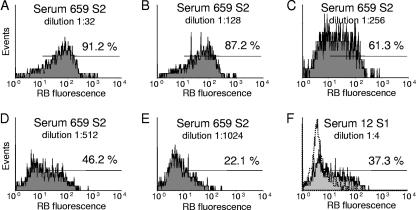
OPA was measured as a respiratory burst, as previously described for group B meningococci (2). Live B. pertussis cells were used as targets, and dihydrorhodamine 123 (DHR 123; Molecular Probes, Invitrogen)-primed PMNs from a donor heterozygous for the FcγRIIa allotype were used as effector cells. DHR 123 is a nonfluorescent probe that is converted to green-fluorescent rhodamine 123 within a phagocyte during a respiratory burst (28). Briefly, on 96-well microtiter plates, twofold dilution series of the various serum samples (50 μl) in Hanks' balanced salt solution supplemented with bovine serum albumin (2 mg/ml) (HBSS/BSA) were mixed with a 5-μl suspension of B. pertussis (OD at 650 nm [OD650] = 0.53) and incubated for 50 min at 37°C under agitation. As a negative control, the test serum was replaced with HBSS/BSA. Next, 5 μl human serum which had been passed through a protein G column (HiTrap protein G HP; GE Healthcare, Oslo, Norway) to remove IgG antibodies against B. pertussis was added as a complement source and the mixture was incubated further for 10 min. Finally, DHR 123-primed PMNs (50 μl) were added and incubation was continued for another 10 min. The PMNs were prepared from 21 ml heparinized venous blood from which the red cells had been removed by lysis in an ammonium chloride solution. The PMNs were washed three times with HBSS/BSA, resuspended in 11 ml HBSS/BSA, and primed with 11 μl DHR 123 (10 mg/ml). The responses were measured by flow cytometry with a Partec CyFlow flow cytometer (Partec GmbH, Münster, Germany), gating on the PMN population with a two-parameter forward scatter/side scatter dot plot. The analytical region for detecting respiratory-burst-positive cells was set on an FL1 histogram while running a negative control sample. The positive region was thus drawn to include less than 15% of the PMNs (exemplified in Fig. 1F, stippled line). The highest reciprocal serum dilution giving a ≥50% respiratory burst of the PMNs was recorded as the serum titer. A late-convalescent-phase serum sample from a subject with a confirmed case of pertussis that was collected 18 months after the onset of the disease was included on every microtiter plate.
FIG. 1.
Flow cytometry histograms showing the respiratory-burst (RB) profiles of five different dilutions of positive S2 serum sample 659 (A to E) and one dilution of S1 serum sample 12 with low activity (F). The histograms are gated on the PMN population in a two-parameter dot plot of forward versus side scatter histograms to discriminate lymphocytes, monocytes, and PMNs (not shown). The horizontal bar defines the area of RB-positive cells, and the value above the bar is the percentage of positive PMNs. S2 serum sample 659 had an OPA titer of 256, whereas S1 serum sample 12 had a titer of <4. Overlaid in panel F is a profile of a negative control sample, i.e., dilution buffer instead of test serum (stippled histogram).
Quantitation of IgG antibodies against live B. pertussis by flow cytometry.
Specific IgG antibodies against B. pertussis were measured with the same preparation of live bacteria that we use in the OPA assay. The method was a slightly modified form of a similar procedure used to quantify IgG antibodies against group B meningococci (2). A twofold dilution, in HBSS/BSA, of a serum sample from an adult who had just recovered from confirmed pertussis was used to create the standard curve, and unknown serum samples were screened at a 1:40 dilution. Onto a U-shaped 96-well microtiter plate, 50 μl of diluted serum or standard serum was mixed with 10 μl of live B. pertussis (OD650 = 0.5). After incubation for 50 min at 37°C with agitation, the bacteria were washed three times in HBSS/BSA by centrifugation at 1,100 × g for 3 min. Finally, bound antibody was visualized after 1 h of incubation at 37°C with a fluorescein isothiocyanate (FITC)-labeled goat anti-human IgG conjugate (Cappel, ICN Pharmaceuticals, Aurora, OH), followed by one washing. The samples were analyzed with the Partec CyFlow flow cytometer, and the pertussis-specific serum IgG concentration was calculated from the geometric mean fluorescence intensity with GraphPad Prism software, version 1.02 (GraphPad Software Inc., San Diego, CA). Pertussis-specific serum IgG concentrations are reported in arbitrary units (AU) with the standard serum IgG concentration designated as 1,000 AU/ml. The upper and lower limits of detection were 8,000 and 62.5 AU/ml, respectively; serum samples falling above or below these limits were set to 8,000 or 31.2 AU/ml.
