Abstract
The genetically detoxified Bordetella pertussis adenylate cyclase is a promising delivery system for immunodominant tuberculosis antigens in gamma interferon release assays. This system has not been evaluated in human immunodeficiency virus (HIV)-infected persons in high tuberculosis prevalence areas. A whole-blood gamma interferon release assay with Mycobacterium tuberculosis antigens (early-secreted antigenic target 6, culture filtrate protein 10, alpha-crystallin 2, and TB10.3) delivered by adenylate cyclase in addition to native tuberculosis antigens (without adenylate cyclase delivery) was evaluated in 119 adults in Khayelitsha Township, Cape Town, South Africa. Results were compared to tuberculin skin test results of 41 HIV-positive and 42 HIV-negative asymptomatic persons, in addition to 36 HIV-positive persons with recently diagnosed smear- or culture-positive pulmonary tuberculosis. Delivery of tuberculosis antigens by adenylate cyclase decreased by 10-fold the amount of antigen required to restimulate T cells. Furthermore, the responses of HIV-positive persons with a low response to native tuberculosis antigens were enhanced when these antigens were delivered by adenylate cyclase. When gamma interferon responses to the tuberculosis antigens (with or without delivery by adenylate cyclase) were combined, a significantly higher number of patients were scored positive than by tuberculin skin testing. Ex vivo responses to tuberculosis antigens delivered by adenylate cyclase are maintained in the context of HIV infection. Our findings suggest that the majority of those in this population are infected with tuberculosis, which is of significant public health importance.
While eradication of tuberculosis (TB) appears a realistic goal in low-prevalence countries, the incidence and mortality rates in sub-Saharan Africa are continuing to rise, fuelled primarily by the coexisting human immunodeficiency virus (HIV) pandemic (12). The Cape Town metropole in South Africa has one of the highest incidences of TB ever recorded, in certain areas in excess of 1,612/100,000, coupled with an antenatal HIV seroprevalence of approximately 33% (8, 9; Provincial and City Data 2005). There is an urgent need for new, accurate, simple, and low-cost diagnostic tests for detecting TB infection and disease (4).
Recent genome-driven advances in Mycobacterium tuberculosis antigen discovery has led to new avenues for the diagnosis of TB (2, 5, 20). Two of the most widely studied antigens are region of difference 1 (RD1)-based early-secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10), which form the basis of two recently commercialized blood-based immunodiagnostic tests for TB (T-SPOT.TB and QuantiFERON TB-Gold) (23). These assays potentially represent a significant improvement over the tuberculin skin test (TST) for the detection of latent TB infection (LTBI) and active TB disease in adults, particularly in areas where BCG vaccination rates are high and exposure to nontuberculous mycobacteria is ubiquitous (2, 10, 23). Furthermore, in HIV-infected (HIV+) persons, the highest-risk group for disease progression and in whom the sensitivity of the TST is significantly reduced (1, 11, 17), the assays may offer a considerable advantage for the diagnosis of TB. Such assays, however, have not been extensively evaluated in HIV+ persons living in high TB prevalence areas. In addition, they require a relatively large volume of blood, which is important when considering their application in pediatric practice.
We have recently shown that the genetically detoxified Bordetella pertussis adenylate cyclase (CyaA) is able to efficiently deliver M. tuberculosis-specific antigens for presentation to both human and bovine T cells (28, 29). CyaA facilitates direct translocation of the inserted antigens across the cytoplasmic membrane of target cells, by using CD11b associated with the β2 integrin (CD11b/CD18) as its specific receptor (14, 15, 25). Since CD11b/CD18 (also known as MAC-1) is mainly expressed on macrophages and dendritic cells, CyaA specifically targets professional antigen-presenting cells. Previous work has shown that the inserted antigen can be efficiently presented via both the classical major histocompatibility complex (MHC) class I and II antigen-processing pathways to CD8+ and CD4+ T cells and that T-cell recognition is sensitive to antibody blockade of MHC classes I and II, anti-CD11b, and chloroquine (inhibitor of endosomal processing that therefore decreases presentation via MHC class II) (19, 24, 28, 29).
We have used this antigen delivery vector in a low-volume whole-blood-based gamma interferon (IFN-γ) release assay (IFNGRA) for detecting LTBI in both adults and school children and showed that delivery by CyaA most efficiently enhanced the detection of IFN-γ in people who had a low or undetectable response to native TB antigens (3, 29). As people with immune suppression might have low TB-specific T-cell responses, we were specifically interested in determining whether LTBI could be detected in HIV+ persons with this novel whole-blood culture system. As part of an existing study comparing the performance of commercial IFNGRA for the detection of LTBI in HIV+ persons (22), we had the opportunity to evaluate T-cell responses to recombinant CyaA-carrying ESAT-6 and CFP-10. We also evaluated two other antigens that are immunodominant in humans, alpha-crystallin 2 (Acr2) and TB10.3. Acr2 is a member of the alpha-crystallin family of small heat shock proteins that we have recently identified and found to be infection stage specific; high frequencies of IFN-γ-secreting T cells responsive to this antigen have been detected in persons with recent exposure to infectious TB (30). TB10.3 is a member of the ESAT-6 family that has recently been implicated in protection against experimental bovine TB (16, 26). By comparing the results of the IFNGRA to those of TST in both HIV+ and HIV-negative (HIV−) persons, we aimed to determine whether these low blood volume assays could be used to diagnose TB infection in high-risk patient groups.
