Abstract
This study presents detailed analyses of total and specific serum antibody levels among 26 and 24 adult volunteers before vaccination and after the third dose of the meningococcal serogroup B outer membrane vesicle (OMV) vaccines MeNZB and MenBvac, respectively, in a clinical trial in New Zealand (V. Thornton, D. Lennon, K. Rasanathan, J. O'Hallahan, P. Oster, J. Stewart, S. Tilman, I. Aaberge, B. Feiring, H. Nokleby, E. Rosenqvist, K. White, S. Reid, K. Mulholland, M. J. Wakefield, and D. Martin, Vaccine 24:1395-1400, 2006). With the homologous vaccine strains as targets, both vaccines induced significant increases in serum bactericidal and opsonophagocytic activities and in the levels of immunoglobulin G (IgG) to OMV antigens in an enzyme-linked immunosorbent assay (ELISA) and to live meningococci by flow cytometry. They also induced high levels of activity against the heterologous strains, particularly in terms of opsonophagocytic activity and IgG binding to live bacteria. The antibody levels with the homologous and heterologous strains in the four assays showed high and significant positive correlations. Specific IgG binding to 10 major OMV antigens in each vaccine was measured by scanning of immunoblots; ELISAs for two antigens, lipopolysaccharide and Neisseria surface protein A (NspA), were also performed. Both vaccines elicited significant increases in IgG binding to all homologous and heterologous OMV antigens except NspA. The total IgG band intensity on the blots correlated significantly with the IgG levels determined by the OMV ELISA and flow cytometry. In conclusion, the results of the various immunological assays showed that both OMV vaccines gave rise to high levels of specific and cross-reacting antibodies.
Since 1991, an epidemic of meningococcal disease in New Zealand has caused over 200 deaths and nearly 6,000 cases of disease in a population of 4 million people (www.moh.govt.nz). Most of the cases are caused by serogroup B strains, and from 1991 through 2004, 86% of these expressed the P1.7-2,4 (P1.7b,4) PorA and belonged to the sequence type 41/44 complex (lineage III) (13, 15). The majority of these strains also expressed the serotype 4 PorB protein (14).
In contrast to the other capsular meningococcal polysaccharides, group B polysaccharide is poorly immunogenic in humans (65); and vaccines based on subcapsular antigens, such as outer membrane proteins or outer membrane vesicles (OMVs) from various group B strains, have been developed and used in clinical trials (6, 9, 12, 18, 48). The experience of the Norwegian Institute of Public Health (NIPH) with the development and production of the OMV vaccine (MenBvac) for the protection trial in Norway (6, 19) led to a partnership with Chiron Vaccines (now Novartis Vaccines & Diagnostics) and the New Zealand Ministry of Health, in which NIPH developed and produced a tailor-made OMV vaccine (MeNZB) from a representative strain of the New Zealand epidemic (NZ98/254) based on the production process for MenBvac (23, 24, 41a). After technology transfer, Chiron Vaccines upscaled the MeNZB production process, and the New Zealand Government committed funding to cover the GMP production of MeNZB, clinical trials, vaccine purchase, and the implementation of a national immunization program that has delivered vaccine to those from 6 weeks to 19 years of age inclusive (41).
In the first of the clinical trials undertaken with adults, the safety and immunogenicity of MeNZB after three doses were compared with those of the parent vaccine, MenBvac (50). The present study describes the vaccine-induced responses to both vaccines in this trial, in which a larger set of immunological assays was applied. The levels of immunoglobulin G (IgG) antibody to OMVs, Neisseria surface protein A (NspA), and lipopolysaccharide (LPS) were measured in enzyme-linked immunosorbent assays (ELISAs); IgG to live meningococci was measured by flow cytometry; functional antibody activities were measured by bactericidal and opsonophagocytic assays; and the specific intensity of IgG binding to 10 major antigenic components of the two OMV vaccines was measured by scanning of immunoblots.
(Parts of the present work were presented at the 14th International Pathogenic Neisseria Conference, Milwaukee, WI [1a, 58a].)
MATERIALS AND METHODS
Clinical trial.
A phase I/II clinical trial (clinical trial V60P1) was performed in 2002 with 75 healthy adults (age range, 18 to 50 years) in Auckland, New Zealand, with the aim of comparing the safety and the immunogenicity of MeNZB with those of MenBvac (50). The clinical trial was approved by the Ministry of Health and the Ethics Committee (Auckland region) (50). Two groups of 25 and 24 individuals received 25 and 50 μg of MeNZB, respectively, whereas the remaining 26 individuals received 25 μg of MenBvac. Three doses of each vaccine were given at 6-week intervals. Blood samples were drawn at the time of each vaccination and 6 weeks after the last dose. The OMV vaccine lots for this trial were produced in the facilities at NIPH (30a). For our study, sera collected prior to vaccination and 6 weeks after the third dose from those receiving 25 μg doses of MeNZB (n = 24) and MenBvac (n = 26) were analyzed. The data for one MeNZB vaccinee were excluded, as only the prevaccination sample was available.
Vaccine strains.
The vaccine strains for MenBvac (seed-lot strain 44/76-SL; B:15:P1.7,16) and MeNZB (NZ98/254; B:4:P1.7-2,4) were used (19, 24, 41a). The strain was called homologous when antibody determination for one vaccine group was measured with the corresponding vaccine strain and heterologous when it was measured with the other vaccine strain.
Specific mouse antibodies.
Monoclonal antibodies to L3,7,9 LPS (9-1-L379), L8 LPS (2-1-L8), and OpaJ (class 5.5 protein) (15-1-P5.5) were kindly provided by W. D. Zollinger, Walter Reed Army Institute of Research, Washington, DC; monoclonal antibody to FbpA (RT 36) was provided by S. A. Morse, Centers for Disease Control and Prevention, Atlanta, GA; monoclonal antibody to L1 LPS (223-D8) was provided by J. Kolberg, Norwegian Institute of Public Health, Oslo, Norway; and mouse polyclonal serum against recombinant NspA was provided by G. Guillén, Center for Genetic Engineering & Biotechnology, Havana, Cuba.
IgG antibodies in ELISA.
The concentrations of IgG to OMVs in serum were measured by ELISA with deoxycholate-extracted OMVs from the vaccine strains as antigen sources (46). Sera were diluted 1:200, and IgG levels were measured as arbitrary units (AU) per ml.
Specific IgG antibodies to NspA protein were measured in microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) coated with OMVs prepared from Escherichia coli cells that did or did not express recombinant NspA (rNspA), as described previously (38, 40). The difference in IgG antibody binding between rNspA-positive and rNspA-negative OMVs was taken to represent the binding of NspA-specific antibodies.
IgG binding to LPS was measured with Maxisorp plates (Nunc) coated overnight at room temperature with 100 μl/well of 10 μg LPS/ml in physiological buffered saline (PBS) with 0.1% sodium deoxycholate (42). LPS of immunotype L3 was prepared from a B:4:P1.7-2,4 meningococcal strain from New Zealand (strain NZ98/228) by using a modification of the method reported by Wu et al. (64). The bacteria were grown for 12 h at 36°C in a modified Frantz medium; LPS was purified by hot phenol extraction and gel filtration on a Sephacryl S-300 column eluted with 20 mM Tris HCl and 2 mM EDTA (pH 8.5) containing 1% sodium deoxycholate. In sodium dodecyl sulfate-polyacrylamide gels, the purified L3 LPS showed no protein bands after staining with Coomassie blue and only one LPS band after staining with silver (52). It reacted strongly with the L3,7,9-specific monoclonal antibody in immunoblots and by ELISA.