Quantitation of C3 deposition on live B. pertussis by flow cytometry.
In the presence of a complement source, specific antibody to B. pertussis may induce complement activation and subsequent deposition of complement activation products at the bacterial surface. The amount of deposits can be measured by using FITC-labeled anti-C3c conjugate (C3c is part of the larger C3b protein that becomes covalently attached to target structures when activated by C3 convertases). Twofold dilutions of a serum sample from a recruit with high anti-B. pertussis IgG levels were used to establish a standard curve ranging from 1:20 to 1:1,280. The C3b binding obtained at 1:20 was given a value of 200 AU ml−1. All serum samples were tested diluted 1:40 in HBSS/BSA. As a negative control, the test serum was replaced with HBSS/BSA. On a microtiter plate, 50 μl of diluted serum was mixed with 10 μl of live suspended B. pertussis (OD650 = 0.5) and incubated for 50 min at 37°C with agitation. Thereafter, human serum previously passed through a protein G column was added as a complement source to a final concentration of 6%, and the incubation was continued for 12 min at 37°C. The bacteria were washed three times in HBSS/BSA by centrifugation at 1,100 × g for 3 min. Finally an FITC-labeled rabbit anti-human C3c reagent (Dako, Glostrup, Denmark) was added. After incubation for 50 min at 37°C with agitation, the bacteria were washed once in HBSS/BSA by centrifugation at 1,100 × g for 3 min. The samples were analyzed with the Partec CyFlow flow cytometer, and the mean fluorescence intensity was used to calculate the amount of C3 deposits on the bacteria with GraphPad Prism software.
Statistical analysis.
Geometric means and 95% confidence intervals (CIs) of the geometric means were used to analyze differences between groups. The Wilcoxon signed-rank test was used for comparisons between paired serum samples. Pearson's correlations were calculated by using log2-transformed data to look for a relationship between the different assays. GraphPad Prism was used for all statistical calculations.
RESULTS
High incidence of pertussis prior to military service.
All serum samples were screened for IgG antibodies against PT by the Serion classic ELISA. Paired serum samples were always analyzed together. The geometric mean levels of IgG against PT at entry into service (S1) was 10.2 FDA U/ml (95% CI, 9.1 to 11.5 FDA U/ml), whereas at the end of service (S2) it had declined significantly to 8.3 FDA U/ml (95% CI, 7.4 to 9.3 FDA U/ml) (P < 0.0001, Wilcoxon signed-rank test). Of a total of 464 recruits, 361 (78%) revealed <30 FDA U/ml anti-PT IgG (Serion) in both serum samples and 34 of these reached borderline level with values of 20 to 30 FDA U/ml in one of the samples (Table 1). Interestingly, 39 recruits (8.4%) demonstrated levels of IgG against PT of >80 FDA U/ml (Serion) in the S1 sample, indicating recent B. pertussis infection prior to enrolment. All but one of these recruits revealed higher values in S1 (165.4 FDA U/ml; 95% CI, 142.3 to 192.3 FDA U/ml) than in S2 (100.0 FDA U/ml; 95% CI, 82.9 to 120.5 FDA U/ml). However, less than half of these were confirmed by the Pertusscan ELISA. Along with this, we found no samples positive for recent pertussis with the Pertusscan assay that was not caught by the Serion assay.
TABLE 1.
Serion anti-PT IgG levels in paired serum samples collected from all participantsa
| Sample groupb (no. of samples) | Geometric mean activity (FDA U/ml)d
|
|
|---|---|---|
| S1 | S2 | |
| 1 (361) | 6.0 (5.6, 6.5) | 4.8 (4.5, 5.2) |
| 2c (64) | 37.8 (31.7, 45.2) | 37.4 (33.5, 41.8) |
| 3 (39) | 165.4 (142.3, 192.3) | 100.0 (82.9, 120.5) |
Samples were collected at entry into military service (S1) and 8 months later (S2). Paired serum samples were grouped on the basis of Serion PT IgG activity.
Serion PT IgG levels were as follows: for group 1, <30 FDA U/ml in both S1 and S2; for group 2, 30 to 80 FDA U/ml in S1 and/or S2; and for group 3, >80 FDA U/ml in S1.
Also included in group 2 is the one subject with seroconversion during service (reaching 103.0 FDA U/ml in the S2 sample).
Values in parentheses are 95% CIs.
Low incidence of pertussis during the military service.
