Abstract
Rhodococcus equi is a facultative intracellular pathogen that causes pneumonia in young foals but does not induce disease in immunocompetent adult horses. Clearance of R. equi depends mainly on gamma interferon (IFN-γ) production by T lymphocytes, whereas the predominance of interleukin 4 (IL-4) is detrimental. Young foals, like neonates of many other species, are generally deficient in the ability to produce IFN-γ. The objective of this study was to compare the cytokine profiles, as well as cell-mediated and antibody responses, of young foals to those of adult horses following intrabronchial challenge with R. equi. The lymphoproliferative responses of bronchial lymph node (BLN) cells to concanavalin A were significantly higher in foals than in adult horses. In contrast, adult horses had significantly higher lymphoproliferative responses to R. equi antigens than did foals. Infected foals had significantly lower IL-4 mRNA expression but significantly higher IFN-γ expression and IFN-γ/IL-4 ratio in R. equi-stimulated BLN lymphocytes than did infected adults. Infection with R. equi in foals resulted in a significant increase in the percentage of T lymphocytes and CD4+ T lymphocytes in bronchoalveolar lavage fluid in association with a significant decrease in the percentage of these cell populations in BLNs. Infection of foals also resulted in a marked increase in serum immunoglobulin Ga (IgGa) and IgGb levels, resulting in concentrations in serum that were significantly higher than those of adult horses. This study demonstrates that the immune response to R. equi in foals is not biased toward IL-4 and is characterized by the predominant induction of IFN-γ.
Rhodococcus equi, a gram-positive facultative intracellular pathogen, is one of the most important causes of pneumonia in foals aged between 3 weeks and 5 months. R. equi has also emerged as a significant opportunistic pathogen in immunosuppressed people, especially those infected with the human immunodeficiency virus (5, 12, 21). As opposed to foals, adult horses are typically resistant to R. equi infections. R. equi, a soil saprophyte, is widespread in the environment (40, 41). Unlike environmental R. equi, isolates from pneumonic foals typically contain an 80- to 90-kb plasmid encoding a family of seven closely related virulence-associated proteins, designated VapA and VapC to VapH (42). Plasmid-cured derivatives of virulent R. equi strains lose the ability to replicate and survive in macrophages (18). Plasmid-cured derivatives also fail to induce pneumonia and are completely cleared from the lungs of foals, confirming the absolute necessity of the large plasmid for the virulence of R. equi (18, 45).
Study of the pathogenesis of R. equi infection has been complicated by the fact that typical granulomatous lung lesions have not been reproduced by R. equi infection in any immunocompetent species other than young horses. The normal murine lung can progressively clear an inoculum of R. equi sufficient to induce severe pneumonia in foals, suggesting that the results of studies of the pathogenesis of this infection in mice may not necessarily be extrapolated to foals. Nevertheless, most of what is known of immunity to R. equi comes from experiments with mouse models. Adoptive transfer of R. equi-specific CD4+ T-lymphocyte lines to R. equi-susceptible nude mice demonstrated that a Th1 response is sufficient to achieve pulmonary clearance, whereas a Th2 response is detrimental (30). In addition, blockage of gamma interferon (IFN-γ) enhances disease in normally resistant immunocompetent mice (29).
Immunity to R. equi in horses likely depends on both humoral and cell-mediated immune responses. Antibodies to the Vap proteins do not provide complete protection but have been shown to enhance the pulmonary clearance of R. equi following heavy intrabronchial challenge in foals (24). Clearance of R. equi in adult horses is associated with a significant increase in bronchoalveolar lavage (BAL) fluid CD4+ and CD8+ lymphocytes, lymphoproliferative responses to R. equi antigens, development of R. equi-specific cytotoxic T lymphocytes, and IFN-γ induction (22, 23, 31, 36). The concentrations of R. equi-specific immunoglobulin Ga (IgGa) and IgGb are also dramatically enhanced in conjunction with pulmonary clearance in adult horses (31).
How these findings with mice and adult horses relate to the foal remains to be determined. Analogy to human immunodeficiency virus-related R. equi pneumonia suggests either that foals are immunocompromised in some way or that infection with virulent R. equi alters the immune responses in foals. The recognized Th2 bias in the immune responses of neonates from many species (1), along with the recent finding that young foals are deficient in the ability to produce IFN-γ in response to mitogens, has led to the hypothesis that an IFN-γ deficiency may be at the basis of their peculiar susceptibility to R. equi infections (8). However, the facts that oral administration of live virulent R. equi to newborn foals confers complete protection against subsequent heavy intrabronchial challenge (10, 26) and that most foals on farms where the disease is endemic do not develop disease or develop subclinical disease and eventually clear the infection suggest that most foals have the ability to mount protective immune responses to R. equi.
