Abstract
Outer membrane vesicle (OMV) and recombinant protein-based vaccines targeted against multiple strains of group B meningococci are under development. The serum bactericidal antibody (SBA) assay has been designated the surrogate of protection, but the exact cutoff has not been determined. We measured the SBA titers in 2,415 serum samples and the anti-OMV IgG antibody concentrations in 2,672 serum samples representative of the English population to establish a baseline of natural immunity. SBA and anti-OMV IgG antibody titers are high in infants in the first 3 months of life, declining thereafter, presumably as maternal immunity wanes. About 6% of the subjects in the 1- to 11-year-old age group had SBA titers ≥4. During the teenage years, there was a marked increase in the percentage of subjects with SBA titers ≥4, rising to over 50% in 19-year-olds, with about 20% of older adults achieving this titer. The peak in SBA and anti-OMV IgG titers coincided with the peak in meningococcal carriage. Simple mathematical models confirm that the relationship between observed seroprevalence and carriage by age is consistent with carriage inducing SBA and that following an episode of carriage, SBA levels may remain elevated for many months. With the exception of children aged 3 to 11 months, there was no clear relationship between disease incidence and seroprevalence.
With the introduction of meningococcal group C oligosaccharide-protein conjugate (MCC) vaccines in the United Kingdom and the decline of group C disease in targeted age groups (21, 28), attention is now being focused on the prevention of group B meningococcal disease. From the trials of outer membrane vesicle (OMV) vaccines in Cuba, Brazil, and Norway, the serum bactericidal antibody (SBA) assay has been designated the surrogate of protection (3); however, the exact cutoff is currently undetermined. Trials of candidate group B vaccines are now being undertaken, and it is imperative that we further our understanding of the correlates of protection for group B Neisseria meningitidis.
For group C disease, a series of studies in the United States in the 1960s established that an SBA titer of 1 in 4 (with human complement) is protective. Direct evidence was obtained from soldiers who were monitored prospectively; those with SBA titers less than 1 in 4 were more susceptible to meningococcal disease (11). Indirect evidence was obtained from cross-sectional seroprevalence studies, where an inverse relationship between the age-specific prevalence of SBA activity and the incidence of disease for groups A, B, and C was observed (11). Further work in the United Kingdom established an SBA titer of 1 in 8 with rabbit complement as a correlate of protection for group C disease (4). MCC vaccines were licensed in the United Kingdom and elsewhere on the basis of safety and immunogenicity data, in the absence of a large efficacy trial. It is likely that efficacy trials of group B vaccines will also be prohibitively large because of the low incidence of disease, and hence, a large sample size will be required. In New Zealand, the MeNZB vaccine was introduced without an efficacy trial, with 95% of adults, 74 to 76% of children aged 6 months to 12 years, and 53% of younger infants responding to three doses of vaccine with a fourfold rise in SBA titers to at least 1 in 8 (23, 24).
The main aims of this study were to describe the age-specific seroprevalence of group B SBA and anti-OMV IgG antibody, to establish a baseline seroprevalence in the United Kingdom, and to inform our understanding of the correlates of protection for group B disease. The strain selected (strain NZ98/254) was B:4:P1.7-2,4, which is of the sequence type 41/44 (ST-41/44 complex). This is identified through serological testing as B:4:P1.4 because P1.7-2 can be identified only by genotyping and not with monoclonal antibodies (20). From 1995-1996 to 2003-2004, B:4:P1.4 was responsible for about one-fifth of the cases of group B disease in England and Wales and was the most common phenotype isolated (14). In order to inform the natural history of meningococcal infection, we also compared the results from this seroprevalence study with available data on meningococcal carriage, meningococcal disease, and the seroprevalence of antibodies to group C meningococci in the prevaccination era.
MATERIALS AND METHODS
Serum samples.