IgG antibodies to live meningococci by flow cytometry.
Specific IgG antibodies were measured as described previously (2) by using live meningococci grown overnight on Columbia sheep blood agar. A twofold dilution of standard serum in the range of 0.4 to 50 AU/ml and twofold dilutions of vaccinees' sera (starting at 1:5 dilutions) were incubated with live bacteria of either vaccine strain. Bound IgG antibodies were detected with a fluorescein isothiocyanate-labeled goat anti-human IgG conjugate (Cappel, ICN Pharmaceuticals, Aurora, OH); the samples were analyzed with a CyFlow ML flow cytometer (Partec GmbH, Münster, Germany). A standard curve was constructed from the geometric mean fluorescence intensity of the standard serum with GraphPad Prism software (GraphPad, San Diego, CA); the corresponding value of the vaccinees' sera was interpolated to calculate the serum antibody concentrations in AU/ml.
Opsonophagocytosis.
Opsonophagocytic activity (OPA) was measured at NIPH as respiratory burst (RB), as described previously (2), with some modifications: the assay was performed with live meningococci grown overnight on Columbia sheep blood agar, and the sera were tested without heat inactivation of complement. Control experiments revealed no differences in viability or antigen expression whether the bacteria were grown overnight or for 4 h (data not shown). Twofold dilutions of the sera (50 μl) were incubated with live bacteria (5 μl; optical density at 620 nm = 0.7) of strain 44/76-SL or NZ98/254 for 45 min at 37°C with agitation. A human serum sample which had been passed through a protein G column to remove the IgG served as the complement source. Five microliters of this preparation was then added, and incubation was continued for 10 min. Finally, polymorphonuclear leukocytes (PMNs; 50 μl; approximately 4 × 106 cells/ml) primed with dihydrorhodamine 123 (Molecular Probes, Eugene, OR) were added and the mixture was incubated for another 10 min. The percentage of RB-positive PMNs was measured by flow cytometry. The reported titer in serum was the highest reciprocal serum dilution that gave ≥50% RB of the PMNs. Sera with activities less than 50% RB at a 1:2 dilution were given a titer of 1. A responder was defined as an individual with a fourfold or greater increase in titer between the paired pre- and postvaccination sera.
SBA.
Levels of bactericidal antibodies were determined at The Institute of Environmental Science and Research Ltd. by a validated serum bactericidal activity (SBA) assay (33) by starting with a 1:2 serum dilution and using the two vaccine strains and 25% human complement (22). This complement source was different from that used in the OPA assays. Titers were expressed as reciprocal values of the serum dilutions that gave ≥50% killing of the target strain, with a titer of <2 defined as 1 (33). A fourfold or greater increase in the titer of the postvaccination sample compared with the titer of the prevaccination sample required an individual with a prevaccination titer of 2 to attain a postvaccination titer of ≥8.
Immunoblotting.
Paired serum samples diluted 1:200 were blotted against OMVs from both vaccine strains as described previously (58, 62). IgG binding to the OMV antigens was detected with 1:500 dilutions of rabbit anti-human IgG conjugated to horseradish peroxidase (DAKO A/S, Denmark). Two different incubation conditions were used: one with the standard buffer of 3% bovine serum albumin in PBS for blocking and serum dilutions and the other with addition of 0.05% Tween 20 to all solutions including the PBS washing fluid (34). Under both conditions, sera were incubated with and without addition of 0.15% Empigen BB reagent (Albright & Wilson, Whiteheaven, United Kingdom). This reagent may increase the level of IgG binding to OMV proteins but suppresses antibody reactions with antigens of lower molecular weight, such as LPS (31, 59). All nitrocellulose strips were stained for 10 min, and care was taken to blot all sera under identical conditions. From each blot of about 25 strips, 3 strips from different locations on the blot were incubated with a human standard serum sample (diluted 1:400) that reacted moderately with the PorA band of both vaccine antigens. The strips were scanned with Kodak 1D image analysis software to determine the intensity of IgG binding to each of the following major OMV antigens: Omp85, FetA (FrpB or 70-kDa protein), PorA, PorB, FbpA, RmpM (class 4 protein), OpcA, Opa129 (class P5.5 protein) (11), NspA, and LPS. The scan values on each blot were adjusted by a factor obtained from the mean PorA intensity on the standard strips relative to a fixed value for PorA. Only blots incubated in the presence of 0.05% Tween 20 were scanned; however, when an additional increase in specific IgG binding to OMV proteins was detected in the presence of Empigen BB, that value was used. To measure antibodies to outer membrane phospholipase A, OMVs from PorA-negative cells of strain 44/76 that did and that did not express the phospholipase A (8), kindly supplied by M. Bos, Utrecht University, Utrecht, The Netherlands, were used.
Statistical analyses.
Differences in antibody levels between paired pre- and postvaccination sera were compared by use of the Wilcoxon signed-rank test, differences between the two vaccine groups were compared by use of the Mann-Whitney rank sum test, and correlations were assessed by the Spearman rank order correlation test by application of the SigmaStat program from Systat Software Inc., Richmond, CA. P values <0.05 were considered significant.
RESULTS
IgG antibody responses to OMVs and live meningococci.
The IgG levels in the pre- and postvacination sera were measured with OMVs by ELISA and with live meningococci by flow cytometry. Both assays showed that MeNZB and MenBvac induced significant IgG responses (P < 0.001) to antigens on both the homologous and the heterologous strains (Table 1). In the ELISA with OMVs from strain NZ98/254 as the antigen, the median IgG level of the postvaccination sera from the MeNZB group was 1.8-fold higher than that for the MenBvac group (456 versus 252 kAU/ml; P < 0.001). With OMVs from strain 44/76-SL, the MenBvac vaccinees showed a 1.7-fold higher level than the other vaccine group (448 versus 268 kAU/ml; P < 0.001). Thus, the vaccine-elicited levels of IgG to the homologous OMVs were about twice those to the heterologous OMVs. Against live meningococci of strain NZ98/254 as the target in flow cytometry, the median IgG level for the MeNZB group postvaccination was similar to that for the MenBvac group (47 versus 53 AU/ml; P = 0.426). With live 44/76-SL bacteria, the response of the MenBvac group was 3.0-fold higher (125 versus 41 AU/ml) than that of the MeNZB group, but this difference was not significant (P = 0.062). These results with live cells demonstrate that both vaccines induce high levels of cross-reactive IgG antibodies against the heterologous strains.
TABLE 1.