MATERIALS AND METHODS
Participants.
This study was approved by the University of Cape Town Research Ethics Committee (UCT REC 443/2004 and 173/2005). Patients were recruited at the joint Médecins Sans Frontières, Provincial Government of the Western Cape, and City Health Ubuntu HIV-TB clinic in the Township of Khayelitsha, South Africa, an area with an extremely high burden of TB and HIV. Informed consent was obtained for all participants. Forty-one HIV+ and 42 HIV− asymptomatic persons, in addition to 36 HIV+ persons with recently diagnosed smear- or culture-positive pulmonary TB (HIV+PTB), were enrolled. In addition to blood testing, all patients underwent the TST. A positive TST result was defined as ≥5 mm in the HIV+ and HIV+PTB groups and ≥15 mm in the HIV− group. The presence of a BCG scar was recorded, and TB contact history was documented. Isoniazid preventive treatment was offered to those with a positive TST result according to South African national guidelines, and HIV+ persons with a CD4 count of less than 200 were referred for antiretroviral treatment.
Recombinant antigen and CyaA toxoid construction.
Antigenic stimulants included purified native recombinant ESAT-6, CFP-10, Acr2, and TB10.3 proteins, as well as their fusions within the recombinant CyaA proteins carrying these antigens inserted at two different permissive positions of the adenylate cyclase activity domain (336 or 224, resulting in detoxified CyaA). The native antigens and CyaA-TB antigen fusions (CyaA/336-ESAT-6, CyaA/336-CFP-10, CyaA/224-CFP-10, CyaA/336-Acr2, and CyaA/336-TB10.3) were constructed, expressed in Escherichia coli, and purified close to homogeneity as previously described (29). The open reading frames that encode Acr2 and TB10.3 were amplified by PCR with primer pairs 5′-GATGTGTACATATGAACAATCTCGCATTGTGGTCG and 3′-CATGTGTACAGTAAGCTTCGTGATGGCGATGCGCGC for the Acr2 antigen and 5′-GTAAAAAGCTTTATGTCGCAGATTATGTACAACTAT and 3′-AGCTAAAGCTTTTGCCGCCCCACTTGGCGGCTTC for TB10.3, respectively. The amplified fragments were cloned as previously described (29) and entirely sequence verified for absence of mutations before production of the corresponding native TB antigens and CyaA-TB antigen fusions (further details will be provided on request to sebo@biomed.cas.cz).
Antigen-specific IFNGRA.
The whole-blood assay and measurement of IFN-γ by enzyme-linked immunosorbent assay (ELISA) were performed as previously described (29). Briefly, venous blood was collected, diluted 1:10 with RPMI 1640 medium supplemented with glutamine, and processed within 4 h of sampling. Aliquots (180 μl) of diluted blood were plated in 96-well U plates (Nunc) with stimulating antigens (20 μl) in duplicate wells. Phytohemagglutinin (PHA; Sigma) at 5 μg/ml was included as a positive control. Negative controls included unstimulated wells (nil) and wells containing mock CyaA toxoid (CyaA-E5) with no antigenic insert. Following a 72-h culture at 37°C in a CO2 incubator, supernatants were harvested and immediately frozen for subsequent IFN-γ measurements by ELISA.
The ELISA was carried out according to the manufacturer's instructions. Briefly, 96-well flat-bottom plates were coated overnight at 4°C with purified mouse anti-IFN-γ (BD Pharmingen 554548). Following washing and blocking, 100 μl of IFN-γ standards (from 10 pg/ml to 10,000 pg/ml) and culture supernatants were added in duplicate wells and incubated overnight at 4°C. After washing, the wells were plated with biotinylated mouse anti-human IFN-γ (BD Pharmingen 554550) and incubated for 45 min at room temperature. Following a further washing step, wells were incubated with streptavidin-peroxidase (Sigma) for 30 min. ortho-Phenylenediamine hydrochloride was used as the substrate for detection, and 2 N H2SO4 was used to stop color development. Optical densities were read at 490 nm on a plate reader, and IFN-γ concentrations were calculated from standard curves.
For analysis, we defined a positive whole-blood IFNGRA according to the criteria in Table 1. Values were chosen to reflect recommended cutoff values in commercially available whole-blood IFNGRAs (21). Background values in the nil and mock CyaA-E5-containing wells were subtracted from values in the native-antigen (ESAT-6, CFP-10, Acr2, and TB10.3) and CyaA-TB antigen fusion (CyaA/336-ESAT-6, CyaA/336-CFP-10, CyaA/224-CFP-10, CyaA/336-Acr2, and CyaA/336-TB10.3) wells, respectively, as previously described (29). A background-corrected value of greater than or equal to 70 pg/ml in response to test antigens, and when the absolute value was more than 1.5 times the background response (nil or mock CyaA-E5), was deemed positive. Results were classified as indeterminate when the corrected test antigen response was less than 70 pg/ml in the presence of an inadequate PHA response (PHA minus nil being <100 pg/ml) or when absolute values were less than 1.5 times the background responses.
TABLE 1.