As a basis for the present study, we hypothesized that, although foals have a naïve immune system, infection with R. equi results in adult-like IFN-γ induction. To address this hypothesis, R. equi-susceptible foals and resistant adult horses were infected intrabronchially with a low inoculum of virulent R. equi. Lymphoproliferative responses, cytokine mRNA expression, lymphocyte subsets in BAL fluid and bronchial lymph node (BLN) cell populations, and Ig concentrations were measured and compared between groups.
MATERIALS AND METHODS
Preparation of R. equi for challenge.
R. equi ATCC 33701, a virulent strain containing an 80-kb virulence plasmid, was used to infect foals (42). Bacteria were kept as frozen stabilates. Aliquots of R. equi were grown on Trypticase soy agar (TSA) plates for 48 h at 37°C. Bacteria were harvested with 4 ml of sterile phosphate-buffered saline (PBS) per plate. The bacterial concentration was determined by counting CFU.
Animals, intrabronchial challenge, and study design.
Ten foals between 7 and 10 days of age and 10 adult horses between 3 and 12 years of age were used in this study. Adequate transfer of passive immunity was confirmed in foals at 12 to 24 h of age by measurement of plasma IgG concentrations with a commercial immunoassay (DVM Stat; Corporation for Advanced Applications, Newburg, WI). Foals, together with their dams, were moved to individual stalls in an isolation facility on the day after birth. Adult horses were moved to the isolation facility at least 2 days prior to the beginning of the study. Prior to initiation of the study, all animals were determined to be healthy on the basis of a thorough physical examination, complete blood count, biochemical profile, cytology and bacterial culture of a tracheobronchial aspirate, and thoracic radiographs.
Prior to infection, animals were sedated with 0.5 mg/kg of xylazine hydrochloride and 0.05 mg/kg of butorphanol tartrate, given intravenously. Five foals and five adult horses were infected intrabronchially with an inoculum of 2 × 104 CFU of R. equi per kg of body weight diluted in 50 ml of PBS. This corresponded to a total inoculum of approximately 1 × 106 CFU for each foal and 1 × 107 CFU for each adult horse. Five foals and five adults were used as controls and were given only PBS intrabronchially. A flexible fiber-optic endoscope was used to deliver 25 ml of the bacterial suspension or PBS into each main bronchus.
The day of infection was designated as day 0. Baseline values for heart rate, respiratory rate, temperature, white blood cell count, and fibrinogen concentration were obtained on day 0 prior to sedation. Serum was also collected for measurement of baseline Ig concentration. Animals were clinically assessed throughout the study based on daily complete physical examinations as well as twice daily heart rate, respiratory rate, and temperature recording. Serum and whole-blood samples for white blood cell counts and measurement of fibrinogen concentrations were collected again on day 15 postinfection, and BAL was performed. Euthanasia was performed immediately following collection of BAL fluid by intravenous administration of a lethal dose of pentobarbital sodium.
BLNs were collected aseptically and placed in sterile PBS for transport to the laboratory. All organs were examined macroscopically, and representative samples of normal and diseased lungs, as well as bronchial lymph nodes, were fixed in 10% buffered formol saline. The fixed tissues were embedded in paraffin, sectioned at 10 μm, stained with hematoxylin and eosin, and examined histologically. The lymph node samples were graded 0 to 3 based on the severity of lymphoid hyperplasia and sinus histiocytosis. The pathologist was unaware of the source of the tissue sample. The numbers of viable R. equi cells in four dispersed and preselected loci of both lungs were enumerated by culturing serial dilutions of lung homogenates on TSA plates and counting the CFU. The eight sites were the craniodorsal, cranioventral, middle, and caudodorsal parts of each lung.
Collection of BAL fluid.
BAL fluid was collected on day 15 postinfection. Animals were sedated with 0.5 mg/kg of xylazine hydrochloride and 0.07 mg/kg of butorphanol tartrate, given intravenously. A 10-mm-diameter and 1.8-m-long bronchoscope was passed via nasal approach into either the left or right lung until wedged in a fourth- to sixth-generation bronchus. The lavage solution consisted of four aliquots of 50 ml of physiologic saline (0.9% NaCl) solution infused and aspirated immediately. The total nucleated cell count in BAL fluid was determined by use of a hemacytometer. Slides of the BAL fluid were prepared by cytocentrifugation, and air-dried slides were stained with Wright-Giemsa stain. A differential count was made by examining 200 cells. BAL fluid was centrifuged at 200 × g for 10 min. Bronchoalveolar cells were washed and resuspended in 1 ml of freezing medium containing 90% fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO). Cells were placed at −70°C for 4 h and then transferred to liquid nitrogen until being used for lymphocyte immunophenotyping by flow cytometry.