Serum antibody activity in pre- and postvaccination sera from MeNZB and MenBvac vaccinees measured in four antibody assays with the two vaccine strainsa
| Target strain | Vaccineb | IgG titer by ELISAc (kAU/ml)
|
Pd | IgG titer by flow cytometryc (AU/ml)
|
Pd | SBA assay resultc (titer)
|
Pd | OPA assay resultc (titer)
|
Pd | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination sera | Postvaccination sera | Prevaccination sera | Postvaccination sera | Prevaccination sera | Postvaccination sera | Prevaccination sera | Postvaccination sera | ||||||
| NZ98/254 (vaccine strain for MeNZB) | MeNZB | 20 (0-244) | 456 (164-660) | <0.001 | 3 (1-28) | 47 (8-895) | NS | 1 (1-32) | 26 (1-416) | 0.038 | 1 (1-32) | 48 (4-256) | NS |
| MenBvac | 20 (0-300) | 252 (24-584) | 2 (1-83) | 53 (5-1,980) | 1 (1-111) | 16 (1-239) | 1 (1-64) | 32 (1-1,024) | |||||
| 44/76-SL (vaccine strain for MenBvac) | MeNZB | 40 (0-264) | 268 (92-568) | <0.001 | 8 (1-147) | 41 (1-712) | NS | 1 (1-28) | 6 (1-169) | NS | 1 (1-32) | 16 (1-128) | NS |
| MenBvac | 28 (0-300) | 448 (184-624) | 2 (1-468) | 125 (6-2,875) | 1 (1-104) | 11 (1-478) | 1 (1-64) | 32 (2-512) | |||||
Values are medians, with ranges given in parentheses. P was <0.001 for all differences between pre- and postvaccination sera. Postvaccination sera were obtained 6 weeks after the third dose.
A total of 24 and 26 individuals received MeNZB and MenBvac, respectively.
OMVs were the antigens in the ELISA, and live meningococci were the antigens in the remaining analyses.
P value for differences in responses to either vaccine strain between the two vaccine groups. NS, not significant.
The IgG levels of the pre- and postvaccination sera from both vaccine groups in the OMV ELISA correlated significantly (P < 0.001) with those obtained with live bacteria by flow cytometry, as shown in Table 2. High correlation coefficients (range, 0.801 to 0.938) were found with both the homologous and the heterologous strains.
TABLE 2.
Correlations between antibody activities in sera from MeNZB and MenBvac vaccinees measured by different antibody assays with the homologous and heterologous vaccine strainsa
| Antibody assaysb | Spearman rank order correlations coefficient
|
|||
|---|---|---|---|---|
| Homologous strains
|
Heterologous strains
|
|||
| MeNZB groupc | MenBvac groupd | MeNZB groupc | MenBvac groupd | |
| IgG OMV ELISA vs IgG ELISA with live bacteria | 0.883 | 0.881 | 0.801 | 0.938 |
| IgG OMV ELISA vs SBA assay | 0.797 | 0.837 | 0.676 | 0.819 |
| IgG OMV ELISA vs OPA assay | 0.836 | 0.895 | 0.851 | 0.912 |
| IgG OMV ELISA vs total IgG band intensity on blots | 0.757 | 0.833 | 0.585 | 0.855 |
| IgG with live bacteria vs total IgG band intensity on blots | 0.615 | 0.901 | 0.474 | 0.788 |
| IgG with live bacteria vs OPA assay | 0.850 | 0.902 | 0.819 | 0.907 |
| SBA assay vs IgG ELISA with live bacteria | 0.902 | 0.851 | 0.799 | 0.797 |
| SBA assay vs OPA assay | 0.835 | 0.799 | 0.726 | 0.843 |
All correlations were significant (P < 0.001).
OMVs were the antigens in the ELISA and the immunoblotting assay; live meningococci were used in the remaining assays.
Pre- and postvaccination sera from 24 individuals.
Pre- and postvaccination sera from 26 individuals in all assays except for the blotting assay, which had 16 individuals.
SBA.
In serum bactericidal assays, the median titers were also low before vaccination, but both vaccines induced significant increases (P < 0.001) in SBA against both strains (Table 1). With NZ98/254 as the target strain, the median postvaccination titer of those immunized with MeNZB was 1.6-fold higher than the titer for those immunized with MenBvac (titers, 26 versus 16; P = 0.038). With strain 44/76-SL, the median titer for the MenBvac group was 1.8-fold higher than that for the MeNZB group (titers, 11 versus 6), but this difference was not significant (P = 0.229). Thus, MeNZB gave slightly higher SBA against strain NZ98/254 than MenBvac, whereas both vaccines induced similar SBAs against strain 44/76-SL.
OPA.
Each vaccine also induced significant increases (P < 0.001) in OPA, measured as RB, with the homologous and the heterologous strains (Table 1). Prevaccination titers against both strains were low, with a median titer of 1 for all vaccinees. With NZ98/254 as the target strain, the MeNZB vaccinees demonstrated a 1.5-fold higher median titer after vaccination compared to that for the MenBvac group (titers, 48 versus 32; P = 0.620). With strain 44/76-SL, the MenBvac group showed a twofold higher titer than the MeNZB group (titers, 32 versus 16; P = 0.062). The nonsignificant differences indicated high cross-reactivities in the OPA responses of the two vaccine groups, as also noted above for IgG binding to live meningococci.
Among the volunteers who received MeNZB, 88% (21/24) demonstrated a fourfold or greater increase in OPA against vaccine strain NZ98/254 and 79% (19/24) demonstrated a fourfold or greater increase in OPA against strain 44/76-SL. Similar results were observed for the MenBvac group, with 92% (24/26) and 81% (21/26) of the volunteers responding to the homologous and heterologous strains, respectively.
The OPA titers of the pre- and postvaccination sera from both vaccine groups against both strains correlated significantly (P < 0.001) with the SBA titers, with correlation coefficients ranging from 0.726 to 0.843 (Table 2).
IgG antibodies to LPS by ELISA.
IgG antibodies to purified L3 LPS were determined by ELISA with sera collected 6 weeks after each of the three doses. Both vaccines induced only weak increases in L3 antibody titers after each immunization (data not shown). The mean IgG levels after the third dose were only 1.4- to 1.5-fold higher (P = 0.004) than the prevaccination levels, and there was no significant difference between the responses of the MenBvac and MeNZB vaccinees.
IgG antibodies to NspA by ELISA.
Antibodies to NspA were determined with OMVs from E. coli cells that expressed or that did not express rNspA. Although MeNZB contained a distinct amount of NspA protein, as previously shown on Coomassie blue-stained polyacrylamide gels (24, 56) and as probed in our study with a specific antibody on immunoblots, most vaccinees showed no increase in IgG to this protein, nor was there any significant difference in the responses between the two vaccine groups (data not shown). MenBvac contained only trace amounts of this antigen.
IgG antibodies to specific OMV antigens on immunoblots.
