Abstract
A fusion protein designated CSU-F36 was constructed that consisted of acylated Rv1411, a potent Toll-like receptor-2 agonist, fused to ESAT-6, a well-characterized immunogenic protein from Mycobacterium tuberculosis. The CSU-F36 fusion protein strongly induced interleukin 12 secretion from macrophages and induced the increased accumulation of CD4 T cells capable of secreting gamma interferon in the lungs of infected mice. These mice were significantly protected from low-dose aerosol challenge with M. tuberculosis, even with CSU-F36 delivered in a simple depot material. This “natural adjuvant”-containing system could potentially bypass the need for more expensive TH1-inducing adjuvants and could be applied to many mycobacterial proteins to provide effective and cheap new vaccines against tuberculosis.
Disease caused by Mycobacterium tuberculosis remains a global epidemic, with 8 million new cases each year and 2 to 3 million deaths (6, 13). Over the past two decades, a variety of new vaccine candidates has been produced, some of which have proven to have activity in various animal models (1, 12). New vaccines are still badly needed, however, but they also need to be relatively cheap to make.
The Toll-like receptors consist of a set of over one dozen pattern recognition receptors that play a major role in the innate immune response to invading microbes. Triggering these receptors has a variety of outcomes involving various signaling pathways and the subsequent induction of cytokine secretion (11). Lipoproteins, such as the 19-kDa lipoprotein (Rv3763) and the 26-kDa lipoprotein (Rv1411), as well as the lipoglycan lipoarabinomannan, all components of the cell wall of M. tuberculosis, are bound by Toll-like receptor-2 (TLR-2) on the surface of macrophages, triggering intracellular signaling, leading to the production of interleukin 12 (IL-12) by these cells, thus polarizing reactive T cells to a TH1 pathway (4, 15).
In earlier studies, we observed that DO11 mice that are T-cell-receptor transgenic for an ovalbumin peptide lived much longer than expected after low-dose aerosol infection with M. tuberculosis. Subsequent analysis of this phenomenon identified a cell wall fraction that potently induced IL-12 production by spleen cells, in turn inducing gamma interferon (IFN-γ) secretion by T cells (16). Subsequent fractionation of the cell wall material revealed the active molecule to be a 26-kDa lipoprotein designated Rv1411c. Culture of macrophages with purified Rv1411c induced IL-12 production in normal mice but not in the TLR-2 gene-disrupted mice, thus indicating that the biological activity of this lipoprotein was mediated via this receptor.
Given the potency of the Rv1411 protein to switch on production of the key protein in the TH1 pathway, we posed the question as to whether this could be exploited as a new vaccine strategy. To test this, we constructed a recombinant fusion protein, designated CSU-F36, consisting of acylated Rv1411 as the N terminus, joined to ESAT-6, used here as a relevant example of an immunogenic protein of M. tuberculosis. The recombinant was expressed in Mycobacterium smegmatis to preserve the correct glycosylation and acylation of Rv1411, to allow it to bind TLR-2; this strategy was necessary because Rv1411 made with Escherichia coli is not correctly acylated, does not trigger TLR-2, and may actually interfere with vaccine-induced immunity (7-9).
The results of this study show that the expressed fusion protein stimulated IL-12 production in vitro, increased the percentage of CD4 cells entering the lungs of infected mice, and protected mice to a degree similar to that of Mycobacterium bovis BCG. In addition, the results also showed that the fusion protein was still protective if delivered in a simple depot adjuvant, raising the possibility that the “built-in” adjuvant properties of the acylated Rv1411 can be exploited to make inexpensive new vaccines.
MATERIALS AND METHODS
Mice.
Studies were performed with C57BL/6 mice purchased from Jackson Laboratories, Bar Harbor, ME. They were kept under barrier conditions in an ABL-III laboratory and fed sterile water and chow.
Fusion proteins.