Only one subject had seroconversion indicating B. pertussis infection during military service, revealed as a concentration of IgG antibodies against PT of 4.0 FDA U/ml (Serion) in the S1 sample, compared to 103.0 FDA U/ml in the S2 sample. Correspondingly, we observed a 4.8-fold increase in the level of IgG antibodies against FHA (Pertusscan), a 7.3-fold increase in IgG against PT (Pertusscan), and an 8.2-fold increase in IgA against FHA (Pertusscan), whereas the OPA titer showed a 32-fold increase, C3 activation showed a 3.5-fold increase, and IgG binding to live B. pertussis showed a 5.7-fold increase. We have no clinical information to confirm the laboratory findings in this case.
Correlation between different assays.
On the basis of the Serion PT IgG levels, the serum samples were divided into three groups, i.e., <30 FDA U/ml (no or low levels of antibodies), >30 and <80 FDA U/ml, and >80 FDA U/ml (high levels of antibodies, indicative of recent infection). Serum pairs from these three groups were selected for further analysis. All pairs (n = 40) with IgG levels of >80 FDA U/ml in pre- and/or postservice serum samples, 42 pairs (out of 62) with activities between 30 and 80 FDA U/ml in pre- and/or postservice serum samples, and 42 pairs (out of 361) with IgG activities of <30 FDA U/ml in both serum pairs were included in the other analysis, i.e., Pertusscan 2 + 2 assay of IgG and IgA antibodies against PT and FHA, OPA, IgG binding, and C3 deposition on B. pertussis with live bacteria.
Table 2 shows the geometric mean activities and the 95% CIs of the geometric mean results of each test of these 248 serum samples sorted by their Serion PT IgG activities. As indicated, we observed nonoverlapping CIs for all assays but IgA antibodies against PT (Pertusscan). In line with this, there was a positive correlation between all assays except for IgA antibodies against PT, which revealed very low correlations with the other tests (Table 3).
TABLE 2.
Geometric mean activities measured by the different assaysa
| Sample group (no. of samples)b | Geometric mean activity (95% CI)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Serion PT IgG (FDA U/ml) | Pert FHA IgG (% of cutoff) | Pert FHA IgA (% of cutoff) | Pert PT IgG (% of cutoff) | Pert PT IgA (% of cutoff) | OPA against live bacteria (titer) | C3b against live bacteria (AU/ml) | IgG against live bacteria (AU/ml) | |
| 1 (92) | 4.9 (4.1, 5.9) | 126.1 (113.1, 140.4) | 54.6 (49.3, 60.6) | 101.0 (92.1, 110.8) | 82.5 (76.6, 88.8) | 49.2 (38.1, 63.5) | 232.6 (206.0, 262.6) | 62.6 (50.1, 78.3) |
| 2 (89) | 47.7 (45.2, 50.5) | 246.6 (229.1, 265.5) | 92.0 (80.1, 105.7) | 267.4 (255.4, 279.9) | 80.5 (75.2, 86.2) | 227.8 (185.8, 279.2) | 520.3 (435.8, 621.1) | 375.4 (291.6, 483.2) |
| 3 (67) | 150.9 (135.3, 168.1) | 309.5 (283.5, 337.9) | 125.8 (108.0, 146.4) | 491.8 (466.2, 518.7) | 91.4 (83.8, 99.8) | 429.4 (343.6, 536.7) | 1,031.0 (799.2, 1,330.0) | 843.4 (620.4, 1,146.0) |
Pert, Pertusscan.
All 248 serum samples (124 paired serum samples) were sorted by IgG anti-PT levels from the Serion assay. Groups were defined as described in footnote b of Table 1.
TABLE 3.
Correlation between different assays of the 248 serum samples testeda
| Test | Pearson's correlation coefficient (r) with:
|
||||||
|---|---|---|---|---|---|---|---|
| Serion PT IgG | Pertusscan FHA IgG | Pertusscan FHA IgA | Pertusscan PT IgG | Pertusscan PT IgA | IgG against live bacteria | OPA against live bacteria | |
| Pertusscan FHA IgG | 0.72 | ||||||
| Pertusscan FHA IgA | 0.51 | 0.65 | |||||
| Pertusscan PT IgG | 0.95 | 0.72 | 0.53 | ||||
| Pertusscan PT IgA | 0.14b | 0.19c | 0.49 | 0.25 | |||
| IgG live bacteria | 0.67 | 0.72 | 0.56 | 0.68 | 0.22d | ||
| OPA live bacteria | 0.68 | 0.79 | 0.64 | 0.69 | 0.27 | 0.83 | |
| C3b live bacteria | 0.59 | 0.52 | 0.47 | 0.61 | 0.21e | 0.76 | 0.70 |
Unless specified otherwise, P < 0.0001.