Sera were selected from the Health Protection Agency (HPA) Seroepidemiology Unit, as described by Osborne et al. (22). Briefly, participating laboratories submit residual sera from routine diagnostic testing to the HPA Seroepidemiology Unit. All samples are anonymous; a unique identity number is assigned, and details about the age, sex, and geographical location of the person from whom the sample was obtained are collated in a database. Other information, such as the reason for the blood test, is not retained, although sera from immunocompromised individuals are excluded. This type of serum sample collection (convenience sampling) has been shown to be comparable to a random-cluster survey (16). For this study, 2,706 samples collected between 2000 and 2004 from individuals aged between 0 and 93 years were selected. Ages were stratified as follows: <0.3 years, 0.3 to 0.59 years, 0.6 to 0.99 years, single-year age bands from age 1 year up to and including 19 years of age, 20 to 24 years, and 10-year age bands thereafter. The results for 2,672 serum samples were available for the OMV enzyme-linked immunosorbent assay (ELISA), and there were 2,415 results for the SBA assay.
SBA assay.
Sera were tested by using a standardized complement-mediated SBA assay against strain NZ98/254, B:4:P1.7-2,4 (ST-42, complex ST-41/44), as described elsewhere (2, 9, 19). The complement source was complement-preserved human plasma. SBA titers were expressed as the reciprocal of the final serum dilution that gave ≥50% killing at 60 min. SBA titers of <2 were assigned a value of 1 for computational purposes. The SBA data are categorized into summary groups of ≥2, ≥4, and ≥8, according to the correlates of protection proposed for OMV vaccines (3). The percentage of individuals in each of these categories was calculated for each age group.
OMV ELISA.
Specific IgG OMV ELISA endpoint titers against OMV antigen preparations from target strain NZ98/254 were determined as described previously (7). Geometric mean titers (GMTs) and 95% confidence intervals were estimated for each age group. The correlation between the immunoglobulin G (IgG) OMV ELISA result and the SBA titer was assessed by using Spearman's rank correlation in Stata software (version 9).
Disease data.
Laboratory surveillance data collected by the HPA Meningococcal Reference Unit between 1995-1996 and 2003-2004 were analyzed to determine the incidence of disease due to group B overall and selected phenotypes (14). Population statistics for England and Wales were obtained from the Office for National Statistics and were used as the denominator.
Carriage data.
Age-specific carriage prevalence data were obtained from the Stonehouse study (5), conducted in Gloucestershire, England, in 1986, which remains the largest cross-sectional study in the United Kingdom covering all age groups. Recent data on the prevalence of meningococcal carrier phenotypes was obtained from the United Kingdom Meningococcal Carriage Study (18), which collected nasopharyngeal swabs from about 15,000 school and college students between the ages of 15 and 18 years in eight sites in England, Scotland, and Wales for 3 years between 1999 and 2001.
Seroprevalence of antibodies to group C meningococci.
An earlier study in the United Kingdom described the seroprevalence of SBAs to a group C meningococcal strain (reference strain C11, phenotype C:16:P1.7-1,1) before the introduction of widespread MCC vaccination (27). We used these data to compare the age-specific profiles of SBAs to group B and group C. Note that the SBA for group C was determined by using baby rabbit complement, and so a cutoff of ≥8 was used (4).
Mathematical modeling.
Natural immunity to meningococci is generated following periods of asymptomatic carriage (12). To assess whether the age-specific seroprevalence data were consistent with (and so could be explained by) age-specific patterns of carriage, we created a simple mathematical model. Since the prevalence of SBA titers ≥2, ≥4, and ≥8 was higher than the prevalence of carriage, it was assumed that a period of immunity ensues after carriage is cleared.
By following a classic susceptible-infected-recovered-susceptible approach, the individuals in the model, aged between 0 and 75 years, were assumed to exist in one of four exclusive categories: category M, “immune,” with SBA titers ≥4 due to maternal antibody; category S, susceptible to carriage with a low SBA titer; category B, carrier of group B meningococci; and category I, “immune,” with SBA titers ≥4 due to prior carriage.
 |
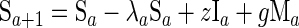 |
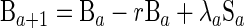 |
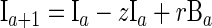 |
where a is age, λ is the force of infection, r is the recovery rate from carriage, g is the rate of waning of maternally derived immunity, and z is the rate of waning of carriage-induced immunity.
Carriers were assumed to have low SBA titers until they cleared their carriage. For the purposes of the model, we chose an SBA titer of ≥4 as our cutoff. Note that we did not model meningococcal disease as an outcome. We estimated the age-specific (monthly) force of infection with group B using a five-parameter function, as described previously (29):
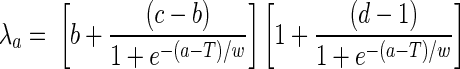 |
where a is age; T is the age at the peak of carriage; w is the width of carriage peak; and b, c, and d are constants.