To generate fully acylated recombinant fusion antigens, Rv1411c was used as the N-terminal fusion partner and was linked at its C terminus to ESAT-6. The gene encoding Rv1411c (including the region that encodes the translocation signal sequence) was amplified by PCR using reversed and forward primers that contained restriction sites to facilitate downstream cloning efforts and a high-fidelity polymerase. The forward primer possessed an NdeI restriction site where the coding sequence of Rv1411c is in-frame with the ATG of the NdeI restriction site, and the reversed primer contained a restriction site that allowed for in-frame fusion of Rv1411c with the ESAT-6 gene. The Rv1411c gene amplification product was cloned into the mycobacterial expression vector pVV2 that allows for a C-terminal six-His tag on the final fusion product and is also a shuttle vector that enables amplification in E. coli. The gene encoding ESAT-6 was PCR amplified using a high-fidelity polymerase, and reverse and forward primers contained restriction sites that allowed for proper cloning into the pVV2:Rv1411c plasmid. The forward primer allowed for in-frame fusion to Rv1411c, and the reverse primer allowed for in-frame fusion to the sequence encoding the six-His tag. The final plasmid construct was transformed into M. smegmatis mc2155, and the fusion protein was produced. The recombinant M. smegmatis clone was grown to late log phase, and cells were harvested by centrifugation at 3,000 × g and lysed by probe sonication. Since Rv1411c possessed a Sec translocation signal sequence and an acylation domain, the recombinant fusion product was a lipoprotein localized in the cell envelope of M. smegmatis. Thus, a lysis buffer (10 mM sodium phosphate [pH 7.4], 0.5 M sodium chloride, 10 mM imidazole) supplemented with 8 M urea to allow for solubilization of the recombinant acylated-fusion protein was used. The lysate was clarified by centrifugation at 27,000 × g, and the supernatant was collected and applied directly to a column packed with Ni-Sepharose for immobilized metal affinity chromatography. The column was washed with lysis buffer, and the recombinant protein was eluted with buffered 1 M imidazole and 8 M urea. The protein was then dialyzed with decreasing levels of urea and ultimately in a buffer compatible with the vaccination protocol.
Vaccinations.
Mice were vaccinated three times with CSU-F36 in 250 μg of dimethyl dioctadecylammonium bromide (DDA; Sigma, St. Louis, MO) adjuvant with or without 25 μg of monophosphoryl lipid A (MPL; Glaxo SmithKline, Hamilton, MN) emulsified in 200 μl of sterile saline, given by subcutaneous injection. Negative control mice were given adjuvant or saline only; positive control mice were vaccinated with a single subcutaneous injection of 106 cells of Mycobacterium bovis BCG Pasteur at the same time as the third inoculation with the fusion vaccine was given. Thirty days later, mice were challenged with a low-dose aerosol infection from M. tuberculosis H37Rv, using a Glas-Col device (Terre Haute, IN) to deposit approximately 100 bacteria into their lungs. The bacterial load in the lungs 30 days later was determined by plating serial dilutions of individual right-lobe homogenates onto nutrient 7H11 agar. Colonies were counted after 21 days of incubation at 37°C.
Real-time PCR.
Peritoneal exudate macrophages were obtained by intraperitoneal injection of 1 ml of thioglycolate broth (BDH, Poole, United Kingdom) 72 h before lavage. On average, each mouse yielded about 2.5 × 107 exudate cells. Cells were then cultured in RPMI 1640 medium (Gibco, Melbourne, Australia) containing 1,000 U/ml heparin. Macrophages were isolated by cell adhesion. Cells were incubated in 24-well plates for 2 h at 37°C in 5% CO2. The nonadherent cells were removed by washing three times with phosphate-buffered saline (PBS; Sigma, St. Louis, MO). Each well contained approximately 5 × 106 adherent macrophages. These cells were then incubated with 20 ng/well of 1411c expressed in M. smegmatis, the Rv1411c-ESAT-6 fusion, Rv1411 expressed in E. coli, or ESAT-6. Cellular suspensions from antigen-stimulated cultures were frozen 24 h later in 1 ml of Ultraspec RNA reagent (Biotex). Total RNA was extracted according to the manufacturer's protocol. RNA samples from each group and each time point were treated with DNase (Ambion) and reverse transcribed with a Bio-Rad iScript cDNA synthesis kit using Moloney murine leukemia virus reverse transcriptase (Invitrogen Life Technology). cDNA was then amplified using TaqMan Universal PCR master mix on an ABI PRISM 7700 sequence detection system (Applied Biotechnology). Samples were run in the absence of the reverse transcription (RT) enzyme to confirm that the signal was derived from RNA. The fold increase in signal over that of the 18S housekeeping gene was determined using the ΔΔ cycle threshold (ΔΔCT) calculation method. Primers and probe sequences for murine IL-12 were used as previously described (5, 10).
Flow cytometry.