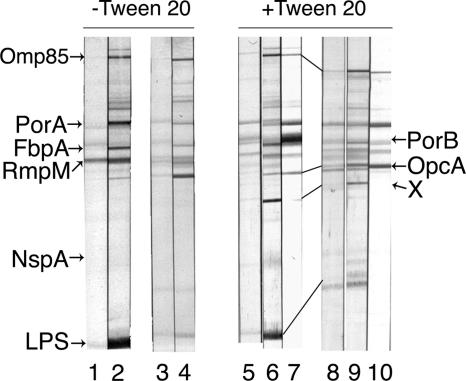
IgG binding to the various OMV antigens was studied on immunoblots under two different experimental conditions: under one condition 0.05% Tween 20 was present in all solutions, and under the other condition this detergent was absent, as shown in Fig. 1. Only blots incubated with Tween 20 were scanned for their IgG binding intensities. Such blots usually had lower levels of background staining. They also showed somewhat stronger antibody binding to the major outer membrane antigens, in addition to bands of unknown nature in the molecular weight range between those for FetA and PorA and, most notably, between those for OpcA/OpaJ and LPS, as shown by band X in Fig. 1. Both vaccines contained LPS of immunotype L3. In addition, MenBvac also contained the L8 immunotype and MeNZB contained the L1 immunotype, as demonstrated on immunoblots developed with specific monoclonal antibodies and silver-stained polyacrylamide gels. In the scanning analysis, IgG binding to LPS was defined as the sum for the L3 band and either the L1 or the L8 band. Although the L1 and L8 LPSs were present in smaller amounts than the L3 LPS in the OMVs, many sera showed distinct binding to these antigens, as demonstrated in Fig. 1. The postvaccination serum from this MeNZB vaccinee showed an L1-specific response with strain NZ98/254 OMV, but the response was suppressed by the Empigen BB reagent. However, this reagent increased IgG binding to PorA and PorB in the NZ98/254 OMV and to PorA and OpcA in the 44/76-SL OMV.
FIG. 1.
Immunoblots demonstrating IgG binding to OMV antigens from the two vaccine strains of pre- and postvaccination sera from one volunteer immunized with three doses of MeNZB. The nitrocellulose strips were incubated without and with 0.02% Tween 20 in the reagents. Strips 1, 3, 5, and 8 were incubated with prevaccination serum; and strips 2, 4, 6, 7, 9, and 10 were incubated with postvaccination serum from volunteer 1043. OMVs from homologous vaccine strain NZ98/254 were antigens for strips 1, 2, 5, 6, and 7; and the heterologous 44/76-SL OMVs were antigens for strips 3, 4, 8, 9, and 10. The positions of the major OMV antigens are shown on both sides of the panel; X designates IgG binding to an unknown antigen visible only in the presence of Tween 20. Specific L1 LPS bands are seen in strips 2 and 6. Strips 7 and 10 were also incubated with 0.15% Empigen BB reagent, which abolished IgG binding to LPS but increased that to PorA, PorB, and/or OpcA. The Adobe Photoshop 8.0 CS program was used to prepare the figure.
Immunoblot analyses were performed with all 24 paired serum samples from the MeNZB group and 16 randomly selected pair serum samples from the MenBvac group. The median IgG binding intensity to the 10 major OMV antigens in strain NZ98/254 of the pre- and postvaccination sera from the two vaccine groups is shown in Table 3; the corresponding results obtained with strain 44/76-SL OMV are presented in Table 4. MeNZB induced significant increases (P < 0.001) in IgG levels to the Omp85, FetA, PorA, PorB, FbpA, RmpM, and LPS antigens in the NZ98/254 vaccine OMV (Table 3). Four prevaccination and nine postvaccination serum samples demonstrated IgG binding to the OpcA protein. Although the median levels were equal to zero, the postvaccination IgG levels were significantly higher (P = 0.02). Except for FetA, MenBvac elicited significant antibody responses (P < 0.001 to 0.005) to the same antigens in the heterologous OMV. A comparison of the postvaccination responses (Table 3) showed no statistical difference between the two vaccine groups in the levels of IgG to PorA, RmpM, OpcA, and LPS in the NZ98/254 OMV. The responses to FetA, PorB, FbpA, and NspA were higher among the MeNZB vaccinees (P = 0.008 to 0.044) than those among the MenBvac vaccinees, whereas the response to Omp85 was slightly lower (P = 0.042) than that among the MenBvac vaccinees. In polyacrylamide gels and on immunoblots, developed with specific monoclonal antibodies, MeNZB was shown to contain a larger amount of FbpA but a smaller amount of OpcA than MenBvac.
TABLE 3.
Levels of serum IgG to antigens in OMVs from strain NZ98/254 measured on immunoblots before vaccination and after the third dose of MeNZB and MenBvaca
| OMV antigen | IgG binding intensityb (kAU) for MeNZB group
|
P for MeNZB group | IgG binding intensityb (kAU) for MenBvac group
|
P for MenBvac group | P for postresponse comparison | ||
|---|---|---|---|---|---|---|---|
| Prevaccination sera | Postvaccination sera | Prevaccination sera | Postvaccination sera | ||||
| Omp85 | 0 (0-1.4) | 1.6 (0-7.9) | <0.001 | 0 (0-1.4) | 3.2 (0-9.2) | <0.001 | 0.042 |
| FetA | 0.4 (0-15.2) | 1.7 (0-15.4) | 0.001 | 0 (0-15.9) | 0 (0-19.5) | NS | 0.044 |
| PorA | 4.3 (2.2-9.1) | 13.0 (3.9-22.7) | <0.001 | 4.4 (2.1-12.6) | 6.7 (2.4-20.4) | <0.001 | NS |
| PorB | 6.5 (0-23.7) | 17.5 (5.0-48.8) | <0.001 | 6.7 (5.2-25.7) | 11.6 (4.6-19.4) | 0.005 | 0.015 |
| FbpA | 0 (0-14.7) | 12.3 (0-18.7) | 0.001 | 0 (0-11.0) | 4.1 (0-16.8) | 0.002 | 0.029 |
| RmpM | 3.4 (0-7.0) | 6.3 (2.3-14.8) | <0.001 | 3.2 (1.3-12.0) | 5.6 (2.8-17.2) | <0.001 | NS |
| OpcA | 0 (0-2.7) | 0 (0-9.2) | 0.020 | 0 (0-6.2) | 1.8 (0-14.0) | 0.004 | NS |
| OpaJc | NDe | ND | ND | ND | |||
| NspA | 1.6 (0-16.1) | 2.4 (0-13.6) | NS | 0 (0-2.4) | 0 (0-3.1) | NS | 0.008 |
| LPSd | 0 (0-29.5) | 4.9 (0-41.3) | <0.001 | 0 (0-9.3) | 2.9 (0-24.8) | 0.002 | NS |
Pre- and postvaccination sera from 24 and 16 individuals receiving MeNZB and MenBvac, respectively, were analyzed.
IgG binding intensities were obtained by scanning; the values are medians, with ranges given in parentheses. The AUs were rounded off to the nearest 100 integer. NS, no significant difference.
OpaJ is not detected in MeNZB.
LPS antibody responses are the sums for the L3 and L1 bands.
ND, not determined.
TABLE 4.