Preparation of R. equi antigen.
Antigen for use in proliferation assays was prepared as previously described (31). Briefly, R. equi ATCC 33701 was grown in brain heart infusion broth for 48 h at 37°C with agitation. The bacteria were harvested by centrifugation at 3,840 × g for 10 min and washed with sterile PBS. Two milliliters of the bacterial pellet was resuspended in 10 ml of PBS, and the bacteria were disrupted by three cycles of freezing at −20°C and thawing in a water bath at 37°C. The sample was centrifuged at 12,000 × g for 15 min at 4°C to separate the pellet of intact bacteria and debris. The resulting supernatant was further centrifuged at 25,000 × g for 20 min at 4°C to obtain the soluble antigens. The same protocol was used to obtain soluble antigen for Corynebacterium pseudotuberculosis for use as a negative control in the proliferation assays.
R. equi antigen for use in enzyme-linked immunosorbent assays (ELISA) was prepared by a similar protocol except that the bacteria were further disrupted by sonication with 5-s pulses for 10 min and passage through a French press at 16,000 lb/in2. Disrupted cells were centrifuged at 13,000 × g for 10 min, and the supernatant was used. The protein content of each resulting soluble antigen preparation was determined independently with the BCA protein assay kit (Pierce, Rockford IL). The preparations were aliquoted and frozen at −70°C until needed.
Preparation of BLN cells, cell stimulation, and proliferation assays.
Cells used for proliferation assays were collected from BLNs. Briefly, BLNs were cut into 125-mm3 pieces, and cell suspensions were prepared in glass tissue grinders. Mononuclear cells were harvested by density gradient centrifugation using endotoxin-free Ficoll-Paque (Amersham Biosciences, Pittsburgh, PA). Aliquots of 3 × 107 cells were placed in 1 ml of freezing medium containing 90% FCS and 10% DMSO. Cells were placed at −70°C for 4 h and then transferred to liquid nitrogen until being assayed.
Immediately after thawing, BLN cells were washed twice and placed in Dulbecco's modified Eagle's medium supplemented with 10% FCS, 2 mM glutamine, 25 mM HEPES, and penicillin-streptomycin (100 U and 100 μg per ml, respectively). More than 70% of the cells were viable after thawing, as assessed by trypan blue exclusion. Proliferative responses were assessed with a nonradioactive colorimetric assay. This assay has been shown to correlate closely with conventional radioactive [3H]thymidine incorporation in many species, including the horse (3, 49). Aliquots (100 μl) of cells (1 × 106 cells/ml) were placed in triplicate wells of 96-well black plates with flat, clear-bottom wells (Corning Inc., Corning, NY). Cells were separately incubated with no antigen (blank), 2 μg/ml of concanavalin A (ConA) (positive control), 10 μg/ml of C. pseudotuberculosis soluble antigens (negative control), or 10 μg/ml of soluble R. equi antigen. Optimal concentrations of antigens and mitogen were determined based on a dose-response curve with soluble R. equi antigen and ConA, respectively. The cells were stimulated at 37°C for 72 h in 6% CO2. Twelve hours before the end of the assay, 20 μl of Alamar blue (Accumed International Inc., Westlake, OH) was added to each well and fluorescence was determined with a fluorometer (Synergy HT; BioTek Instruments Inc., Winooski, VT) using an excitation wavelength of 530 nm. Emission was measured at 590 nm. The change in fluorescence was calculated as the mean of the stimulated cells minus the mean of the cells without antigen or mitogen (blank).
BLN cells used for quantification of mRNA expression were prepared exactly as described above with the exception that the cells were stimulated with the soluble R. equi antigen for 12 h. This time, selection was based on a time-response curve for IFN-γ and interleukin 4 (IL-4) mRNA expression.
RNA isolation from BLN cells, DNase treatment of RNA samples, and cDNA synthesis.
Isolation of total RNA from BLN cells was performed with the RNeasy kit (QIAGEN Inc., Valencia, CA) according to the manufacturer's instructions. The RNA concentration was measured by optical density at 260 nm (OD260). All RNA samples were treated with amplification-grade DNase I (Gibco BRL, Rockville, MD) to remove any trace of genomic DNA contamination. Briefly, 1 U of DNase I and 1 μl of 10× DNase I reaction buffer were mixed with 1 μg of total RNA for a total volume of 10 μl. The mixture was incubated for 10 min at room temperature and then inactivated by the addition of 1 μl of 25 mM of EDTA and heating at 65°C for 10 min.
cDNA was synthesized with the Advantage RT-for-PCR kit (Clontech, Palo Alto, CA) by using the protocol of the manufacturer. Briefly, 1 μg of DNase-treated total RNA was mixed with 1 μl of oligo(dT)18 primer (20 μM) and heated at 70°C for 2 min. After cooling to room temperature, the following reagents were added: 4 μl of 5× reaction buffer, 1 μl of deoxynucleoside triphosphates (10 mM each), 0.5 μl of RNase inhibitor, (40 U/μl) and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl). The mixture was incubated at 42°C for 1 h, heated at 94°C for 5 min, diluted to a final volume of 100 μl, and stored at −70°C until being used for PCR analysis.