To prepare lung cells, mice were euthanized by CO2 asphyxiation, and the thoracic cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml of ice-cold PBS containing 50 U/ml heparin (Sigma, St Louis, MO). Lungs were aseptically removed, teased apart, and treated with a solution of DNase IV (30 g/ml; Sigma Chemical) and collagenase XI (0.7 mg/ml; Sigma) for 45 min at 37°C. To obtain a single-cell suspension, the organs were gently passed through cell strainers. The remaining erythrocytes were lysed with Gey's solution (0.15 m NH4Cl, 10 mm HCO3), and the cells were washed with Dulbecco's modified Eagle's minimal essential medium. Total cell numbers were determined by using a Neubauer chamber. Single-cell suspensions were then resuspended in PBS containing 0.1% sodium azide. Cells were incubated in the dark for 25 min at 4°C with specific fluorescein isothiocyanate-labeled anti-CD4 (GK1.5) monoclonal antibody (BD Pharmingen). Measurement of intracellular IFN-γ was conducted by preincubating lung cells with monensin (3 μM) and anti-CD3 and anti-CD28 (both at 0.2 μg/106 cells) for 4 h at 37°C in 5% CO2. The cells were then surface stained, washed, and fixed and permeabilized, followed by staining with anti-IFN-γ (clone XMG1.2) or its respective isotype control. Samples were analyzed on a Becton Dickinson LSR-II multicolor flow cytometer, and data were analyzed using CELLQUEST software (Becton Dickinson immunocytometry systems). A cytometric bead array kit (CBA; BD Biosciences, San Jose, CA) was used to measure inflammatory cytokines. Cellular suspensions were incubated for 3 days at 37°C with the different antigens and analyzed as described above.
Histology.
Lung samples were fixed in 10% formal saline, embedded in paraffin wax, and sectioned. Slides were stained with hematoxylin and eosin, then read by a board-certified veterinary pathologist without knowledge of the treatment groups.
Statistical analysis.
Data are presented using the mean values (n = 5) of results using replicated samples and duplicate or triplicate assays. Standard deviations were used to evaluate the differences between groups of data.
RESULTS
Immunogenicity of CSU-F36.
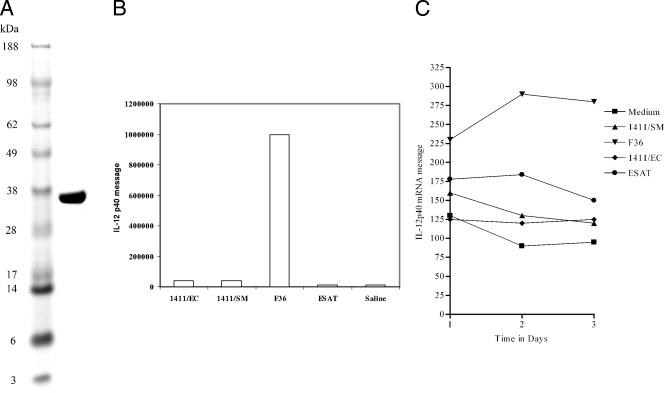
Production of the recombinant fusion in M. smegmatis resulted in a polyprotein of approximately 36 kDa (Fig. 1A), reflecting acylation and glycosylation. The CSU-F36 fusion protein strongly induced IL-12 production from cultures of murine macrophages assayed, both in terms of mRNA expression (Fig. 1B) and verification that the protein was secreted (Fig. 1C). As shown here, there were only background levels of mRNA detected in the various components of the vaccine, but a large response to the fusion protein was observed after expression in M. smegmatis. We then verified that IL-12 was indeed being secreted by using a cytometric bead assay. Here the fusion protein induced the raised levels of IL-12p70, whereas the components gave responses barely above the saline control response. None of the materials tested induced any IL-10 secretion, whereas the fusion protein, the ESAT, and the Rv1411 made in E. coli all induced low levels of IL-6 (data not shown).
FIG. 1.
(A) Gel electrophoresis of the expressed fusion protein shows that it had an approximate molecular size of 36 kDa. (B) RT-PCR analysis of mouse adherent peritoneal macrophages cultured for 3 days in the presence of the test materials for expression of mRNA encoding the IL-12p40 molecule. Data shown are means ± standard errors of the means (SEM) of the results for four wells per material. (C) Data demonstrate that macrophages stimulated with the fusion protein vaccine in vitro secreted IL-12p70, as determined by a cytometric bead assay. Data are shown as means ± SEM of the results for four wells.