Levels of serum IgG to antigens in OMVs from strain 44/76-SL measured on immunoblots before vaccination and after the third dose of MeNZB and MenBvaca
| OMV antigen | IgG binding intensityb (kAU) for MeNZB group
|
P for MeNZB group | IgG binding intensityb (kAU) for MenBvac group
|
P for MenBvac group | P for postresponse comparison | ||
|---|---|---|---|---|---|---|---|
| Prevaccination sera | Postvaccination sera | Prevaccination sera | Postvaccination sera | ||||
| Omp85 | 0 (0-8.7) | 3.6 (0-13.9) | <0.001 | 0 (0-7.7) | 7.3 (0-14.6) | <0.001 | NS |
| FetA | 0.9 (0-26.4) | 1.8 (0-27.8) | NS | 0 (0-27.8) | 0 (0-23.9) | NS | NS |
| PorA | 9.2 (4.2-27.7) | 13.2 (4.3-31.0) | <0.001 | 6.5 (1.4-23.8) | 13.4 (9.4-32.6) | <0.001 | NS |
| PorB | 7.6 (0-21.4) | 8.0 (0-26.0) | NS | 6.4 (2.2-27.2) | 16.2 (3.8-32.3) | <0.001 | <0.001 |
| FbpA | 0 (0-12.3) | 4.3 (0-17.9) | <0.001 | 0 (0-10.7) | 0 (0-21.4) | 0.031 | NS |
| RmpM | 2.9 (0.8-7.1) | 7.1 (2.6-32.7) | <0.001 | 2.5 (0-12.7) | 7.1 (0-22.6) | <0.001 | NS |
| OpcA | 4.8 (1.6-13.7) | 6.8 (3.0-23.3) | 0.001 | 4.3 (1.4-18.9) | 8.9 (3.6-22.2) | <0.001 | NS |
| OpaJ | 1.7 (0-13.7) | 2.2 (0-14.3) | NS | 0 (0-18.2) | 6.7 (0-20.1) | <0.001 | 0.01 |
| NspA | NDd | ND | ND | ND | |||
| LPSc | 0 (0-15.2) | 5.1 (0-41.8) | <0.001 | 0 (0-5.8) | 9.4 (0-47.6) | <0.001 | NS |
Pre- and postvaccination sera from 24 and 16 individuals receiving MeNZB and MenBvac, respectively, were analyzed.
IgG binding intensities were obtained by scanning; the values are medians, with ranges given in parentheses. AUs were rounded off to the nearest 100 integer. NS, no significant difference.
LPS antibody responses are the sums for the L3 and L8 bands.
ND, not determined.
With the homologous 44/76-SL OMV, MenBvac induced significant responses (P < 0.001) to Omp85, PorA, PorB, RmpM, OpcA, OpaJ129, and LPS (Table 4). Only four prevaccination and seven postvaccination serum samples showed reactions with FbpA. Although the median levels were zero, as noted above for OpcA, the increase after vaccination was significant (P = 0.031). In the vaccinees receiving MeNZB, the levels of IgG to the Omp85, PorA, FbpA, RmpM, OpcA, and LPS antigens in the 44/76-SL OMV also increased significantly (P < 0.001). Apart from the serotype 15 PorB and OpaJ129 antigens, which were expressed only in the 44/76-SL OMV, no significant difference in the IgG responses to the remaining eight OMV antigens was found between the two vaccine groups (Table 4). These results indicated that the vaccines generally gave rise to high levels of cross-reacting antibodies. Neither of the vaccines induced significant responses to FetA in the 44/76-SL OMV. This antigen was present in roughly equal amounts in both vaccines, but the FetA in MeNZB had a slightly lower molecular weight in polyacrylamide gels, consistent with a deletion in the main variable immunogenic region of this protein compared with the FetA in MenBvac (49, 53). Although outer membrane phospholipase A is present in strain 44/76 and was identified in OMVs from NZ98/254 (8, 57), no IgG reaction was observed with this protein expressed in OMVs from a PorA-negative 44/76 strain, in support of the findings of a recent study (8).
We also analyzed if the sum of the intensities of all IgG bands on the blots shown by the pre- and postvaccination sera would correspond to the IgG levels measured by the OMV ELISA and with live bacteria by flow cytometry. For the MenBvac group with 44/76-SL OMV as the antigen, the sum of the band intensities correlated significantly with the IgG OMV ELISA and flow cytometric determinations, with correlation coefficients of 0.833 (P < 0.001) and 0.901 (P < 0.001), respectively (Table 2). For the MeNZB group with NZ98/254 OMV as the antigen, the corresponding correlation coefficients were 0.757 (P < 0.001) and 0.615 (P < 0.001). With the heterologous strains, the MenBvac group also demonstrated high correlation coefficients, while those for MeNZB group were somewhat lower (Table 2).
Correlations of specific IgG levels with functional antibody levels.
The levels of IgG against the various OMV antigens in the postvaccination sera were analyzed for a possible statistical correlation with the corresponding SBA and OPA activities. For the MenBvac vaccinees, this analysis demonstrated a positive correlation with SBA and IgG binding to the P1.7,16 PorA, serotype 15 PorB, RmpM, OpcA, and LPS antigens in the 44/76-SL OMV, with Spearman rank correlation coefficients ranging from 0.543 to 0.797 (P = 0.001 to 0.029). A corresponding analysis for OPA showed significant correlations for the RmpM antibody (correlation coefficient, 0.619; P = 0.010) and the LPS antibody (correlation coefficient, 0.511; P = 0.042). For the MeNZB vaccinees, only the intensity of IgG binding to OpcA correlated significantly with SBA (correlation coefficient, 0.635; P = 0.001) and OPA (correlation coefficient, 0.465; P = 0.022).
DISCUSSION
The first clinical trial with MeNZB was performed with healthy adults and indicated that the vaccine was safe and immunogenic and had a reactogenicity profile similar to that of the parent vaccine, MenBvac (50). The primary immunogenicity measure used was a fourfold or greater rise in SBA 4 to 6 weeks postvaccination. Fourfold rises in serum IgG concentrations, determined by the OMV ELISA, were also reported (50). Our study provides a more detailed investigation of the functional and nonfunctional antibody responses in the individuals after the third dose of MeNZB or MenBvac in that trial.
Both vaccines elicited significant increases in functional antibodies, measured as both SBA and OPA, as well as in the levels of IgG to OMVs by ELISA and to live meningococci in flow cytometry (Table 1). The postvaccination responses to the homologous OMVs by ELISA of either vaccine group were significantly higher than those of the other group, indicating the induction of OMV-specific antibodies. In bactericidal assays with target strain NZ98/254, the MeNZB vaccinees also showed higher SBAs than the MenBvac vaccinees. With strain 44/76-SL, however, no significant difference between the SBAs of the two groups was observed, nor were significant differences in OPA against live bacteria or in IgG binding to live bacteria found with any of the strains. There is no clear explanation for these observations of similar antibody activities, but the results may be explained by a combination of factors, including differences in OMV-induced antibody accessibility to the antigens on live cells, as shown for PorB3 antibodies (36); differences in the levels of cross-reactive antibodies induced by common antigens in the two OMV vaccines; and the impact of secondary antibody responses due to previous exposure to meningococci. Our results suggested that strain NZ98/254 was slightly easier to kill in the bactericidal in vitro assay than strain 44/76-SL, as the SBA titers against these two strains were in the same range for the MenBvac group (titers, 16 versus 11; P = 0.429). However, this serosensitivity may possibly be caused by the complement source.