Quantification of cytokine mRNA.
Gene-specific primers and internal oligonucleotide probes for equine G3PDH (glyceraldehyde-3-phosphate dehydrogenase), IL-2, IL-4, IL-10, and IFN-γ have been previously described (4, 16). The internal probes were labeled at the 5′ end with the reporter dye 6-carboxyfluorescein and at the 3′ end with the quencher dye 6-carboxytetramethyl-rhodamine. Amplification of 2 μl of cDNA was performed in a 25-μl PCR mixture containing 900 nM concentrations of each primer, 250 nM TaqMan probe, and 12 μl of TaqMan Universal PCR Mastermix (Applied Biosystems). Amplification and detection were performed with the ABI Prism 7700 Sequence Detection System (Applied Biosystems) with initial incubation steps at 50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Serial dilutions of cDNA from equine blood mononuclear cells stimulated for 24 h with ConA were used to generate a standard curve for relative quantification of each gene of interest. Each sample was assayed in triplicate, and the mean value was used for comparison. Samples without cDNA were included in the amplification reactions to determine background fluorescence and to check for contamination. To account for variations in the amount and quality of the starting material, all results were normalized to G3PDH expression.
Flow cytometry for lymphocyte immunophenotyping.
Immunophenotyping was performed on BAL and BLN mononuclear cells to determine lymphocyte subsets. All incubations and washes were performed at 4°C. After thawing, cells were washed twice in a PBS buffer solution containing 2% FCS and 0.1% sodium azide. Aliquots of 50 μl containing 5 × 105 cells were stained for 30 min with murine monoclonal antibodies binding to equine B lymphocytes (B29A; VMRD Inc., Pullman, WA), T lymphocytes (HB19A; VMRD Inc.), CD4+ T lymphocytes (HB61A; VMRD, Inc.), and CD8+ T lymphocytes (MCA2385; Serotec, Raleigh, NC). A monoclonal antibody of the same isotype but not reactive with equine cells was used as the negative control (MCA928; Serotec). After two washes in cold buffer solution, cells were incubated with rabbit F(ab′)2 anti-mouse IgG conjugated to fluorescein isothiocyanate (Serotec) for 30 min. Cells were washed and fixed in 1% paraformaldehyde. Analyses were performed with a FACSort flow cytometer equipped with Cell Quest software (BD Biosciences, Rockville MD). Data were collected from 10,000 events for each sample, with forward scatter and side scatter parameters used to gate the lymphocyte populations. Data were expressed as percentages of each subset within the gated population.
Determination of Ig concentrations.
R. equi-specific IgM, IgGa, IgGb, IgGc, and IgG(T) concentrations in serum collected on day 0 (preinfection) and again on day 15 postinfection were determined by ELISA. Optimal dilutions of reagents were obtained by checkerboard titrations. Wells in Immulon II 96-well microtiter plates (Thermo Fisher Scientific, Waltham, MA) were coated at 4°C overnight with 10 μg/ml of soluble R. equi antigen in carbonate-bicarbonate buffer (pH 9.6; total volume, 100 μl/well). Plates were washed four times with PBS-0.05% Tween 20 between each of the following incubations. Plates were blocked with PBS-1% bovine serum albumin for 1 h at room temperature. Serum from each experimental animal was diluted 1:100, and 100 μl was added to each well for 1 h of incubation at room temperature. To determine isotype-specific responses, 100 μl of peroxidase-conjugated goat anti-equine IgGa (1:5,000), IgGb (1:5,000), IgGc (1:1,000), IgG(T) (1:1,000), or IgM (1:2,500) (Serotec) was added to the wells for 1 h of incubation at room temperature. After the addition of substrate (ABTS; Roche Diagnostics, Indianapolis, IN), plates were incubated for 45 min in the dark at room temperature and the OD405 was measured. For each Ig subisotype measured, serum from a high responder was serially diluted to generate a standard curve for relative quantification of Ig concentrations in the experimental animals. The standard curve was run on each plate to correct for interplate variability. Wells incubated without serum were used as blanks to subtract out the background absorbance. Each sample was run in triplicate, and the mean OD was used.