Exponential decay was assumed for the recovery rate from carriage (r), the rate of waning if maternally derived immunity (g), and the rate of waning of carriage-induced immunity (z) so that the average duration in that category was equal to the reciprocal of the rate (e.g., duration of carriage = 1/r). The average duration of carriage was fixed at 3, 6, or 9 months. The eight remaining parameters in the nine-parameter model were fitted to age-specific carriage (14 datum points, 5-year age bands up to age 65 years) and SBA seroprevalence (28 datum points) data by the maximum-likelihood method, as described previously (29). The model was created in Microsoft Excel software, and the “solver” tool was used to minimize the model deviance (calculated as twice the difference between the saturated and the model log likelihood).
RESULTS
SBAs.
The results for the 2,415 serum samples are presented in Fig. 1. An additional 295 serum samples were tested; but 221 had an insufficient volume for titer determination, and in 74 samples non-complement-mediated lysis was present and, thus, a final SBA titer could not be reported. In the youngest children, nearly 30% of infants less than 5 months of age had titers ≥4, presumably as a result of maternal immunity. In older infants the titers declined, and at 8 to 11 months of age only 5% had SBA titers ≥4. The SBA titers remained low through childhood and then increased in the teenage years, with the highest proportion above putatively protective levels occurring at 19 years of age. In older adults, the titers were lower, with about 20% of adults older than 25 years of age having titers ≥4.
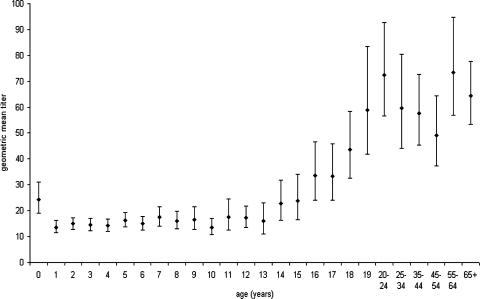
FIG. 1.
Percentage of sera with SBA titers ≥2, ≥4, and ≥8, by age.
IgG OMV ELISA.
A total of 2,672 serum samples were assayed by the OMV ELISA. Anti-OMV IgG GMTs by age (95% confidence intervals) are shown in Fig. 2. There was a significant decline in the anti-OMV IgG GMT from those under 1 year old to toddlers. The GMTs remained fairly constant until the early teenage years, when there was an age-dependent increase in the anti-OMV IgG titer up to 19 years of age, after which antibody levels plateaued.
FIG. 2.
Age-specific IgG OMV ELISA GMTs and 95% confidence intervals.
Correlation between SBA titer and IgG OMV ELISA result.
The Spearman's correlation coefficient for the 2,415 serum samples for which both SBA titers and ELISA results were available was 0.48 (P < 0.001), suggesting a positive correlation between the two assays.
Relationship between SBA seroprevalence and disease incidence.
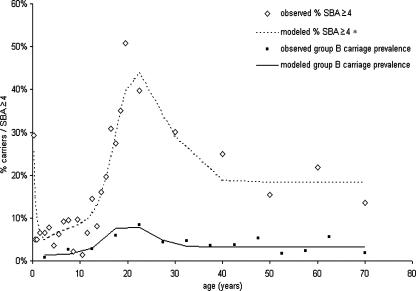
The relationship between the incidence of group B disease by age and the prevalence of SBA titers ≥4 can be seen in Fig. 3. The disease incidence was the highest in infants aged 3 to 7 months, and this coincided with low SBA titers. However, as the disease incidence declined through childhood, there was no apparent increase in the proportion of children with protective SBA titers. In teenagers, while SBA levels were high, there was a small secondary peak in disease incidence. In adults, the disease incidence was very low; and approximately 32%, 20%, and 15% of adults ages 25 years old or older had SBA titers ≥2, ≥4, and ≥8, respectively. Disease due to B:4:P1.4 and subtype P1.4 associated with any serogroup and serotype followed an age distribution very similar to that for group B (data not shown).
FIG. 3.
Age-specific incidence of serogroup B disease compared to the percentage of individuals with SBA titers ≥4.
Relationship between carriage and SBA seroprevalence.