Criteria for interpretation of the whole-blood IFNGRA for both native antigens and CyaA constructs
| PHA − nil (pg/ml)a | [CyaA-ESAT-6] − [CyaA-E5], [CyaA-CFP-10] − [CyaA-E5], [ESAT-6] − nil, or [CFP-10] − nil (pg/ml) | Result and interpretation |
|---|---|---|
| ≥100 | ≥70 and ≥1.5 times WTb or nil | Positive |
| <100 | ≥70 and ≥1.5 times WT or nil | Positive |
| ≥100 | <70 | Negative |
| <100 | <70 | Indeterminate |
| ≥100 | ≥70 and less than 1.5 times WT or nil | Indeterminate |
| <100 | ≥70 and less than 1.5 times WT or nil | Indeterminate |
The minimum specific activity of the IFN-γ standard used in the assay was 20 U/ng. Therefore, a value recorded as 100 pg/ml is a minimum of 2 U/ml.
WT = background wild-type response (CyaA-E5 alone).
Analytic and statistical methods.
Clinical and laboratory data sets were created and maintained separately and subsequently merged for analysis by an investigator who had not contributed to either clinical recruitment or the laboratory assays. The normality of data was assessed by the D'Agostino and Pearson omnibus normality test. Nonparametric unpaired data were analyzed by the Mann-Whitney U test with paired data analyzed with the Wilcoxon signed-rank test. Fischer's exact test of probability was used to compare the proportions of positive, negative, and indeterminate assay results between individual groups. Significance was assigned to P < 0.05.
RESULTS
Characteristics of the study groups.
A total of 119 adults were enrolled, comprising 41 asymptomatic HIV+ and 42 HIV− individuals and 36 HIV+ patients with pulmonary TB. The groups did not significantly differ in age (mean ages, 31.5 versus 29.9 versus 32.1 years; P > 0.05), gender, BCG status (65% versus 50% versus 55%; P = >0.05), or history of recent contact with infectious TB (17% versus 5% versus 11%; P = >0.05).
Determination of the optimum concentrations of CyaA-TB antigen fusions.
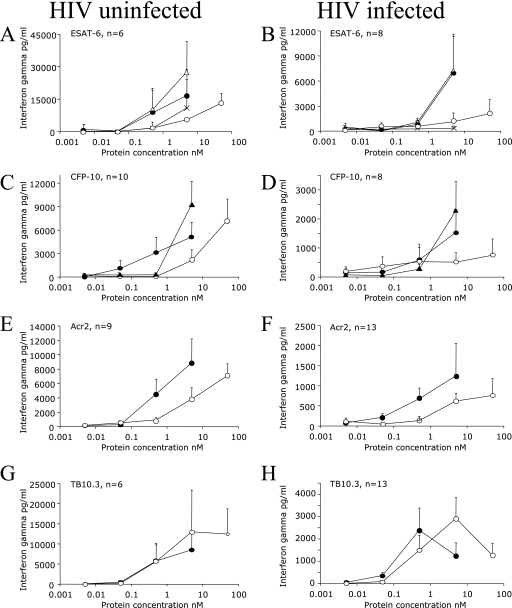
A range of concentrations of 0.005 to 50 nM, representing the same molar amounts of native (recombinant) TB antigens and CyaA-TB antigen fusions, were tested in HIV+ and HIV− persons in a series of dose-response experiments. As not all of the persons responded to all of the antigens and the amount of blood available restricted the number of antigens we could test at any single time, the number of persons tested varied between antigenic preparations. HIV+ persons had lower IFN-γ responses overall compared to HIV− persons (Fig. 1). Delivery of ESAT-6, CFP-10, and Acr2 by CyaA reduced the amount of antigen required to obtain optimal recognition by approximately 10-fold in both HIV+ and HIV− persons (Fig. 1A to F). Interestingly, in the case of the TB10.3 antigen, CyaA presentation did not reduce the amount of antigen required for optimal recognition (Fig. 1G and H). On the basis of these results, we elected to use equivalent concentrations of CyaA-TB antigen fusions at 5 nM and the native TB antigens at a 50 nM final concentration in further experiments, except for the TB10.3 antigen, which was used at 5 nM for both native and CyaA fusion experiments.
FIG. 1.
Dose-response curves of HIV-uninfected and HIV-infected persons. (A and B) Responses of six HIV-uninfected and eight HIV-infected persons to CyaA-ESAT-6 (▵), CyaA alone (×), [CyaA-ESAT-6]-CyaA (•), and ESAT-6 (○). Panels C to H represent corrected results, where the response to mock CyaA-E5 is already subtracted (see Materials and Methods). Open circles represent the native TB antigen, black filled circles represent CyaA carrying the antigen inserted at position 336, and black triangles (panels C and D) represent CyaA carrying the antigen inserted at position 224. Data are means ± standards errors.
Assessment of CyaA-TB antigen fusions in HIV+ and HIV− persons.
We next assessed the whole-blood responses of 41 HIV+ and 42 HIV− persons. The median (interquartile range [IQR]) PHA responses of HIV+ and HIV− persons were 1,741 (337 to 6,475) pg/ml and 4,976 (0 to 18,914) pg/ml, respectively (P = 0.5). The median (IQR) background responses of HIV+ and HIV− persons to mock CyaA-E5 were 295 (0 to 1,149) pg/ml and 201 (0 to 1,265) pg/ml, respectively (P = 0.31). Although the median IFN-γ responses to both native TB antigens and CyaA-TB antigen fusions were greater in HIV− persons, the differences were not statistically significant, except in the case of CyaA-ESAT-6 (Table 2). On the basis of our criteria (Table 1), the number of assays scored positive, negative, or indeterminate with the CyaA-TB antigen fusions (5 nM) was similar to that with the native TB antigens (50 nM) for both HIV+ and HIV− persons, confirming the overall equivalence between antigens at these concentrations (Table 3).