Statistical analysis.
The normality of the data and equality of variances were assessed with the Kolmogorov-Smirnov and Levene tests, respectively. A one-way analysis of variance (ANOVA) was used to compare lymphoproliferative responses, BLN hyperplasia scores, cytokine mRNA expression, and percentages of lymphocyte subsets between experimental groups (control foals, infected foals, control adults, and infected adults). Data that did not meet the assumptions for parametric testing were log transformed. In rare instances when a normal distribution of the data was not achieved despite transformation, data were analyzed with the Kruskal-Wallis ANOVA on ranks. A two-way ANOVA for repeated measurements was used to determine the effects of time (pre- versus postinfection), the experimental group (control foals, infected foals, control adults, and infected adults), and the interaction between time and experimental group on antibody concentrations. Variables that did not meet the assumptions of the ANOVA were rank transformed prior to analysis. When appropriate, multiple pairwise comparisons were done with the Student-Newman-Keuls test. Pearson product moment correlations were used to determine the strength of the relationship between each IgG subisotype and IL-4 or IFN-γ. The z statistic was used to assess significant differences between 2 coefficients of correlation. For each test, significance was set at a P value of <0.05.
RESULTS
Disease process and pathological findings.
To compare the immune responses of foals (susceptible to R. equi infection) and adult horses (resistant to R. equi), five foals and five adult horses were infected intrabronchially with a low inoculum of R. equi. Five foals and five adult horses were not infected and were used as controls. Animals were euthanized for sample collection on day 15 postinfection. All animals maintained a normal temperature, heart rate, and respiratory rate and showed no clinical evidence of disease. White blood cell counts and fibrinogen concentrations also remained within normal limits throughout the study. All five infected foals had macroscopic pulmonary lesions. Approximately 5 to 15% of the lung tissue was firm and reddened, and there were multiple small nodular lesions up to 1 cm in diameter. The BLNs of infected foals were considerably larger than those of the other groups. All infected foals had histologic lesions of suppurative to pyogranulomatous bronchopneumonia. R. equi was cultured from the lung of each infected foal. The mean number of R. equi CFU (log10 ± standard deviation [SD]) in the lung tissue of infected foals was 5.77 ± 1.22 per g of lung tissue. The lungs of control animals and those of infected adult horses were free of lesions, and bacterial culture was negative. Lymphoid hyperplasia and sinus histiocytosis were present in the BLNs of multiple animals in each group. There was no significant difference in the BLN hyperplasia scores between groups.
R. equi-specific proliferative responses and cytokine profiles of BLN cells.
To determine whether differences in proliferative responses and cytokine profiles contribute to the susceptibility of foals to infection with R. equi, responses of BLN mononuclear cells from susceptible, infected foals were compared to those of R. equi-resistant adult horses. Uninfected foals and adult horses were used as controls in these experiments. Proliferative responses of BLN cells to ConA were significantly higher in both groups of foals than in both groups of adult horses (Fig. 1A). In contrast, infected and control adult horses had significantly higher proliferative responses to the soluble R. equi antigen than both control and infected foals (Fig. 1B). There was no proliferation in response to stimulation with C. pseudotuberculosis (negative control; data not shown).
FIG. 1.
Proliferative responses of BLN cells from five foals and five adult horses at 15 days after challenge with virulent R. equi. Five foals and five adult horses were used as uninfected controls. BLN cells were stimulated either with ConA (A) or with soluble R. equi antigens (B). The change in fluorescence was calculated as the mean fluorescence of the stimulated cells minus that of the same cells cultured without mitogen or antigen. The results are displayed as mean ± SD. Different letters between experimental groups (a, b) indicate a statistically significant difference (P < 0.05).
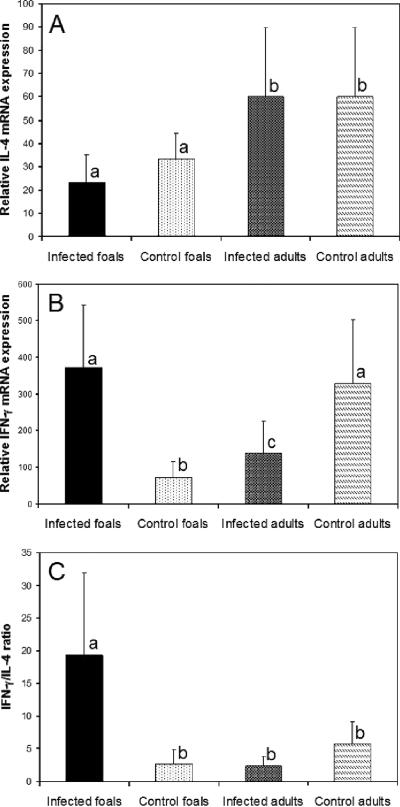
Infected and control foals had significantly lower IL-4 mRNA expression in response to in vitro stimulation of their BLN cells with R. equi antigen than both infected and control adults (Fig. 2A). Infected foals had had significantly higher IFN-γ mRNA expression than control foals or infected adults (Fig. 2B). Infected adult horses had significantly higher IFN-γ mRNA expression than control foals but lower IFN-γ expression than control adults (Fig. 2B). The IFN-γ/IL-4 ratio of infected foals was significantly higher than that of all three other groups (Fig. 2C). There was no significant difference in IL-2 or IL-10 mRNA expression between groups (data not shown).