P = 0.03.
P = 0.0027.
P = 0.0006.
P = 0.001.
IgG antibodies against PT were measured by the Serion and Pertusscan ELISAs. The two assays gave similar results (r = 0.95) (Table 3). There was also a high correlation between anti-PT and anti-FHA IgG levels (r = 0.72).
In addition to measuring specific IgG antibodies against PT and FHA by ELISA, we also quantified levels of IgG against live B. pertussis by flow cytometry. Since it is unlikely that the study participants had received any pertussis vaccine after 1 year of age, any raised antibody level must have been induced by B. pertussis or a cross-reacting organism. It was thus of interest to measure antibody activity against whole, live bacteria. In the serum samples with Serion PT levels of <30 FDA U/ml, the geometric mean level of IgG against live B. pertussis was 62.6 AU/ml (Table 2). The serum samples with higher levels of IgG against PT demonstrated similarly raised levels of IgG against live bacteria. Thus, there was a significant correlation between IgG binding to live bacteria and IgG antibodies against PT (Serion) (r = 0.67) and to IgG antibodies against FHA (r = 0.72) (Table 3).
Complement activation leading to deposition of the C3 split product on target cells may add a substantial contribution to the opsonophagocytic process by interaction with CRs on phagocytic cells. A high correlation between C3 deposition and IgG binding to live bacteria was observed (r = 0.76), whereas the corresponding correlations between anti-PT and anti-FHA IgG levels were lower, at r = 0.59 and r = 0.52, respectively (Table 3). Several serum samples, particularly those with low levels of IgG antibody against live B. pertussis, revealed low C3 deposition activity, similar to that of the negative control (i.e., HBSS/BSA instead of test serum).
Opsonophagocytosis was quantified as a respiratory burst induced in PMNs by the recruits' serum samples against live B. pertussis. This respiratory-burst protocol proved to be reliable and reproducible against B. pertussis. There was no respiratory burst of the negative control (sample without test serum), indicating that the bacteria alone in the presence of complement serum and PMNs did not induce any burst. A positive control (late-convalescent-phase) serum sample revealed a titer of 128 to 256 throughout the whole experiment. Figure 1A to E show a representative example of a respiratory burst from a twofold dilution of a recruit serum sample giving a titer of 256. Experiments following the same respiratory-burst protocol, but with fluorescence-labeled live B. pertussis and omitting DHR 123 labeling of the effector cells, indicate that the bacteria are internalized by the PMNs with a positive serum sample but not with a negative serum sample (data not shown).
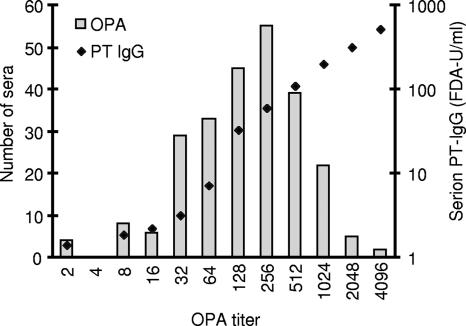
Most of the 248 serum samples analyzed demonstrated OPA. Forty-seven serum samples revealed OPA titers of ≤32, 133 had titers ranging from 64 to 256, and 68 had OPA titers of ≥512 (Fig. 2). In the absence of test serum, there was no OPA. Only two recruits demonstrated OPA titers of <4, i.e., without detectable activity (Fig. 1F). We observed a significant antibody-induced respiratory burst that correlated with IgG binding to live B. pertussis (r = 0.83) and to C3 deposition (r = 0.70) (Table 3), suggesting that opsonization may play an important role in immunity against B. pertussis. There was a higher correlation with IgG anti-FHA than with anti-PT IgG (Serion) (r = 0.79 and r = 0.68, respectively).
FIG. 2.
Distribution of OPA titers and corresponding anti-PT IgG levels. The bars represent the number of samples at each OPA titer, and the diamonds reflect the geometric mean Serion PT IgG level at each OPA titer. All serum samples, both S1 and S2, are included.
Can OPA be used as a marker of protective activity against pertussis?