TABLE 2.
IFN-γ results for HIV-uninfected, HIV-infected, and HIV+PTB patients
| Patient group and antigen | IFN-γ (pg/ml)a | P valueb |
|---|---|---|
| HIV− | ||
| ESAT-6 | 1,510 (0-3,642) | |
| CyaA-ESAT-6 | 1,388 (176-5,634) | |
| CFP-10 | 1,862 (263-5,478) | |
| CyaA/224-CFP-10 | 1,408 (0-7,156) | |
| CyaA/336-CFP-10 | 355 (0-2,450) | |
| Acr2 | 460 (0-1,672) | |
| CyaA-Acr2 | 867 (132-3,060) | |
| TB10.3 | 320 (15-4,011) | |
| CyaA-TB10.3 | 898 (47-5,450) | |
| HIV+ | ||
| ESAT-6 | 894 (0-2,685) | 0.34 |
| CyaA-ESAT-6 | 111 (0-1,890) | 0.02 |
| CFP-10 | 935 (0-2,784) | 0.13 |
| CyaA/224-CFP-10 | 463 (0-2,800) | 0.08 |
| CyaA/336-CFP-10 | 85 (0-769) | 0.06 |
| Acr2 | 260 (0-644) | 0.41 |
| CyaA-Acr2 | 200 (0-1,316) | 0.11 |
| TB10.3 | 360 (31-1,015) | 0.56 |
| CyaA-TB10.3 | 134 (0-1,692) | 0.06 |
| HIV+PTB | ||
| ESAT-6 | 377 (0-1,276) | 0.39 |
| CyaA-ESAT-6 | 669 (14-1,790) | 0.40 |
| CFP-10 | 488 (20-1,599) | 0.55 |
| CyaA/224-CFP-10 | 709 (0-1,618) | 0.87 |
| CyaA/336-CFP-10 | 174 (0-1,590) | 0.24 |
| Acr2 | 469 (0-1,296) | 0.38 |
| CyaA-Acr2 | 56 (0-441) | 0.57 |
| TB10.3 | 599 (0-1,714) | 0.71 |
| CyaA-TB10.3 | 510 (12-2,869) | 0.58 |
Values are medians. Values in parentheses are IQRs.
P values were obtained by comparisons between HIV-uninfected and HIV-infected patients and between HIV-infected and HIV+PTB patients with the Mann-Whitney U test.
TABLE 3.
Whole-blood IFNGRA results for HIV-infected and HIV-uninfected persons
| Patient group | Antigen (no. of serum samples tested) | IFNGRA result | No (%) of serum samples tested with:
|
P value | |
|---|---|---|---|---|---|
| Antigen with CyaAb | Antigen alonea | ||||
| HIV infected | ESAT-6 (41) | Positive | 18 (44) | 25 (61) | 0.18 |
| Negative | 14 (34) | 10 (24) | 0.47 | ||
| Indeterminate | 9 (22) | 6 (15) | 0.57 | ||
| CFP-10c (41) | Positive | 22 (54) | 26 (63) | 0.5 | |
| Negative | 9 (22) | 8 (20) | 1.0 | ||
| Indeterminate | 10 (24) | 7 (17) | 0.59 | ||
| Acr2 (24) | Positive | 13 (54) | 14 (58) | 1.0 | |
| Negative | 7 (29) | 5 (21) | 0.74 | ||
| Indeterminate | 4 (17) | 5 (21) | 1.0 | ||
| TB10.3 (24) | Positive | 14 (58) | 17 (71) | 0.55 | |
| Negative | 9 (38) | 4 (17) | 0.19 | ||
| Indeterminate | 1 (4) | 3 (12) | 0.61 | ||
| HIV uninfected | ESAT-6 (42) | Positive | 30 (72) | 28 (67) | 0.81 |
| Negative | 6 (14) | 8 (19) | 0.77 | ||
| Indeterminate | 6 (14) | 6 (14) | 1.0 | ||
| CFP-10 (42) | Positive | 28 (67) | 34 (81) | 0.21 | |
| Negative | 9 (21) | 5 (12) | 0.38 | ||
| Indeterminate | 5 (12) | 3 (7) | 0.71 | ||
| Acr2 (35) | Positive | 25 (71) | 20 (57) | 0.32 | |
| Negative | 6 (17) | 10 (29) | 0.4 | ||
| Indeterminate | 4 (12) | 5 (14) | 1.0 | ||
| TB10.3 (35) | Positive | 25 (72) | 23 (66) | 0.80 | |
| Negative | 5 (14) | 6 (17) | 1.0 | ||
| Indeterminate | 5 (14) | 6 (17) | 1.0 | ||
The concentrations of antigens used alone were as follows: ESAT-6, 50 nM; CFP-10, 50 nM; Acr2, 50 nM; TB10.3, 5 nM.
The antigens paired with CyaA are termed CyaA-ESAT-6, CyaA-CFP-10, CyaA-Acr2, and CyaA-TB10.3. Each was used at a concentration of 5 nM.