FIG. 2.
Relative IL-4 (A) and IFN-γ (B) mRNA expression, as well as the IFN-γ/IL-4 ratio (C), following stimulation of BLN cells with soluble R. equi antigens. BLN cells were collected from five foals and five adult horses at 15 days after challenge with virulent R. equi. Five foals and five adult horses were used as uninfected controls. The results are displayed as mean ± SD. Different letters between experimental groups (a, b, c) indicate a statistically significant difference (P < 0.05).
BAL fluid cytology and lymphocyte subsets in BAL fluid and BLN lymphocytes.
Cytological examination of BAL fluid and immunophenotyping of BAL and BLN cells were performed to determine whether differences in BAL fluid composition exist between R. equi-infected foals and adult horses and to characterize the subsets of BLN cells used for the proliferation assays and cytokine induction experiments described above. The total nucleated cell counts in BAL fluid were not significantly different between groups. The percentage of neutrophils in the BAL fluid of infected foals (17.2% ± 12.3%) was significantly higher than that of all three other groups (mean ± SD, 4.7% ± 3.8%). The percentage of macrophages in the BAL fluid of control foals (84.4% ± 7.2%) was significantly higher than that of both groups of adult horses (54.9% ± 19.1%). The percentage of lymphocytes in the BAL fluid of control horses (37.8% ± 18.6%) was significantly higher than that of both groups of foals (10.7% ± 6.9%). There were no significant differences between groups for other cell types upon cytological examination.
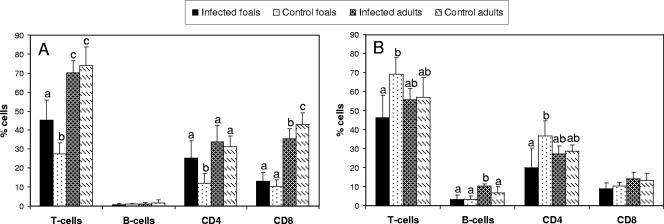
The BAL fluid contained a significantly higher percentage of T cells in adult horses than in foals (Fig. 3A). Adult horses and infected foals had a significantly higher percentage of CD4+ T cells in BAL fluid than did control foals (Fig. 3A). Adult horses also had a significantly higher percentage of CD8+ T cells in BAL fluid than did both groups of foals (Fig. 3A). The BLN cells contained a significantly higher percentage of T cells in control foals than in infected foals (Fig. 3B). Infected adult horses had a significantly higher percentage of B cells in their BLNs than did the other three experimental groups (Fig. 3B). Infected foals had a significantly lower percentage of CD4+ T cells in BLNs than did control foals (Fig. 3B).
FIG. 3.
Lymphocyte subpopulations in BAL fluid (A) and BLNs (B) from five foals and five adult horses at 15 days after challenge with virulent R. equi. Five foals and five adult horses were used as uninfected controls. The subpopulations are displayed as a percentage of the total number of gated cells (mean ± SD). Different letters between experimental groups (a, b, c) indicate a statistically significant difference (P < 0.05). The difference is not significant when the bars have at least one letter in common.
Antibody responses and correlations between IgG subisotypes and the cytokine profile.