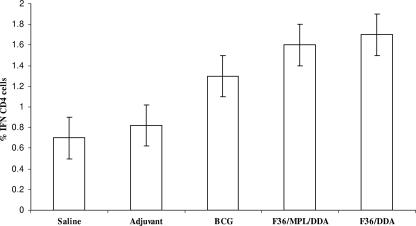
The ability of the fusion protein to influence the course of the challenge infection was assessed by determining the influx of CD4 T cells into the infected lungs, by determining the bacterial load, and by assessing the lung immunopathology. The results of the flow cytometric analysis are shown in Fig. 2. Both the BCG and the fusion protein vaccine increased the numbers of IFN-γ+ CD4 cells entering the lungs by approximately twofold. The increased numbers induced by immunization with the fusion protein were significantly higher than for the adjuvant control (P < 0.05) but not higher than the BCG controls.
FIG. 2.
Mice immunized with the fusion protein vaccine and then challenged by low-dose aerosol infection with M. tuberculosis accumulated approximately twice as many IFN-γ-positive CD4 cells as mice immunized with adjuvant controls (P < 0.05). Cells were analyzed by flow cytometry after processing for intracellular cytokine staining. Data shown are means ± standard errors of the means of results for four separate mice.
The CSU-F36 fusion protein confers protection similar to that of BCG.
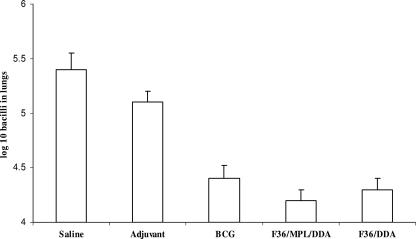
The protection observed in the lungs of mice vaccinated with CSU-F36 was approximately 1 log, comparable to that engendered by the BCG vaccine positive control (Fig. 3). These reductions were statistically significant (P < 0.05). Importantly, CSU-F36 gave similar results regardless of whether it was formulated in an adjuvant mixture of MPL and DDA or in DDA alone.
FIG. 3.
All three vaccine formulations tested protected aerosol-infected mice by approximately 1 log. Data shown are means ± standard errors of the means of results for five mice per group.
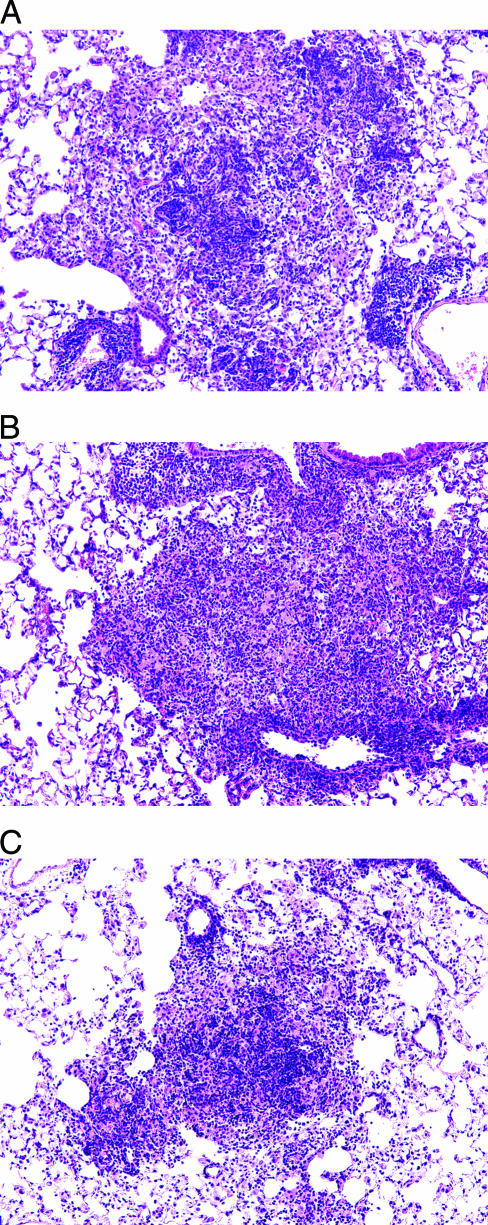
Pathology analysis of lungs at this time showed large, disorganized lesions in the saline controls, characteristic of this early stage of the granulomatous response (Fig. 4A). Lesions in the adjuvant controls were similar, with large numbers of macrophages and few lymphocytes, although many of the latter could be seen beginning to accumulate around adjacent vessels (Fig. 4B). In the lungs of mice vaccinated with the fusion protein, lesions were generally smaller and differed in that they had larger numbers of lymphocytes aggregating in the centers (Fig. 4C).