The increase in SBA titers during the teenage years corresponds to an increase in group B carriage, as observed in the Stonehouse carriage study (5). More recent carriage data are available only for 15- to 18-year-olds (18) and not across the whole age range, but this showed that the prevalence of B:4:P1.4 strains between 1999 and 2001 was 0.4% (representing 2% of all carrier isolates). The prevalence of strains with a PorA variable region 2 P1.4, regardless of the group or the type, was 0.7%, representing 4% of all carrier isolates (S. Gray, personal communication).
The simple susceptible-infected-recovered-susceptible model was able to capture the observed SBA titer and carriage data (Fig. 4). The estimated duration of immunity (SBA titer, ≥4) following carriage varied according to the assumed duration of carriage (Table 1). For a longer duration of carriage, the estimated force of infection was lower, and thus, the period of immunity following carriage was also longer. The model helps to illustrate that the age distributions of the SBA titers and carriage data are highly consistent.
FIG. 4.
Model fit to group B SBA and carriage data. This model assumes that the average duration of group B carriage is 6 months. Other parameters were estimated (Table 1). *, categories M (maternal immunity) and I (acquired immunity) are combined.
TABLE 1.
Model estimates of duration of immunity under different assumptions for duration of carriage
| Avg duration (mo) of carriage (fixed) | Prevalence (%) of maternal immunity at birth | Avg duration (mo) of maternal immunity | Avg duration (mo) of immunity after carriage | Model deviance |
|---|---|---|---|---|
| 3 | 41 | 4.4 | 16.2 | 28.4 |
| 6 | 32 | 8.3 | 33.8 | 29.7 |
| 9 | 26 | 12.6 | 52.5 | 31.6 |
Seroprevalence of SBAs to group B compared with those to group C.
The seroprevalence profiles for group B and group C (in the prevaccine era) are illustrated in Fig. 5. A greater proportion of young infants had putatively protective SBA titers to group B strains than to group C strains, although the 95% confidence limits overlap. There was also a more marked peak in the titers of SBAs to the group B strain in 15- to 24-year-olds (P = <0.001), perhaps reflecting the greater prevalence of related strains in carriage populations. In older adults, the group B SBA titers declined, but the group C SBA titers were maintained.
FIG. 5.
Age-specific seroprevalence of SBA antibodies to group B and group C reference strains in the United Kingdom.
DISCUSSION
We have described the age-specific prevalence of bactericidal and OMV antibodies to group B meningococci in sera collected in England between 2000 and 2004. We performed several ecological analyses of the SBA titer data (the established correlate of protection [3]) compared to the carriage and disease data, which gives some useful insights into the natural history of meningococcal infection. The visual consistency between the age-related carriage and the seroprevalence data are supported by simple mathematical models, which provide evidence that natural immunity is being induced by carriage, with the persistence of SBA levels for many months after clearance. The model chosen here is intended to be illustrative and may not give a correct description of the course of group B infection. It does, however, show that simple models can adequately capture the data; it is not necessary, for example, to invoke the presence of “cross-reacting” organisms that induce immunity. Since contemporary carriage data across all age groups were not available from the United Kingdom, we used carriage data collected in 1986. While the specific meningococcal strains circulating are likely to have changed over this time, group B meningococci are still commonly carried (18), the age-specific prevalence of carriage is consistent with that from more recent studies conducted elsewhere in Europe (6), and the age distribution of the cases was similar between the late 1980s and the early 2000s (14, 15), suggesting that no major changes in age-specific transmission patterns have occurred.