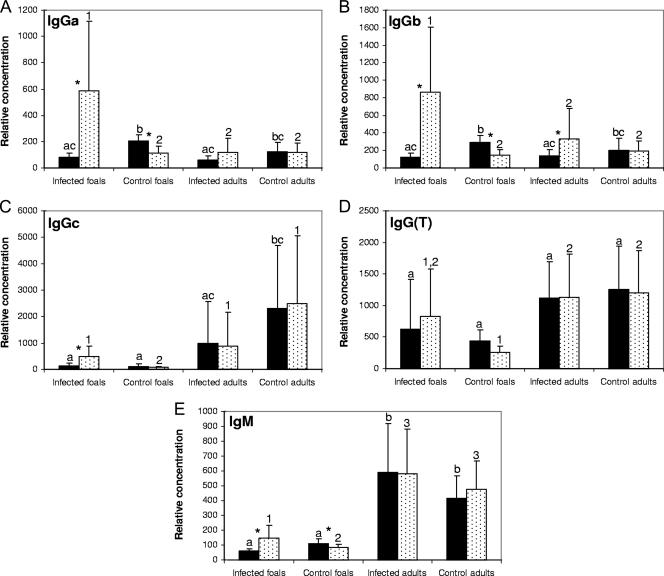
Because antibody has been shown to contribute to protection against R. equi infection in foals, we sought to investigate the differences in IgM and IgG subisotype responses between infected foals and adult horses. Relative antibody concentrations between groups were determined by ELISA prior to infection with R. equi and again on day 15 postinfection. Infection of foals with R. equi resulted in a significant increase in IgGa, IgGb, IgGc, and IgM concentrations compared to preinfection values (Fig. 4). Control foals had significantly reduced IgGa, IgGb, and IgM concentrations on day 15 postinfection compared to preinfection values, as a result of the waning of maternal antibodies (Fig. 4). Postinfection IgGa and IgGb concentrations were significantly higher in infected foals than in both groups of adult horses (Fig. 4A and B). Postinfection IgGc and IgG(T) levels were not significantly different between infected foals and adult horses (Fig. 4C and D). Infection of adult horses with R. equi was associated only with a significant increase in IgGb concentrations (Fig. 4B). There were no significant changes in antibody concentrations in control adult horses during the study. IgM concentrations were significantly higher in adult horses than in foals regardless of infection status (Fig. 4E).
FIG. 4.
Relative serum IgM and IgG subisotype concentrations in five foals and five adult horses before (solid bars) and 15 days after (dotted bars) challenge with virulent R. equi. Five foals and five adult horses were used as uninfected controls. The results are displayed as mean ± SD. Different letters between experimental groups (a, b, c) indicate a statistically significant difference in preinfection Ig concentrations. Different numbers between experimental groups (1, 2) indicate a statistically significant difference in postinfection Ig concentrations. *, significant difference between pre- and postinfection Ig concentrations for a given group (P < 0.05).
To determine whether equine IgG subisotypes, like those of mice and humans, can reflect the Th1-Th2 bias of the immune response, we determined the strength of the relationship between each IgG subisotype and IL-4 or IFN-γ. There was a significant correlation between IgG(T) (P = 0.04) and IgGc (P < 0.0001) and IL-4 (Table 1). The coefficient of correlation between IgGc and IL-4 was significantly higher (P = 0.02) than that between IgGc and IFN-γ (Table 1). In contrast, the coefficient of correlation between IgGa and IFN-γ was significantly higher (P = 0.04) than that between IgGa and IL-4 (Table 1).
TABLE 1.
Correlation between IgG subisotypes in serum and IL-4 or IFN-γ mRNA expression in BLN cellsa
| IgG subisotype | Coefficient of correlationb
|
|
|---|---|---|
| IL-4 | IFN-γ | |
| IgGa | −0.27* | 0.38* |
| IgGb | −0.12 | 0.33 |
| IgGc | 0.79*† | 0.30* |
| IgG(T) | 0.46† | 0.06 |
Five foals and five adult horses were infected intrabronchially with virulent R. equi, and serum and BLN cells were collected at 15 days postinfection. Five uninfected foals and five adult horses were used as controls.
Symbols: *, significantly different coefficient of correlation between a given subisotype and IL-4 versus that of the same subisotype and IFN-γ (P < 0.05); †, statistically significant correlation (P < 0.05).
DISCUSSION
Despite a central role for cell-mediated immune responses in protection against R. equi, most studies of foals have focused on antibody responses. The present study is the first to compare cell-mediated and humoral immune responses of R. equi-susceptible foals to those of resistant adult horses following a controlled experimental challenge. Many studies of the pathogenesis of R. equi in foals have used an overwhelming challenge dose (>109 CFU/foal) that induces considerable pulmonary lesions within 3 days and fulminating clinical signs within 10 days of infection (18, 24, 28). Epidemiological evidence indicates that most foals on farms where the disease is endemic become infected very early in life (27). However, the median age at the time of diagnosis of R. equi pneumonia on such farms is approximately 37 days (17). These findings indicate that the incubation period under natural conditions is much longer than that after overwhelming experimental challenge, presumably because of a lower infective dose. The present study used a lower inoculum of R. equi (approximately 106 CFU/foal) to induce subclinical disease and to more closely reproduce the situation encountered following natural infection. The small and focal nature of pulmonary lesions may indicate that the foals of the present study were in the process of clearing the infection. Alternatively, it may just reflect a longer incubation period as a result of using a lower inoculum of R. equi. Disease resolution following experimental infection of foals with R. equi has been reported previously (33).