In the previous study of this trial (50), the number of MeNZB vaccinees showing fourfold or greater increases in SBAs against strains NZ98/254 and 44/76-SL were 100% and 48%, respectively, while the corresponding numbers for the MenBvac group were 42% and 65%. Thus, more vaccinees had SBA responses with the homologous strain than with the heterologous strain. In the opsonic assay, we found fewer differences in the numbers of vaccinees with fourfold or greater OPA responses to the strains, and the numbers were generally higher than those seen for the SBA responses (range, 79 to 92%). However, significant correlations were obtained between SBA and OPA in the pre- and postvaccination sera for both vaccine groups and with both target strains (Table 2). Such a correlation has previously been documented for adult MenBvac vaccinees with strain 44/76-SL (1, 3). Both assays depend on antibodies and complement, and although several studies indicate that SBA correlates with protection against group B disease, as recently reviewed by Borrow et al. (7), OPA is also believed to confer protection, especially against group B disease (45). Our results indicated that OPA may constitute an important supplement to SBA for the evaluation of the two vaccines. The levels of IgG to OMVs and to live bacteria from strain 44/76-SL in the serum of the MenBvac group correlated significantly with the SBA and OPA responses (Table 2), as reported previously (1, 3, 17, 39, 51). Significant correlations for the corresponding antibody activities among the MeNZB vaccinees were also observed (Table 2).
IgG binding to the blots, measured by scanning, also reflected the IgG levels determined by the other assays. In support of previous findings (62), significant correlations between the intensities of all IgG bands and the corresponding IgG levels in the OMV ELISA (Table 2) were found. In addition, the IgG band intensities correlated with the levels of IgG binding to live bacteria. The majority of the prevaccination sera gave distinct immunoreactive bands, most often in the 70- to 90-kDa range, possibly because of the previous carriage of meningococci. However, these antibody specificities were unlikely to be functional, as judged from the low SBA and OPA titers of the prevaccination sera (Table 1).
Each vaccine group showed significant increases in IgG to purified immunotype L3 LPS in the ELISA after the third dose. This component was present in both vaccines. In addition, MeNZB contained immunotype L1 LPS (16) and MenBvac contained immunotype L8 LPS (5, 42, 62). The blotting method, which measured IgG binding to the L3 immunotype, as well as to the L1 and L8 immunotypes, also demonstrated that both vaccines induced significant IgG responses to LPS in the homologous as well as the heterologous OMVs. Immunotype L8, which is present in smaller amounts than L3 in MenBvac, was generally more immunogenic than L3 among the MenBvac vaccinees, as reported previously (42, 62). The same was also observed for L1 in the sera from subjects given MeNZB. The L1 and L8 immunotypes differ by only one galactose residue (28, 47), but distinct IgG binding to one immunotype was seldom reflected by the corresponding binding to the other.
NspA is a conserved surface-exposed meningococcal protein that gives rise to bactericidal and protective antibodies in mice (32) but not in humans (21). Although it is expressed by strain 44/76 (38), MenBvac contained only trace amounts of NspA, whereas this antigen was distinctly present in MeNZB, in support of the findings presented in recent reports (16, 56). However, we did not detect any increase in IgG antibodies to NspA among the MeNZB vaccinees by ELISA or immunoblotting. Interestingly, sera from mice vaccinated with a group A OMV vaccine demonstrated distinct NspA antibody reactions in the same ELISA (40), so a species difference in the immune response to this antigen seems possible.
Both vaccines induced increased IgG antibody binding on blots to the Omp85, PorA, FbpA, RmpM, OpcA, and LPS antigens in the homologous and heterologous OMVs. We found no significant difference in the postvaccination responses of the two vaccine groups with each of the PorA antigens (Tables 3 and 4). This antigen is defined as P1.7-2,4 PorA in strain NZ98/254 and as P1.7,16 PorA in strain 44/76. The variable regions 1 (VR1's) of the two PorA antigens (the P1.7-2 and P1.7 epitopes) are the same except for a deletion of three amino acids in P1.7-2, masking this epitope from interacting with a P1.7-specific monoclonal antibody (35, 60), whereas the two VR2's (the P1.4 and P1.16 epitopes) are very different (35). Studies performed by the use of various antibody assays, including immunoblotting, have demonstrated that PorA antibodies induced by MenBvac, MeNZB, or the corresponding PorA components in the Dutch PorA OMV vaccines are mainly directed against VR2 in P1.7,16 and P1.7-2,4 PorA (26, 34, 42, 55, 62, 63). The VR1 epitopes seem to be less immunogenic than VR2. Thus, MenBvac did not induce distinct levels of P1.7-specific antibodies in adults (42, 62, 63), nor was such specificity found in sera from children receiving MeNZB (34). The cross-reactive PorA antibodies, observed by blotting in our study, were also less likely to be induced by the semivariable loop VR3, as this is different in the two vaccine strains (10, 25). The results may possibly be explained by the carriage of meningococcal strains expressing the P1.7,16 PorA or by other unknown cross-reacting antigens.
PorA is a key antigen for the bactericidal responses of OMV vaccines, but OpcA and LPS also induce such activity in humans (22, 26, 34, 37, 42, 43, 54, 61, 66). In the present study, the levels of IgG to these three antigens, in addition to those to PorB and RmpM, in the MenBvac group correlated statistically with SBA against strain 44/76-SL, while only OpcA antibodies showed a corresponding relation with SBA against the homologous strain in the MeNZB vaccinees. No correspondence with the FetA or FbpA responses, which are reported to induce bactericidal antibodies in animals, was found (4, 20, 29, 30). OPA with the homologous strains also correlated with the OpcA antibody responses of the MeNZB group and with the RmpM and LPS antibody responses in the MenBvac group. RmpM and the serotype 15 PorB are not likely to be expressed on the surface of live bacteria (27, 36, 44), and the correlations obtained with the functional SBA and OPA responses probably represent covariation rather than a causal relationship. As the blotting method measures antibodies with both functional and nonfunctional activities, the results from such statistical analyses should be interpreted with caution. Absorption experiments with purified antigens might elucidate the contributions of the various antigens to the functional activities.
In conclusion, three doses of MeNZB or MenBvac induced significant increases in SBA and OPA and in IgG antibodies to OMVs by ELISA and to live bacteria by flow cytometry, as measured with both vaccine strains. High and significant correlations between the various antibody activities were observed. The two vaccine groups showed similar OPA and IgG levels with either live strain, indicating high levels of cross-reactive antibodies. Immunoblotting also demonstrated significant increases in IgG binding to most of the 10 selected antigens in the homologous and heterologous OMVs. The contribution of some of these responses to the functional activities has yet to be determined. Significant correlations between the total IgG band intensity on the blots and the IgG levels to OMVs by ELISA and to live meningococci by flow cytometry were observed.
Acknowledgments
We are grateful for the skilled technical support of E. Fritzsønn and U. Heggelund; the generous gifts of recombinant OMVs from M. Bos; the specific antibodies from G. Guillén, J. Kolberg, S. A. Morse, and W. D. Zollinger; and the preparation of Fig. 1 by P. K. Svendsen. We acknowledge the members of the Vaccine Trials Team, led by Diana Lennon, UniServices, Auckland University, who delivered the vaccine during the adult trial and collected serum samples. We particularly thank Anne Glennie and Nicola Ruijne, who undertook parts of the serum antibody assays.
This study was funded by the Ministry of Health and Care Services in Norway and by the Ministry of Health in New Zealand.
Footnotes
Published ahead of print on 9 May 2007.
REFERENCES
- 1.Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 633531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Aase, A. T. K. Herstad, L. M. Næss, T. E. Michaelsen, and D. R. Martin. 2004. Abstr. 14th Int. Pathogenic Neisseria Conf., p. 140.