CFP-10 refers to CyaA/224-CFP-10.
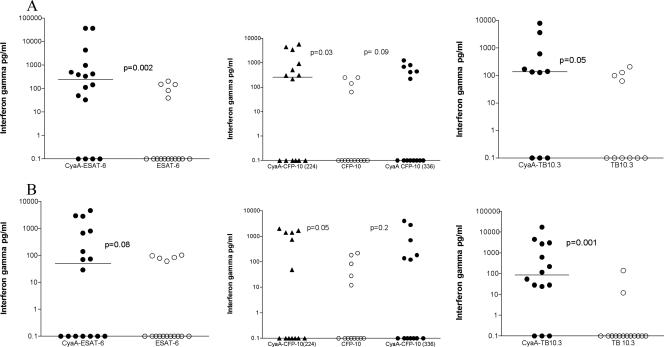
Assessment of CyaA-TB antigen fusions in HIV+ persons with a low response to native antigen.
A previous finding was enhanced in vitro responses to TB antigens delivered by CyaA in HIV− persons with a low response to native TB antigens (29). We therefore stratified our results to assess the responses of low-responding HIV+ persons in detail. We classified the responses to the native TB antigens in the IFNGRA into low (<250 pg/ml), intermediate (250 to 1,000 pg/ml), and high (>1,000 pg/ml). The response to ESAT-6 was significantly enhanced in HIV+ low responders when the antigen was delivered by CyaA (Fig. 2A). Interestingly, six HIV+ persons with no response to ESAT-6 became responders when their blood was stimulated with CyaA-ESAT-6, while four individuals had no response to either ESAT-6 or CyaA-ESAT-6. Similarly, the median response to CFP-10 increased when whole blood was stimulated with CyaA-CFP-10 (Fig. 2A). In this group, seven HIV+ persons with no initial response to CFP-10 responded to at least one CyaA-CFP-10 preparation (CyaA/224-CFP-10 or CyaA/336-CFP-10), and only three CFP-10 nonresponders remained unresponsive to both CyaA-CFP-10 TB antigen fusions. By contrast, delivery of Acr2 and TB10.3 by CyaA did not significantly increase the responses of low responders (Fig. 2A and data not shown).
FIG. 2.
(A) Whole-blood IFN-γ responses of HIV-infected people with low responses (<250 pg/ml) to the native TB antigen, CyaA-ESAT-6 (n = 16), CyaA-CFP-10 (n = 14), and CyaA-TB10.3 (n = 10). (B) Whole-blood IFN-γ responses of HIV-infected patients with TB with low responses (<250 pg/ml) to the native TB antigen, CyaA-ESAT-6 (n = 16), CyaA-CFP-10 (n = 13), and CyaA-TB10.3 (n = 14). Horizontal bars represent median values.
Assessment of CyaA-TB antigen fusions in HIV+ patients with active TB and comparison with the TST.
To estimate the potential diagnostic sensitivity of the CyaA whole-blood IFNGRA, we next assessed the performance of the CyaA-TB antigen fusions in 36 HIV+ persons with active pulmonary TB. Although there was a trend for median IFN-γ responses to mock CyaA-E5 to be higher in the HIV+PTB group compared to those of the HIV+ group, the difference was not statistically significant (295 [0 to 1,149] versus 652 [170 to 1,870] pg/ml; P = 0.08). Within the HIV+PTB group, the median IFN-γ responses were higher for ESAT-6 and CFP-10 when these antigens were delivered by CyaA although no difference was statistically significant (Table 2). When the patients were classified as low responders according to their responses to the native TB antigens, delivery by CyaA led to a significant increase in responses to CyaA/224-CFP-10 and TB10.3 but not to ESAT-6 or Acr2, respectively (Fig. 2B and data not shown).
On the basis of our criteria for defining a positive response, the IFNGRA was more likely to be positive when the TB antigens were presented in the form of CyaA rather than as native TB antigens. Of 29 (81%) patients who returned for TST readings, a positive result (>5 mm) was recorded in 19 (65%). When the results of the IFNGRA (both native TB antigens and CyaA-TB antigen fusions) were combined, 25 (86%) HIV+PTB patients yielded positive test results. Twenty (69%) were positive for ESAT-6 and/or CFP-10, and 23 (79%) were positive for CyaA-ESAT-6 and/or CyaA-CFP-10. Therefore, within this group with relatively advanced immunosuppression (median CD4 level, 167 cells/μl), responses in the IFNGRA were fairly well maintained.
Comparison of IFNGRA results with those of the TST in HIV+ and HIV− persons.
TST results were available for 102 (86%) of the 119 patients. The TST was positive for 20 (55%) HIV+ persons and 25 (68%) HIV− persons (Table 4). In the HIV+ group, 25 (69%) persons responded to native TB antigens in the IFNGRA, with 20 (56%) responding to the CyaA-TB antigen fusions (Table 4). However, when the results of the two assays were combined, 31 (86%) individuals scored a positive test result, significantly more than that for the TST (P = 0.008). In the HIV− group, a similar proportion responded to the native and CyaA-TB antigen fusions. When the results of the two assays were combined, 37 (100%) HIV− persons were scored positive, compared to 25 by the TST (P = 0.0002).
TABLE 4.