Comparisons of the seroprevalence profiles and disease incidence are also of interest. The age patterns of the SBA titers were very similar when SBA titer cutoffs of ≥2, ≥4, or ≥ 8 were used; so this study does not help to determine the exact correlate of protection for group B disease. Nonetheless, this provides further evidence that the SBA assay measures natural exposure to group B meningococci. An inverse relationship between “protective” SBA titers and the incidence of disease by age for groups A, B, and C was first described by Goldschneider and colleagues in the 1960s (11). This relationship is not so clearly observed from these data. Disease incidence is the highest among infants older than 3 months of age, when most maternal immunity has waned and infants are the most vulnerable to infection (25). However, disease incidence declines throughout childhood, even though there is no significant change in the proportion of children with putatively protective SBA titers. Clearly, other explanations for the falling disease incidence must be considered. It may be that the rate of exposure to group B organisms—and hence, the risk of infection—is much lower in children between 2 and 12 years of age than in those of younger or older ages. The increasing prevalence of carriage and the secondary peak in disease incidence in teenagers clearly indicates higher levels of exposure in older children, which is a consequence of behavioral changes (17). However, it is not clear why 2- to 12-year-olds might be at a much lower risk of encountering group B infection than younger children. It has been suggested that anti-capsular IgM antibodies (which are not measured by the anti-OMV IgG ELISA or the SBA assay with human complement [30], as used here) are protective in an infant rat model. These antibodies were also shown not to be opsonic (26); thus, their mechanism of putative protection remains unknown. Neisseria lactamica is commonly carried in infants and young children (1, 5, 10), and it has been postulated that carriage of N. lactamica has a role in generating protection against meningococcal disease (10). Our study showed that there is little change in SBA activity or anti-OMV IgG titers in young children, at a time when N. lactamica carriage is common. If there is indeed a protective effect from N. lactamica, this may be mediated through competition (N. lactamica carriage prevents N. meningitidis colonization), through local immunity in the nasopharyngeal mucosa, or through innate immunity such as non-antibody-mediated opsonophagocytosis, none of which will be measured by the SBA assay or the OMV IgG ELISA. Further studies may help to elucidate these effects (13). It should be noted that the study by Gold et al. (10), which showed that carriers of N. lactamica were more likely than noncarriers to develop SBA activity against meningococci, was based on a small number of observations and that little work has been done to confirm these findings.
The group B capsule is known to be poorly immunogenic, and previous studies have shown that SBA to group B meningococci is primarily directed at noncapsular antigens (8). In MenZB vaccine trials, SBA specifically targeted the variable region 2 P1.4 epitope of the NZ98/254 strain (20). A recent large United Kingdom carriage study (18) showed that the P1.4 epitope was present in 4% of isolated carrier strains, so it seems likely that the serological profiles shown here reflect exposure to these strains. In an earlier seroprevalence study performed to measure antibody against group C meningococci (27), we used the C11 reference strain C:16:P1.7-1,1. These subcapsular antigens are now very uncommon in the United Kingdom, so most of the SBA activity measured was likely to be directed against the group C capsule. Carriage of group C meningococci was rare, even in the prevaccine era, and the differences in the group B and group C seroprevalence profiles may reflect the differences in exposure to the group C capsule and the P1.4 antigen and, possibly, the differences in the immunizing capacity of the polysaccharide capsule compared with those of immunodominant subcapsular protein antigens.
It is important to understand natural immunity to group B meningococci so that the effect of vaccination can be properly appreciated, and this description of group B SBA and anti-OMV IgG provides a baseline of prevaccination immunity. This study raises some interesting questions worthy of further study, including the reason for the decline in disease incidence in young children, despite low SBA activity and anti-OMV IgG titers, and the role of N. lactamica in providing protection against disease.
Acknowledgments
We are grateful to the HPA Seroepidemiology Unit for providing the sera for this study. We thank the United Kingdom Meningococcal Carriage Study for access to unpublished data. We also thank the Norwegian Institute for Public Health for the gift of meningococcal strain NZ98/254.
The laboratory testing for this study was supported by a grant from Novartis Vaccines, and we thank Philipp Oster for his assistance. Caroline Trotter is funded by a Personal Award Scheme Post-Doctoral Award from the National Institute of Health Research (Department of Health). The Immunization Department and the Vaccine Evaluation Unit of the Health Protection Agency have received grants from vaccine manufacturers, in accordance with their code of conduct (http://www.hpa.org.uk/infections/about/dir/psp.pdf).
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Bennett, J. S., D. T. Griffiths, N. D. McCarthy, K. L. Sleeman, K. A. Jolley, D. W. Crook, and M. C. Maiden. 2005. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect. Immun. 732424-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, R., I. S. Aaberge, G. F. Santos, T. L. Eudey, P. Oster, A. Glennie, J. Findlow, E. A. Hoiby, E. Rosenqvist, P. Balmer, and D. Martin. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 245093-5107. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., and E. Miller. 2006. Surrogates of protection, p. 323-341. In M. Frosch and M. C. Maiden (ed.), Handbook of meningococcal disease: infection biology, vaccination, clinical management. Wiley-VCH, Weinheim, Germany.