- 2.Aase, A., E. A. Hoiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47388-396. [DOI] [PubMed] [Google Scholar]
- 3.Aase, A., L. M. Naess, R. H. Sandin, T. K. Herstad, F. Oftung, J. Holst, I. L. Haugen, E. A. Hoiby, and T. E. Michaelsen. 2003. Comparison of functional immune responses in humans after intranasal and intramuscular immunisations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine 212042-2051. [DOI] [PubMed] [Google Scholar]
- 4.Ala'aldeen, D. A., H. A. Davies, and S. P. Borriello. 1994. Vaccine potential of meningococcal FrpB: studies on surface exposure and functional attributes of common epitopes. Vaccine 12535-541. [DOI] [PubMed] [Google Scholar]
- 5.Andersen, S. R., G. Bjune, J. Lyngby, K. Bryn, and E. Jantzen. 1995. Short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis of potential value for production of outer membrane vesicle vaccines. Microb. Pathog. 19159-168. [DOI] [PubMed] [Google Scholar]
- 6.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. Holst Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Froholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 3381093-1096. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 232222-2227. [DOI] [PubMed] [Google Scholar]
- 8.Bos, M. P., B. Tefsen, P. Voet, V. Weynants, J. P. van Putten, and J. Tommassen. 2005. Function of neisserial outer membrane phospholipase A in autolysis and assessment of its vaccine potential. Infect. Immun. 732222-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, and J. Mays. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15-P1.3) outer-membrane protein vaccine in Iquique, Chile. Vaccine 13821-829. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, S. C., M. A. Diggle, P. Molling, M. Unemo, and P. Olcen. 2003. Analysis of PorA variable region 3 in meningococci: implications for vaccine policy? Vaccine. 212468-2473. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge, M. I., M. P. Bos, H. J. Hamstra, W. Jiskoot, P. van Ulsen, J. Tommassen, L. van Alphen, and L. P. van der Ley. 2002. Conformational analysis of opacity proteins from Neisseria meningitidis. Eur. J. Biochem. 2695215-5223. [DOI] [PubMed] [Google Scholar]
- 12.de Moraes, J. C., B. A. Perkins, M. C. C. Camargo, N. T. R. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. L. Gral, V. L. Gattas, H. D. Vasconcelos, B. D. Plikaytis, J. D. Wenger, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao-Paulo, Brazil. Lancet 3401074-1078. [DOI] [PubMed] [Google Scholar]
- 13.Devoy, A. F., K. H. Dyet, and D. R. Martin. 2005. Stability of PorA during a meningococcal disease epidemic. J. Clin. Microbiol. 43832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyet, K. H., and D. R. Martin. 2005. Sequence variation in the porB gene from B:P1.4 meningococci causing New Zealand's epidemic. J. Clin. Microbiol. 43838-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyet, K. H., and D. R. Martin. 2006. Clonal analysis of the serogroup B meningococci causing New Zealand's epidemic. Epidemiol. Infect. 134377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari, G., I. Garaguso, J. Adu-Bobie, F. Doro, A. R. Taddei, A. Biolchi, B. Brunelli, M. M. Giuliani, M. Pizza, N. Norais, and G. Grandi. 2006. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 61856-1866. [DOI] [PubMed] [Google Scholar]
- 17.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 744557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasch, C., G. Coetzee, J. M. Zahadrik, H. A. Feldman, and H. J. Kornhoof. 1983. Development and evaluation of group B serotype 2 protein vaccines: report of a group B field trial. Med. Trop. (Marseille) 43177-180. [Google Scholar]
- 19.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, A. Aase, and O. Closs. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1467-79. [PubMed] [Google Scholar]
- 20.Gomez, J. A., M. T. Criado, and C. M. Ferreiros. 1998. Bactericidal activity of antibodies elicited against the Neisseria meningitidis 37-kDa ferric binding protein (FbpA) with different adjuvants. FEMS Immunol. Med. Microbiol. 2079-86. [DOI] [PubMed] [Google Scholar]
- 21.Halperin, S. A., J. M. Langley, B. Smith, P. Wunderli, L. Kaufman, A. Kimura, and D. Martin. 2007. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine 25450-457. [DOI] [PubMed] [Google Scholar]
- 22.Hoiby, E. A., E. Rosenqvist, L. O. Froholm, G. Bjune, B. Feiring, H. Nokleby, and E. Ronnild. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 14147-155. [PubMed] [Google Scholar]
- 23.Holst, J. 2006. A ‘taylor made’ vaccine trialled as part of public health response to group B meningococcal epidemic in New Zealand. Eurosurv. Wkly. http:\www.eurosurveillance.org/ew/2003/031016.asp#1.
- 24.Holst, J., B. Feiring, L. M. Naess, G. Norheim, P. Kristiansen, E. A. Hoiby, K. Bryn, P. Oster, P. Costantino, M. K. Taha, J. M. Alonso, D. A. Caugant, E. Wedege, I. S. Aaberge, R. Rappuoli, and E. Rosenqvist. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 232202-2205. [DOI] [PubMed] [Google Scholar]
- 25.Humphries, H. E., J. N. Williams, R. Blackstone, K. A. Jolley, H. M. Yuen, M. Christodoulides, and J. E. Heckels. 2006. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 2436-44. [DOI] [PubMed] [Google Scholar]
- 26.Idanpaan-Heikkila, I., E. A. Hoiby, P. Chattopadhyay, U. Airaksinen, T. M. Michaelsen, and E. Wedege. 1995. Antibodies to meningococcal class 1 outer-membrane protein and its variable regions in patients with systemic meningococcal disease. J. Med. Microbiol. 43335-343. [DOI] [PubMed] [Google Scholar]
- 27.Jansen, C., A. Wiese, L. Reubsaet, N. Dekker, H. de Cock, U. Seydel, and J. Tommassen. 2000. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim. Biophys. Acta 1464284-298. [DOI] [PubMed] [Google Scholar]
- 28.Jennings, M. P., Y. N. Srikhanta, E. R. Moxon, M. Kramer, J. T. Poolman, B. Kuipers, and P. van der Ley. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology 145(Pt 11)3013-3021. [DOI] [PubMed] [Google Scholar]
- 29.Kortekaas, J., S. A. Muller, P. Ringler, M. Gregorini, V. E. Weynants, L. Rutten, M. P. Bos, and J. Tommassen. 2006. Immunogenicity and structural characterisation of an in vitro folded meningococcal siderophore receptor (FrpB, FetA). Microbes Infect. 82145-2153. [DOI] [PubMed] [Google Scholar]
- 30.Kortekaas, J., A. Pettersson, J. van der Biezen, V. E. Weynants, P. van der Ley, J. Poolman, M. P. Bos, and J. Tommassen. 2007. Shielding of immunogenic domains in Neisseria meningitidis FrpB (FetA) by the major variable region. Vaccine 2572-84. [DOI] [PubMed] [Google Scholar]
- 30a.Kristiansen, P. A., I. S. Aaberge, K. Møyner, L. M. Næss, K. Bryn, K. Nord, A. G. Skryten, E. Namork, E. Rosenqvist, and K. Harbak. 2002. Abstr. 13th Int. Pathogenic Neisseria Conf., p. 268.