Of the 248 serum samples that were analyzed by all of the assays, 92 had <30 FDA U/ml of IgG antibodies against PT by the Serion assay (Fig. 2). Of these 92 serum samples, 50% (46 serum samples) demonstrated OPA titers of ≥64 (median, 128; range, 64 to 1,024), and consequently, the other 46 serum samples had OPA titers of <64 (median, 32; range, 2 to 32). Of the 89 serum samples with Serion PT IgG levels ranging from 30 to 80 FDA U/ml, 1 revealed an OPA titer of <64 (titer = 2); all of the rest had OPA titers of ≥64 (median, 256; range, 64 to 2,048). And of the 67 serum samples with Serion PT IgG levels of >80 FDA U/ml, all demonstrated OPA titers of ≥64 (median, 512; range, 64 to 4,096).
DISCUSSION
This study confirms that an immunization regimen of three doses of pertussis vaccine during the first year of life does not sustain elevated levels of antibody against B. pertussis in late adolescence. Thus, 78% of the recruits tested had low levels of IgG antibody against PT (<30 FDA U/ml) and 8.4% had serological indications of recent B. pertussis infection at entry into military service (anti-PT IgG levels of >80 FDA U/ml) (8). Only one recruit demonstrated significant seroconversion against B. pertussis during the 8 months of service. It might be that people in the relatively closed community of a military camp are less exposed to circulating B. pertussis than are those in society at large, e.g., in a school setting. The participants performed their military service at three different camps in northern Norway, and in line with our serological results, there was no recorded pertussis outbreak during that period.
There was a low but significant decrease in the level of IgG antibodies against PT during the study period. Even excluding the paired S1 serum samples with >80 FDA U/ml, there was a significant decrease in anti-PT IgG levels from S1 to S2 (Table 1). Similar results were observed for anti-FHA IgG levels. This indicates that many of the participants had experienced contact with B. pertussis or related organisms prior to enrolment and that these antigens were less prevalent among the recruits during their service.
Although the correlation between the anti-PT IgG levels measured by the Serion and Pertusscan ELISAs was very high (r = 0.95), fewer than half of the recruits with serological indications of recent pertussis obtained by the Serion ELISA were confirmed by the Pertusscan ELISA. It may be that the Pertusscan criteria for defining actual pertussis (combined high levels of IgG and/or IgA against PT and/or FHA) are stricter than the Serion criterion (>80 FDA U/ml).
We observed a positive correlation between the level of IgA against FHA and other serological assays, whereas there was a very low correlation between the IgA responses against PT and the other assays. Generally, the levels of IgA against PT were low (Table 2). Even among the subjects with high levels of IgG against PT (>80 FDA U/ml), the IgA levels remained low. Since we do not know when these subjects were exposed to B. pertussis prior to enrolment, any raised anti-PT IgA level may thus have returned to the baseline level by the time the S1 serum was collected. Heininger et al. demonstrated that the IgA response against PT declined to the baseline within a few months (10), whereas the anti-PT IgG level remained increased for a longer period.
When looking for a better correlate for protection, it may be relevant to look at antibodies against other antigens in addition to PT and FHA. PT is perhaps the only species-specific antigen for B. pertussis. A number of antigens that may confer protective immunity against pertussis are shared with Bordetella bronchiseptica, Bordetella parapertussis, and possibly other Bordetella spp. (21, 24). Cross-reacting antibodies have been ascribed to non-Bordetella species like Chlamydia pneumoniae or Mycoplasma pneumoniae (39), and nonencapsulated Haemophilus influenzae expresses antigens that induce antibodies cross-reacting with FHA (3, 6). By measuring antibodies against live B. pertussis, we may quantify all antibody specificities binding to the surface of intact bacteria. These are the sum of antibodies that initiate in vivo effector functions like phagocytosis, complement-mediated bactericidal activity, and inhibition of bacterial adherence. Accordingly, we observed the highest correlation between OPA and IgG antibody binding to live bacteria. Our flow cytometry method is gentle, with no coating of plastic and no fixative or detergents, and all reactions happen in physiological solution, causing minimal denaturing of bacterial epitopes, as has been previously established for group B meningococci (1, 18).
For the OPA analysis, all serum dilutions were supplemented with an external complement source to obtain a 10% complement concentration. This approximates the amount of complement found in mucosal secretions (25). In some experiments in which we omitted external complement, a drop in activity of 1 to 3 titer units was observed (data not shown). Complement activation leading to C3b deposition, together with IgG antibodies against FHA, may specifically block the interaction between FHA and CR3, thus stimulating phagocytosis and a respiratory burst rather than silent internalization (20, 29).