- 5.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect 99591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caugant, D. A., E. A. Hoiby, P. Magnus, O. Scheel, T. Hoel, G. Bjune, E. Wedege, J. Eng, and L. O. Froholm. 1994. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J. Clin. Microbiol. 32323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenport, V., T. Guthrie, J. Findlow, R. Borrow, N. A. Williams, and R. S. Heyderman. 2003. Evidence for naturally acquired T cell-mediated mucosal immunity to Neisseria meningitidis. J. Immunol. 1714263-4270. [DOI] [PubMed] [Google Scholar]
- 8.Derrick, J., J. E. Heckels, and M. Virji. 2006. Major outer membrane proteins of meningococci, p. 181-215. In M. Frosch and M. C. Maiden (ed.), Handbook of meningococcal disease: infection biology, vaccination, clinical management. Wiley, Weinheim, Germany.
- 9.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 744557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137112-121. [DOI] [PubMed] [Google Scholar]
- 11.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1291307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 1291327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorringe, A., D. Halliwell, M. Matheson, K. Reddin, M. Finney, and M. Hudson. 2005. The development of a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Vaccine 232210-2213. [DOI] [PubMed] [Google Scholar]
- 14.Gray, S. J., C. L. Trotter, M. E. Ramsay, M. Guiver, A. J. Fox, R. Borrow, R. H. Mallard, and E. B. Kaczmarski. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55887-896. [DOI] [PubMed] [Google Scholar]
- 15.Jones, D. M. 1995. Epidemiology of meningococcal disease in Europe and the USA, p. 147-157. In K. Cartwright (ed.), Meningococcal disease. John Wiley & Sons, Chichester, United Kingdom.
- 16.Kelly, H., M. A. Riddell, H. F. Gidding, T. Nolan, and G. L. Gilbert. 2002. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 203130-3136. [DOI] [PubMed] [Google Scholar]
- 17.MacLennan, J., G. Kafatos, K. Neal, N. Andrews, J. C. Cameron, R. Roberts, M. R. Evans, K. Cann, D. N. Baxter, M. C. Maiden, and J. M. Stuart. 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiden, M. C. J., and J. M. Stuart, on behalf of the United Kingdom Meningococcal Carriage Group. 2002. Carriage of serogroup C meningococci one year after meningococcal C conjugate polysaccharide vaccination. Lancet 3591829-1830. [DOI] [PubMed] [Google Scholar]
- 19.Martin, D., L. McCallum, A. Glennie, N. Ruijne, P. Blatchford, J. O'Hallahan, and P. Oster. 2005. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 232218-2221. [DOI] [PubMed] [Google Scholar]
- 20.Martin, D. R., N. Ruijne, L. McCallum, J. O'Hallahan, and P. Oster. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, E., D. M. Salisbury, and M. E. Ramsay. 2001. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20S58-S67. [DOI] [PubMed] [Google Scholar]
- 22.Osborne, K., N. J. Gay, L. Hesketh, P. Morgan-Capner, and E. Miller. 2000. Ten years of serological surveillance in England and Wales: methods, results, implications and action. Int J. Epidemiol. 29362-368. [DOI] [PubMed] [Google Scholar]
- 23.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 232191-2196. [DOI] [PubMed] [Google Scholar]
- 24.Oster, P., J. O'Hallahan, I. Aaberge, S. Tilman, E. Ypma, and D. Martin. 2007. Immunogenicity and safety of a strain-specific MenB OMV vaccine delivered to under 5-year olds in New Zealand. Vaccine 253075-3079. [DOI] [PubMed] [Google Scholar]
- 25.Pollard, A. J., and C. Frasch. 2001. Development of natural immunity to Neisseria meningitidis. Vaccine 191327-1346. [DOI] [PubMed] [Google Scholar]
- 26.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, T. E. Michaelsen, A. Aase, and H. Kayhty. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 734694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trotter, C., R. Borrow, N. Andrews, and E. Miller. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 211094-1098. [DOI] [PubMed] [Google Scholar]
- 28.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364365-367. [DOI] [PubMed] [Google Scholar]
- 29.Trotter, C. L., N. J. Gay, and W. J. Edmunds. 2006. The natural history of meningococcal carriage and disease. Epidemiol. Infect. 134556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]