- 31.Mandrell, R. E., and W. D. Zollinger. 1984. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J. Immunol. Methods 671-11. [DOI] [PubMed] [Google Scholar]
- 32.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 1851173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin, D., L. McCallum, A. Glennie, N. Ruijne, P. Blatchford, J. O'Hallahan, and P. Oster. 2005. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 232218-2221. [DOI] [PubMed] [Google Scholar]
- 34.Martin, D. R., N. Ruijne, L. McCallum, J. O'Hallahan, and P. Oster. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuinness, B. T., P. R. Lambden, and J. E. Heckels. 1993. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol. Microbiol. 7505-514. [DOI] [PubMed] [Google Scholar]
- 36.Michaelsen, T. E., A. Aase, J. Kolberg, E. Wedge, and E. Rosenqvist. 2001. PorB3 outer membrane protein on Neisseria meningitidis is poorly accessible for antibody binding on live bacteria. Vaccine 191526-1533. [DOI] [PubMed] [Google Scholar]
- 37.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 624419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 675664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naess, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Hoiby, R. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17754-764. [DOI] [PubMed] [Google Scholar]
- 40.Norheim, G., A. Aase, D. A. Caugant, E. A. Hoiby, E. Fritzsonn, T. Tangen, P. Kristiansen, U. Heggelund, and E. Rosenqvist. 2005. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine 233762-3774. [DOI] [PubMed] [Google Scholar]
- 41.O'Hallahan, J., D. Lennon, P. Oster, R. Lane, S. Reid, K. Mulholland, J. Stewart, L. Penney, T. Percival, and D. Martin. 2005. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine 232197-2201. [DOI] [PubMed] [Google Scholar]
- 41a.Rosenqvist, E., K. Bryn, K. Harbak, J. Holst, E. A. Høiby, P. Kristiansen, H. Lange, D. Martin, K. Møyner, K. Nord, L. M. Næss, A. G. Skryten, I. Aaberge, A. Aase, and H. Nøkleby. 2002. Abstr. 13th Int. Pathogenic Neisseria Conf., p. 64.
- 42.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 634642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenqvist, E., E. A. Hoiby, E. Wedege, B. Kusecek, and M. Achtman. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 1671065-1073. [DOI] [PubMed] [Google Scholar]
- 44.Rosenqvist, E., A. Musacchio, A. Aase, E. A. Hoiby, E. Namork, J. Kolberg, E. Wedege, A. Delvig, R. Dalseg, T. E. Michaelsen, and J. Tommassen. 1999. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect. Immun. 671267-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 1551266-1275. [DOI] [PubMed] [Google Scholar]
- 46.Ruijne, N., R. A. Lea, J. O'Hallahan, P. Oster, and D. Martin. 2006. Understanding the immune responses to the meningococcal strain-specific vaccine MeNZB measured in studies of infants. Clin. Vaccine Immunol. 13797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41236-243. [DOI] [PubMed] [Google Scholar]
- 48.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14195-207. [PubMed] [Google Scholar]
- 49.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 1491849-1858. [DOI] [PubMed] [Google Scholar]
- 50.Thornton, V., D. Lennon, K. Rasanathan, J. O'Hallahan, P. Oster, J. Stewart, S. Tilman, I. Aaberge, B. Feiring, H. Nokleby, E. Rosenqvist, K. White, S. Reid, K. Mulholland, M. J. Wakefield, and D. Martin. 2006. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine 241395-1400. [DOI] [PubMed] [Google Scholar]
- 51.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, P. H. Makela, and H. Kayhty. 2005. Passive protection in the infant rat protection assay by sera taken before and after vaccination of teenagers with serogroup B meningococcal outer membrane vesicle vaccines. Vaccine 234821-4833. [DOI] [PubMed] [Google Scholar]
- 52.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119115-119. [DOI] [PubMed] [Google Scholar]
- 53.van der Ley, P., J. van der Biezen, R. Sutmuller, P. Hoogerhout, and J. T. Poolman. 1996. Sequence variability of FrpB, a major iron-regulated outer-membrane protein in the pathogenic neisseriae. Microbiology 142(Pt 11)3269-3274. [DOI] [PubMed] [Google Scholar]
- 54.van der Voort, E. R., P. van der Ley, J. van der Biezen, S. George, O. Tunnela, H. van Dijken, B. Kuipers, and J. Poolman. 1996. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 642745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vermont, C. L., H. H. van Dijken, A. J. Kuipers, C. J. van Limpt, W. C. Keijzers, A. van der Ende, R. de Groot, L. van Alphen, and G. P. van den Dobbelsteen. 2003. Cross-reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine. Infect. Immun. 711650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vipond, C., J. X. Wheeler, I. M. Feavers, J. Suker, and C. Jones. 2005. Characterization of the protein content of a meningococcal outer membrane vesicle vaccine by polyacrylamide gel electrophoresis and mass spectrometry. Hum. Vaccine 180-84. [DOI] [PubMed] [Google Scholar]
- 57.Vipond, C., J. Suker, C. Jones, C. Tang, I. M. Feavers, and J. X. Wheeler. 2006. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics 63400-3413. [DOI] [PubMed] [Google Scholar]
- 58.Wedege, E. 2001. Immunoblot analysis of sera from patients and vaccinees, p. 275-288. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 58a.Wedege, E., I. Aaberge, K. Bolstad, E. Fritzsønn, U. Heggelund, L. McCallum, L. M. Næss, E. Rosenqvist, and D. Martin. 2004. Abstr. 14th Int. Pathogenic Neisseria Conf., p. 196.
- 59.Wedege, E., K. Bryn, and L. O. Froholm. 1988. Restoration of antibody binding to blotted meningococcal outer membrane proteins using various detergents. J. Immunol. Methods 11351-59. [DOI] [PubMed] [Google Scholar]
- 60.Wedege, E., R. Dalseg, D. A. Caugant, J. T. Poolman, and L. O. Froholm. 1993. Expression of an inaccessible P1.7 subtype epitope on meningococcal class 1 proteins. J. Med. Microbiol. 3823-28. [DOI] [PubMed] [Google Scholar]
- 61.Wedege, E., and L. O. Froholm. 1986. Human antibody response to a group B serotype 2a meningococcal vaccine determined by immunoblotting. Infect. Immun. 51571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wedege, E., E. A. Hoiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 663223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wedege, E., B. Kuipers, K. Bolstad, H. van Dijken, L. O. Froholm, C. Vermont, D. A. Caugant, and G. van den Dobbelsteen. 2003. Antibody specificities and effect of meningococcal carriage in Icelandic teenagers receiving the Norwegian serogroup B outer membrane vesicle vaccine. Infect. Immun. 713775-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, L. H., C. M. Tsai, and C. E. Frasch. 1987. A method for purification of bacterial R-type lipopolysaccharides (lipooligosaccharides). Anal. Biochem. 160281-289. [DOI] [PubMed] [Google Scholar]
- 65.Wyle, F. A., M. S. Artenstein, B. L. Brandt, E. C. Tramont, D. L. Kasper, P. L. Altieri, S. L. Berman, and J. P. Lowenthal. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126514-521. [DOI] [PubMed] [Google Scholar]
- 66.Zollinger, W., E. Moran, D. Schmiel, and B. Brandt. 2006. Abstr. 15th Int. Pathogenic Neissseria Conf., abstr. P8.3.19.