Resistance against bacterial killing depends on the growth phase and the growth medium of the bacteria (17). The presence of BrkA protein is reported to inhibit complement activation by the classical pathway (5). We did not analyze the actual BrkA content of the bacterial preparations we used, but our bacterial growth procedures on Bordet-Gengou agar have been reported to sustain BrkA expression (4). Various amounts of C3b deposition on the bacterial surface were induced by the serum samples, and we observed a positive correlation with the levels of IgG antibody against live B. pertussis, supporting classical complement activation.
The high correlation between IgG antibodies to FHA and OPA may indicate a beneficial role for this antibody specificity; this in contrast to a previous study (11) in which a correlation with antibodies to pertactin only, and not to PT, FHA, or fimbriae, was reported. Indeed, in an other study, Weingart et al. observed that a serum sample with a strong response against FHA showed an activity that inhibited the phagocytosis of B. pertussis (42). Other reports point to the importance of antibodies against ACT (19, 43), as this protein otherwise may block phagocytosis by neutrophils (7). We have not quantified the level of antibodies against ACT in our serum samples.
Some of the discrepancies observed may be related to the use of different OPA protocols. We added an external complement source to all of our test serum dilutions, and all of our serum samples were tested in twofold serial dilutions to give a titer, avoiding problems with a possible prozone phenomenon (observed as a lower percentage of OPA at higher serum concentrations compared to the activity of more diluted serum). Also, since the method we used allows a comparatively short incubation time with bacteria and neutrophils, bacterial toxins that might be present would get less time to interfere with cellular activities. The high correlation we observed between OPA and other serological measurements, like levels of IgG against live B. pertussis, C3b deposition, and specific IgG toward PT and FHA, may suggest that opsonophagocytosis plays an in vivo role in immunity to pertussis.
OPA, C3b deposition, and IgG binding to live bacteria cannot be used as criteria for diagnosing pertussis because of cross-reactivity with B. parapertussis or other microorganisms (6, 12, 13, 39). Accordingly, several serum samples with rather low levels of IgG antibodies to PT were highly positive in these assays. High levels of antibodies to PT, pertactin, and fimbriae have been reported to correlate with protection against pertussis (23), and conversely, low anti-PT levels are associated with increased susceptibility to pertussis (35). However, this is probably not a perfect surrogate marker for protection. Anti-PT IgG probably underestimates protection. In our study, 78% of the subjects had very low anti-PT IgG activity (<30 FDA U/ml). We believe that this may be representative of the general Norwegian population of this age, yet the corresponding prevalence of pertussis in this age group is rather low. It is likely that the many other antibodies that bind specifically to live B. pertussis also contribute to protection, e.g., by inducing phagocytosis. Thus, our assay with IgG binding to live bacteria, and in particular OPA, may possibly better reflect protective immunity against pertussis. Most of the serum samples analyzed demonstrated medium-to-high OPA activity. Even the serum samples with Serion PT IgG values of <30 FDA U/ml revealed a geometric mean OPA titer of 49.2 (Table 2). On the basis of the comparison between the Serion PT IgG levels (and the Pertusscan results) and the corresponding OPA titer, it is tempting to speculate that increased OPA (e.g., an OPA titer of ≥64) may better predict protective immunity against pertussis. Although these are serum values, the airway mucosa is drained with plasma exudates that increase during local inflammation such as that caused by B. pertussis infection (25). Hence, both antibody and complement concentrations are present to facilitate opsonophagocytosis by extravasated PMNs.
Still, the importance of opsonophagocytosis for protective immunity against pertussis remains elusive. In a nonimmune individual, B. pertussis may enter phagocytes silently via FHA-CR3 interaction and thus survive intracellularly (29). In studies where phagocytic cells have been pretreated with soluble toxins like PT or ACT, aberrant phagocytic responses like reduced chemotaxis, reduced internalization, and reduced superoxide generation were observed (7, 22, 30). However, if specific antibodies are present at the time of exposure, the toxin activity may be inhibited by neutralizing antibodies and conventional Fc receptor and CR engagement by different antibody specificities and complement, respectively, may trigger cellular bactericidal processes like a respiratory burst.