Numbers of positive IFNGRA results for HIV-infected and HIV-uninfected persons with presumed LTBI
| Antigen(s) | No. (%) of positive results
|
P value | |
|---|---|---|---|
| HIV+ (total n = 36)a | HIV− (total n = 37)a | ||
| ESAT-6 and/or CFP-10 | 25 (69) | 31 (84) | 0.17 |
| CyaA-ESAT-6 and/or CyaA-CFP-10 | 20 (56) | 30 (81) | 0.02 |
| Any antigens (native and with CyaA) | 31 (86) | 37 (100) | 0.02 |
| Tuberculin (by TST) | 20 (55) | 25 (68) | 0.34 |
Patients returning for TST reading.
Magnitudes of IFN-γ responses and relationships to absolute CD4 counts.
The magnitudes of IFN-γ responses were assessed in relation to absolute CD4 counts. CD4 counts were available for 62 (82%) HIV+ persons (31 HIV+, 31 HIV+PTB). The median (IQR) CD4 counts in the HIV+ and HIV+PTB groups were 462 (287 to 628) and 167 (83 to 403) cells/μl, respectively (P = 0.0006). Although the median CD4 count was lower in the HIV+PTB group than in the HIV+ group, there was no statistically significant difference between the median PHA IFN-γ responses of the groups (592 [0 to 4,680] versus 1,741 [337 to 6,475] pg/ml; P = 0.09). A significantly higher proportion of patients in the HIV+PTB group had a CD4 count of less than 200 cells/μl (17/31 versus 6/31; P = 0.008). The median PHA responses of HIV+ persons with absolute CD4 counts of less than 200 cells/μl were similar to those of persons with absolute CD4 counts of greater than 200 cells/μl (1,366 [0 to 5,142] versus 1,022 [167 to 6,990] pg/ml; P = 0.553). Within the HIV+ and HIV+PTB groups, we found no relationship between the CD4 count and the response to ESAT-6, CFP-10, or the recombinant CyaA-TB antigen fusions.
DISCUSSION
The immunodiagnosis of TB has received considerable attention with the discovery of immunodominant antigens encoded by the RD1 region of M. tuberculosis. Currently available assays incorporating these antigens are considered an improvement over the TST for the diagnosis of LTBI and active TB disease in adults (2, 23). Indeed, recent recommendations advocate the use of an IFNGRA as a replacement test for the TST in all situations where the TST is currently used (contact tracing, immigrant screening, etc.) (21). Currently, however, there are limited data on the utility of these assays in HIV+ persons. In one of the earlier studies, Chapman et al. reported a relatively high sensitivity (90%) of an enzyme-linked immunospot assay for the detection of active TB in HIV-infected Zambian patients (7). In a study of 29 HIV-infected adults, Dheda et al. found an enzyme-linked immunospot assay to be relatively unimpaired by HIV infection with the magnitude of IFN-γ responses independent of the CD4 counts (13). As part of a larger cohort, Liebeschuetz et al. reported a sensitivity of 70% for the detection of active TB disease in HIV+ children residing in an area where TB is endemic (18). In a low-incidence setting, Brock et al. reported promising findings obtained with a whole-blood IFNGRA for the detection of LTBI in HIV+ persons but found a higher rate of indeterminate results in those with advanced immunosuppression (6). Finally, a recent study comparing two forms of an IFNGRA (T-Spot.TB and QuantiFERONTB Gold) with the TST for the detection of LTBI in HIV+ and HIV− adults found the sensitivity of an IFNGRA to be relatively unimpaired by moderately advanced HIV infection (22).
In a previous study, we showed that CyaA carrying ESAT-6 and CFP-10 was able to restimulate T cells from more than 90% of HIV-negative patients with active TB and healthy sensitized donors and that CyaA delivery decreased the molar amount of antigen required to restimulate T cells (29). In the present study, we found that HIV+ persons had a lower magnitude of IFN-γ responses overall in response to test antigens. However, the ex vivo responses to TB antigens were maintained in the context of HIV infection, and CyaA delivery decreased by approximately 10-fold the amount of ESAT-6, CFP-10, and Acr2 required to restimulate T cells. Furthermore, the responses of low-responding HIV-infected people with presumed LTBI were enhanced when ESAT-6 and CFP-10 were delivered by CyaA. Thus, our confirmatory findings in this study including both HIV+ and HIV− persons is significant for two reasons; they show that (i) these results are reproducible in a different ethnic group on a different continent and (ii) the ex vivo stimulation of M. tuberculosis-specific T cells by CyaA-TB antigen fusions is maintained in the context of HIV infection.
To investigate the diagnostic potential of the CyaA vector, we assessed its performance in HIV-positive persons with active pulmonary TB. With predefined cutoff criteria that relate closely to commercial assays, we found that 79% of our patients tested positive in response to TB antigens delivered by CyaA, with this value increasing to 86% when the responses to both CyaA-TB and native TB antigens were combined. Although we expected the magnitudes of the responses in the whole-blood assays to relate to CD4 counts, we found no such correlation, in agreement with the findings by Dheda et al. (13). Importantly, the sensitivity of this IFNGRA for the detection of active TB disease compares favorably with data from other available studies with different IFNGRAs in HIV+ persons (7, 18).