FIG. 4.
Representative histologic appearance of lesions in the lungs of mice inoculated with saline (A), MPL/DDA adjuvant alone (B), or the fusion protein vaccine (C). The photomicrographs show immunization effects with the vaccine in MPL and DDA; there were no obvious differences if DDA alone was used. Lesions in the vaccinated mice were generally smaller and more lymphocytic. (hematoxylin and eosin stain; magnification, ×100).
DISCUSSION
These results provide the first demonstration that a fusion protein vaccine can be designed that is a potent IL-12 inducer and hence drives immunity toward a needed TH1 response capable of protecting the animal against a virulent challenge infection with M. tuberculosis. Moreover, our observation that mice could be immunized with CSU-F36 given simply in a depot material (DDA) is a very important observation. DDA is an extremely cheap material, and therefore, if a vaccine only needed this cationic depot material, such vaccines would be inexpensive to make. The data suggest that, when given in DDA only, the TLR-2-inducing properties of the vaccine were obviously sufficient to generate good protective immunity without adding MPL. This is in contrast to studies using ESAT-6 by itself, in which it has been clearly shown that a minimum of MPL and DDA is needed to generate any degree of immunity (2). Our own unpublished results reach the same conclusions both for Rv1411 used by itself and for ESAT-6. This seems to suggest that Rv1411 itself does not generate protective T cells, and indeed, if anything, it seems to inhibit this process (8, 9).
Fusion proteins have certain attractions in terms of manufacturing and quality control. One such fusion protein vaccine, Mtb72F, has shown excellent results in animal studies and has reached clinical trials (3, 14), and others are in development (12). The approach taken in the current study, fusing a TLR agonist to a protein or proteins of interest, has widespread applicability, as well as the potential to produce efficacious, inexpensive vaccines against tuberculosis.
Acknowledgments
This work was supported by a grant from the College of Veterinary Medicine and Biomedical Sciences, Colorado State University.
We thank John Belisle for providing a culture of M. smegmatis.
The authors have no competing financial interests.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22160-168. [DOI] [PubMed] [Google Scholar]
- 2.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 726622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285732-736. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., J. E. Callahan, J. P. Griffin, A. D. Roberts, and I. M. Orme. 1995. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect. Immun. 633259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dye, C., M. A. Espinal, C. J. Watt, C. Mbiaga, and B. G. Williams. 2002. Worldwide incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 1851197-1202. [DOI] [PubMed] [Google Scholar]
- 7.Hovav, A. H., and H. Bercovier. 2006. Pseudo-rationale design of efficient TB vaccines: lesson from the mycobacterial 27-kDa lipoprotein. Tuberculosis (Edinburgh) 86225-235. [DOI] [PubMed] [Google Scholar]
- 8.Hovav, A. H., J. Mullerad, L. Davidovitch, Y. Fishman, F. Bigi, A. Cataldi, and H. Bercovier. 2003. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect. Immun. 713146-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovav, A. H., J. Mullerad, A. Maly, L. Davidovitch, Y. Fishman, and H. Bercovier. 2006. Aggravated infection in mice co-administered with Mycobacterium tuberculosis and the 27-kDa lipoprotein. Microbes Infect. 81750-1757. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara, M., T. Nakasone, and M. Honda. 2002. Dynamics of gamma interferon, interleukin-12 (IL-12), IL-10, and transforming growth factor β mRNA expression in primary Mycobacterium bovis BCG infection in guinea pigs measured by a real-time fluorogenic reverse transcription-PCR assay. Infect. Immun. 706614-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8452-456. [DOI] [PubMed] [Google Scholar]
- 12.Orme, I. M. 2006. Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine 242-19. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis (Edinburgh) 834-14. [DOI] [PubMed] [Google Scholar]
- 14.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 1727618-7628. [DOI] [PubMed] [Google Scholar]
- 15.Stenger, S., and R. L. Modlin. 2002. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr. Opin. Immunol. 14452-457. [DOI] [PubMed] [Google Scholar]
- 16.Turner, J., K. M. Dobos, M. A. Keen, A. A. Frank, S. Ehlers, I. M. Orme, J. T. Belisle, and A. M. Cooper. 2004. A limited antigen-specific cellular response is sufficient for the early control of Mycobacterium tuberculosis in the lung but is insufficient for long-term survival. Infect. Immun. 723759-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]