Although high levels of antibodies to PT, pertactin, and fimbriae correlate with protection, their exact mechanism of action remains elusive. Direct complement-mediated bactericidal activity has been shown to be of minor importance (42, 45), leaving opsonophagocytosis a more likely effector mechanism for B. pertussis elimination.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Aase, A., E. A. Hoiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47388-396. [DOI] [PubMed] [Google Scholar]
- 2.Aase, A., L. M. Naess, R. H. Sandin, T. K. Herstad, F. Oftung, J. Holst, I. L. Haugen, E. A. Hoiby, and T. E. Michaelsen. 2003. Comparison of functional immune responses in humans after intranasal and intramuscular immunisations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine 212042-2051. [DOI] [PubMed] [Google Scholar]
- 3.Barenkamp, S. J., and E. Leininger. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 601302-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, M. G., and A. A. Weiss. 2002. Growth phase influences complement resistance of Bordetella pertussis. Infect. Immun. 70403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, M. G., and A. A. Weiss. 2003. Activation of the complement cascade by Bordetella pertussis. FEMS Microbiol. Lett. 220271-275. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson, R. M., B. A. Claesson, T. Lagergård, and B. Trollfors. 1999. Acquisition of serum antibodies against filamentous hemagglutinin and pertactin unrelated to Bordetella pertussis infection. Clin. Microbiol. Infect. 5709-712. [Google Scholar]
- 7.Confer, D. L., and J. W. Eaton. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217948-950. [DOI] [PubMed] [Google Scholar]
- 8.de Melker, H. E., F. G. Versteegh, M. A. Conyn-van Spaendonck, L. H. Elvers, G. A. Berbers, Z. A. van Der, and J. F. Schellekens. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334349-355. [DOI] [PubMed] [Google Scholar]
- 10.Heininger, U., J. D. Cherry, and K. Stehr. 2004. Serologic response and antibody-titer decay in adults with pertussis. Clin. Infect. Dis. 38591-594. [DOI] [PubMed] [Google Scholar]
- 11.Hellwig, S. M. M., M. E. Rodriguez, G. A. M. Berbers, J. G. J. V. de Winkel, and F. R. Mooi. 2003. Crucial role of antibodies to pertactin in Bordetella pertussis immunity. J. Infect. Dis. 188738-742. [DOI] [PubMed] [Google Scholar]
- 12.Hodder, S. L., J. D. Cherry, E. A. Mortimer, Jr., A. B. Ford, J. Gornbein, and K. Papp. 2000. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin. Infect. Dis. 317-14. [DOI] [PubMed] [Google Scholar]
- 13.Isacson, J., B. Trollfors, J. Taranger, and R. Lagergard. 1995. Acquisition of IgG serum antibodies against 2 Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr. Infect. Dis. J. 14517-521. [DOI] [PubMed] [Google Scholar]
- 14.Jagels, M. A., P. J. Daffern, B. L. Zuraw, and T. E. Hugli. 1999. Mechanisms and regulation of polymorphonuclear leukocyte and eosinophil adherence to human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 21418-427. [DOI] [PubMed] [Google Scholar]
- 15.Kerr, J. R., and R. C. Matthews. 2000. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur. J. Clin. Microbiol. Infect. Dis. 1977-88. [DOI] [PubMed] [Google Scholar]
- 16.Lenz, D. H., C. L. Weingart, and A. A. Weiss. 2000. Phagocytosed Bordetella pertussis fails to survive in human neutrophils. Infect. Immun. 68956-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melton, A. R., and A. A. Weiss. 1993. Characterization of environmental regulators of Bordetella pertussis. Infect. Immun. 61807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaelsen, T. E., A. Aase, J. Kolberg, E. Wedge, and E. Rosenqvist. 2001. PorB3 outer membrane protein on Neisseria meningitidis is poorly accessible for antibody binding on live bacteria. Vaccine 191526-1533. [DOI] [PubMed] [Google Scholar]
- 19.Mobberley-Schuman, P. S., B. Connelly, and A. A. Weiss. 2003. Phagocytosis of Bordetella pertussis incubated with convalescent serum. J. Infect. Dis. 1871646-1653. [DOI] [PubMed] [Google Scholar]
- 20.Mobberley-Schuman, P. S., and A. A. Weiss. 2005. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 737317-7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser, J. M., E. L. Hewlett, M. S. Peppler, and R. K. Selander. 1986. Genetic diversity and relationships in populations of Bordetella spp. J. Bacteriol. 166230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nourshargh, S., and T. J. Williams. 1990. Evidence that a receptor-operated event on the neutrophil mediates neutrophil accumulation in vivo. Pretreatment of 111In-neutrophils with pertussis toxin in vitro inhibits their accumulation in vivo. J. Immunol. 1452633-2638. [PubMed] [Google Scholar]