Applying similar criteria to the group of asymptomatic HIV+ and HIV− persons, we found a very high rate of positive assay results, with 56% of HIV+ and 81% of HIV− persons responding in the IFNGRA incorporating CyaA as a delivery vector for TB antigens. Again, combining the responses to both native antigens and CyaA-TB antigen fusions increased the proportion of individuals testing positive in both of these groups. Thus, our findings suggest that a significant proportion of this population is infected with TB, which is of significant public health importance.
A significant drawback of the commercial IFNGRA, particularly for use in children, is the volume of blood required (up to 4 ml). Being a whole-blood assay, the CyaA-TB antigen fusion assay offers the advantage of requiring minute volumes of blood (<0.5 ml), as well as being operationally less complex. A recent evaluation of a commercial assay for the diagnosis of LTBI in South African school children reported a high rate of inadequate phlebotomy in 5- to 15-year-old children (27). Further studies are required to evaluate the usefulness of this whole-blood assay system in young children.
A possible limitation, however, of the CyaA-TB antigen fusion assays relates to the background CyaA-E5 response. As the CyaA toxoid derives from genetically detoxified Bordetella adenylate cyclase, it is unknown whether prior vaccination will lead to high background responses and limit the potential usefulness of the assay in young children. This potential problem may, however, be less important with modern acellular pertussis vaccines that do not contain CyaA. In the present study, there was no difference in responses to mock CyaA-E5 between HIV+, HIV+PTB, or HIV− adults. However, when the response to mock CyaA-E5 was high, a significant number of assays yielded indeterminate results.
The CyaA-TB antigen fusion low blood volume IFNGRA represents a novel development for the immunodiagnosis of TB. We have shown that this assay has the potential to identify HIV+ individuals with active TB disease and LTBI. Further work will characterize the nature and effect of the mock CyaA-E5 responses and assess whether these assays hold potential for the diagnosis of active TB disease and LTBI in children.
Acknowledgments
This work was supported by the Wellcome Trust of Great Britain (072070) and facilitated by a donation toward clinical facilities by the Rotary Club of Bad Oldesloe, Germany. T.G.C. is supported by a fellowship from the European Society of Pediatric Infectious Diseases. Z.M. is supported by Czech Ministry of Education Youth and Sports grant 2B06161.
The European Society of Pediatric Infectious Diseases and the Czech Ministry of Education Youth and Sports had no role in the decision to publish this work or the preparation of the manuscript.
We are grateful to Virginia Azevedo and Helene Visser of the Health Department of the City of Cape Town and to Eric Goemaere of Médecins Sans Frontières for constructive discussions prior to commencing the study. We thank Priscilla Mouton for help with recruitment and obtaining blood samples.
R.J.W., C.L., and P.S. are joint signatories to a patent pending on the diagnostic application of the CyaA-TB proteins. None of the rest of us have a competing interest (financial or otherwise) in the publication of this report.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Anastos, K., L. A. Kalish, H. Palacio, C. A. Benson, R. Delapenha, K. Chirgwin, L. Stonis, and E. E. Telzak. 1999. Prevalence of and risk factors for tuberculin positivity and skin test anergy in HIV-1-infected and uninfected at-risk women. Women's Interagency HIV Study (WIHS). J. Acquir. Immune Defic. Syndr. 21141-147. [PubMed] [Google Scholar]
- 2.Andersen, P., M. E. Munk, J. M. Pollack, and M. T. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. The Lancet. 3561099-1104. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, S. T., A. J. Williams, J. R. Brown, S. M. Newton, M. Simsova, M. P. Nicol, P. Sebo, M. Levin, R. J. Wilkinson, and K. A. Wilkinson. 2006. Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing. Am. J. Respir. Crit. Care Med. 1731038-1042. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 2006. The global plan to stop TB, 2006-2015: summary. Wkly. Epidemiol. Rec. 8186-88. [PubMed] [Google Scholar]
- 5.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144(Pt. 11)3195-3203. [DOI] [PubMed] [Google Scholar]
- 6.Brock, I., M. Ruhwald, B. Lundgren, H. Westh, L. R. Mathiesen, and P. Ravn. 2006. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir. Res. 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman, A. L., M. Munkanta, K. A. Wilkinson, A. A. Pathan, K. Ewer, H. Ayles, W. H. Reece, A. Mwinga, P. Godfrey-Faussett, and A. Lalvani. 2002. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 162285-2293. [DOI] [PubMed] [Google Scholar]
- 8.Coetzee, D., K. Hildebrand, A. Boulle, G. Maartens, F. Louis, V. Labatala, H. Reuter, N. Ntwana, and E. Goemaere. 2004. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 18887-895. [DOI] [PubMed] [Google Scholar]
- 9.Coetzee, D., K. Hilderbrand, E. Goemaere, F. Matthys, and M. Boelaert. 2004. Integrating tuberculosis and HIV care in the primary care setting in South Africa. Trop. Med. Int. Health 9A11-A15. [DOI] [PubMed] [Google Scholar]