- 23.Olin, P., H. O. Hallander, L. Gustafsson, E. Reizenstein, and J. Storsaeter. 2001. How to make sense of pertussis immunogenicity data. Clin. Infect. Dis. 33(Suppl. 4)S288-S291. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. G. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 3532-40. [DOI] [PubMed] [Google Scholar]
- 25.Persson, C. G., I. Erjefalt, U. Alkner, C. Baumgarten, L. Greiff, B. Gustafsson, A. Luts, U. Pipkorn, F. Sundler, C. Svensson, et al. 1991. Plasma exudation as a first line respiratory mucosal defence. Clin. Exp. Allergy 2117-24. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds, E., B. Walker, D. Xing, J. Southern, C. Asokanathan, B. Dagg, M. Corbel, and E. Miller. 2006. Laboratory investigation of immune responses to acellular pertussis vaccines when used for boosting adolescents after primary immunisation with whole cell pertussis vaccines: a comparison with data from clinical study. Vaccine 243248-3257. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez, M. E., S. M. Hellwig, D. F. Hozbor, J. Leusen, W. L. van der Pol, and J. G. Van de Winkel. 2001. Fc receptor-mediated immunity against Bordetella pertussis. J. Immunol. 1676545-6551. [DOI] [PubMed] [Google Scholar]
- 28.Rothe, G., A. Oser, and G. Valet. 1988. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften 75354-355. [DOI] [PubMed] [Google Scholar]
- 29.Saukkonen, K., C. Cabellos, M. Burroughs, S. Prasad, and E. Tuomanen. 1991. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J. Exp. Med. 1731143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 697635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steed, L. L., E. T. Akporiaye, and R. L. Friedman. 1992. Bordetella pertussis induces respiratory burst activity in human polymorphonuclear leukocytes. Infect. Immun. 602101-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steed, L. L., M. Setareh, and R. L. Friedman. 1991. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukoc. Biol. 50321-330. [DOI] [PubMed] [Google Scholar]
- 33.Stefanelli, P., R. Ippoliti, C. Fazio, and P. Mastrantonio. 2002. Role of immune sera in the in-vitro phagocytosis of Bordetella pertussis strains. Microb. Pathog. 32135-141. [DOI] [PubMed] [Google Scholar]
- 34.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 161907-1916. [DOI] [PubMed] [Google Scholar]
- 35.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 2003. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 213542-3549. [DOI] [PubMed] [Google Scholar]
- 36.Vainio, K., H. H. Samdal, G. Anestad, D. H. Skutlaberg, K. T. Bransdal, R. Mundal, and I. Aaberge. 2007. Seroprevalence of measles among Norwegian military conscripts in 2004. Eur. J. Clin. Microbiol. Infect. Dis. 26(3)217-220. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg, B. M., H. Beekhuizen, F. R. Mooi, and R. van Furth. 1999. Role of antibodies against Bordetella pertussis virulence factors in adherence of Bordetella pertussis and Bordetella parapertussis to human bronchial epithelial cells. Infect. Immun. 671050-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van't Wout, J., W. N. Burnette, V. L. Mar, E. Rozdzinski, S. D. Wright, and E. I. Tuomanen. 1992. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect. Immun. 603303-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent, J. M., J. D. Cherry, W. F. Nauschuetz, A. Lipton, C. M. Ono, C. N. Costello, L. K. Sakaguchi, G. Hsue, L. A. Jackson, R. Tachdjian, P. A. Cotter, and J. A. Gornbein. 2000. Prolonged afebrile nonproductive cough illnesses in American soldiers in Korea: a serological search for causation. Clin. Infect. Dis. 30534-539. [DOI] [PubMed] [Google Scholar]
- 40.von König, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2744-750. [DOI] [PubMed] [Google Scholar]
- 41.Ward, J. I., J. D. Cherry, S. J. Chang, S. Partridge, W. Keitel, K. Edwards, M. Lee, J. Treanor, D. P. Greenberg, S. Barenkamp, D. I. Bernstein, and R. Edelman. 2006. Bordetella pertussis infections in vaccinated and unvaccinated adolescents and adults, as assessed in a national prospective randomized acellular pertussis vaccine trial (APERT). Clin. Infect. Dis. 43151-157. [DOI] [PubMed] [Google Scholar]
- 42.Weingart, C. L., W. A. Keitel, K. M. Edwards, and A. A. Weiss. 2000. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect. Immun. 687175-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weingart, C. L., P. S. Mobberley-Schuman, E. L. Hewlett, M. C. Gray, and A. A. Weiss. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 687152-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weingart, C. L., and A. A. Weiss. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect. Immun. 681735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, A. A., A. K. Patton, S. H. Millen, S. J. Chang, J. I. Ward, and D. I. Bernstein. 2004. Acellular pertussis vaccines and complement killing of Bordetella pertussis. Infect. Immun. 727346-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]