- 10.Connell, T. G., M. X. Rangaka, N. Curtis, and R. J. Wilkinson. 2006. QuantiFERON-TB Gold: state of the art for the diagnosis of tuberculosis infection? Expert Rev. Mol. Diagn. 6663-677. [DOI] [PubMed] [Google Scholar]
- 11.Converse, P. J., S. L. Jones, J. Astemborski, D. Vlahov, and N. M. Graham. 1997. Comparison of a tuberculin interferon-gamma assay with the tuberculin skin test in high-risk adults: effect of human immunodeficiency virus infection. J. Infect. Dis. 176144-150. [DOI] [PubMed] [Google Scholar]
- 12.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 1631009-1021. [DOI] [PubMed] [Google Scholar]
- 13.Dheda, K., A. Lalvani, R. F. Miller, G. Scott, H. Booth, M. A. Johnson, A. Zumla, and G. A. Rook. 2005. Performance of a T-cell-based diagnostic test for tuberculosis infection in HIV-infected individuals is independent of CD4 cell count. Aids 192038-2041. [DOI] [PubMed] [Google Scholar]
- 14.Guermonprez, P., N. Khelef, E. Blouin, P. Rieu, P. Ricciardi-Castagnoli, N. Guiso, D. Ladant, and C. Leclerc. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J. Exp. Med. 1931035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guermonprez, P., D. Ladant, G. Karimova, A. Ullmann, and C. Leclerc. 1999. Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway. J. Immunol. 1621910-1916. [PubMed] [Google Scholar]
- 16.Hogarth, P. J., K. E. Logan, H. M. Vordermeier, M. Singh, R. G. Hewinson, and M. A. Chambers. 2005. Protective immunity against Mycobacterium bovis induced by vaccination with Rv3109c—a member of the esat-6 gene family. Vaccine 232557-2564. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. L., S. Nyole, A. Okwera, C. C. Whalen, P. Nsubuga, V. Pekovic, R. Huebner, R. S. Wallis, P. N. Mugyenyi, R. D. Mugerwa, and J. J. Ellner. 1998. Instability of tuberculin and Candida skin test reactivity in HIV-infected Ugandans. The Uganda-Case Western Reserve University Research Collaboration. Am. J. Respir. Crit. Care Med. 1581790-1796. [DOI] [PubMed] [Google Scholar]
- 18.Liebeschuetz, S., S. Bamber, K. Ewer, J. Deeks, A. A. Pathan, and A. Lalvani. 2004. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet 3642196-2203. [DOI] [PubMed] [Google Scholar]
- 19.Loucká, J., G. Schlecht, J. Vodolánová, C. Leclerc, and P. Šebo. 2002. Delivery of a MalE CD4+-T-cell epitope into the major histocompatibility complex class II antigen presentation pathway by Bordetella pertussis adenylate cyclase. Infect. Immun. 701002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1781274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazurek, G. H., J. Jereb, P. Lobue, M. F. Iademarco, B. Metchock, and A. Vernon. 2005. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. Morb. Mortal. Wkly. Rep. Recommend. Rep. 5449-55. [PubMed] [Google Scholar]
- 22.Rangaka, M. X., K. A. Wilkinson, R. Seldon, G. van Cutsem, G. A. Meintjes, C. Morroni, P. Mouton, L. Diwakar, T. G. Connell, G. Maartens, and R. J. Wilkinson. 2007. Effect of HIV-1 Infection on T-cell-based and skin test detection of tuberculosis infection. Am. J. Respir. Crit. Care Med. 175514-520. [DOI] [PubMed] [Google Scholar]
- 23.Richeldi, L. 2006. An update on the diagnosis of tuberculosis infection. Am. J. Respir. Crit. Care Med. 174736-742. [DOI] [PubMed] [Google Scholar]
- 24.Saron, M. F., C. Fayolle, P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1997. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA 943314-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlecht, G., J. Loucka, H. Najar, P. Sebo, and C. Leclerc. 2004. Antigen targeting to CD11b allows efficient presentation of CD4+ and CD8+ T cell epitopes and in vivo Th1-polarized T cell priming. J. Immunol. 1736089-6097. [DOI] [PubMed] [Google Scholar]
- 26.Skjøt, R. L., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. M. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 705446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsiouris, S. J., J. Austin, P. Toro, D. Coetzee, K. Weyer, Z. Stein, and W. M. El-Sadr. 2006. Results of a tuberculosis-specific IFN-γ assay in children at high risk for tuberculosis infection. Int. J. Tuberc. Lung Dis. 10939-941. [PubMed] [Google Scholar]
- 28.Vordermeier, H. M., M. Simsova, K. A. Wilkinson, R. J. Wilkinson, R. G. Hewinson, P. Sebo, and C. Leclerc. 2004. Recognition of mycobacterial antigens delivered by genetically detoxified Bordetella pertussis adenylate cyclase by T cells from cattle with bovine tuberculosis. Infect. Immun. 726255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson, K. A., M. Simsova, E. Scholvinck, P. Sebo, C. Leclerc, H. M. Vordermeier, S. J. Dickson, J. R. Brown, R. N. Davidson, G. Pasvol, M. Levin, and R. J. Wilkinson. 2005. Efficient ex vivo stimulation of Mycobacterium tuberculosis-specific T cells by genetically detoxified Bordetella pertussis adenylate cyclase antigen toxoids. Infect. Immun. 732991-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson, K. A., G. R. Stewart, S. M. Newton, H. M. Vordermeier, J. R. Wain, H. N. Murphy, K. Horner, D. B. Young, and R. J. Wilkinson. 2005. Infection biology of a novel alpha-crystallin of Mycobacterium tuberculosis: Acr2. J. Immunol. 1744237-4243. [DOI] [PubMed] [Google Scholar]