In contrast to foals, adult horses are immune and very rarely develop R. equi pneumonia. Prior studies have shown that clearance of R. equi from the lungs of adult horses following intrabronchial challenge is associated with lymphoproliferative responses to R. equi antigens in BAL but not in peripheral blood lymphocytes (22). Because of the paucity of lymphocytes in the BAL fluid of newborn foals (6, 50), the present study used BLN cells for assessment of immune function. It was found that R. equi-infected foals have significantly reduced proliferative responses to R. equi antigens compared to adult horses. Poor lymphocyte proliferation in response to R. equi in foals is not the result of impaired proliferative ability of neonatal lymphocytes, as evidenced by the significantly higher proliferation of BLN cells from both groups of foals compared to adult horses in response to stimulation with ConA. ConA, a mitogen, mediates T-cell proliferation through T-cell-receptor ligation and activation of the downstream signaling pathway, hence indicating normal signaling through the T-cell receptors of equine neonates (37).
In one study, pulmonary lymphocytes from adult horses collected 7 days after challenge with R. equi expressed predominantly IFN-γ, but they also expressed IL-4 mRNA in response to in vitro stimulation with R. equi antigen (31). Almost identical results were obtained in the present study, with a mean IFN-γ/IL-4 ratio of 2.4 in infected adult horses. Although the present study is limited to the quantification of mRNA expression, a recent study has documented an excellent correlation between IFN-γ gene expression and actual protein production in foals (8).
The mechanisms regulating neonatal immune responses are not completely understood, regardless of the species. Cell-mediated immune responses of murine and human neonates are generally thought to be biased toward a Th2 response (1). In a recent study, peripheral blood and BAL mononuclear cells from newborn foals were deficient in the ability to produce IFN-γ following ex vivo stimulation with phorbol myristate acetate (8). In the same study, IFN-γ production increased in an age-dependent manner, reaching adult levels around 3 months of age (8). These findings have led to the hypothesis that foals are born with an inherent inability to mount a Th-1-based cell-mediated immune response, which may contribute to their susceptibility to R. equi (8). The present study clearly shows that young foals can mount strong Th1-based immune responses to R. equi, as evidenced by their significantly higher IFN-γ mRNA expression in BLN cells following stimulation with R. equi antigens and significantly higher IFN-γ/IL-4 ratio than those of adult horses. However, consistent with the findings of Breathnach et al. (8), IFN-γ induction in the uninfected control foals was considerably lower than that of uninfected control adult horses. These findings suggest that, like human and murine neonates, foals have the ability to mount adult-like Th1-based responses provided the appropriate stimulus. Consistent with these results, a previous study documented the presence of IFN-γ but not IL-4 in the lungs of foals experimentally infected with R. equi (19). Previous studies have shown that, although the default response is of the Th2 phenotype, murine neonates can mount Th1 responses provided the right antigen, dose of antigen, costimulatory signal, or type of adjuvant (2, 7, 15, 34, 38). Similarly, human neonates also have the ability to mount strong Th1 responses. For example, vaccination of infants with Mycobacterium bovis BCG, a microorganism closely related to R. equi, induces IFN-γ production of a magnitude similar to that produced by adults (32, 35, 44).
Consistent with previous studies, the present study also found a significantly higher percentage of macrophages and significantly lower percentage of T lymphocytes, CD4+ T lymphocytes, and CD8+ T lymphocytes in BAL fluid of healthy foals than in healthy adult horses (6, 13). Infection with R. equi in foals does not result in significant alterations in lymphocyte subpopulations in peripheral blood (14). In contrast, infection of foals with R. equi in the present study resulted in a significant increase in the percentage of T lymphocytes and CD4+ T lymphocytes in BAL fluid. This was associated with a significant decrease in the percentage of these two cell populations in the BLNs. Work with adult horses has also shown that immune responses to R. equi are compartmentalized, being detectable in the lungs but not in peripheral blood (22). In the present study, inoculation of adult horses with R. equi resulted only in a slight but significant increase in the percentage of B cells compared to uninfected adults. This is in contrast to a previous study in which infection of adult horses with R. equi resulted in a significant increase in both CD4+ and CD8+ T lymphocytes (22). Differences between studies may be explained by the different methods of inoculation. Although the size of inoculum was similar, the aforementioned study delivered the entire inoculum in a focal area of a lung (22), whereas the present study delivered the bacteria into both main bronchi in an attempt to induce more generalized changes. Consistent with this theory is the fact that the BAL fluid changes reported by Hines et al. (22) were much more pronounced in BAL fluid from the infected lung segment than from that of the contralateral lung.
Focal pulmonary challenge with R. equi in adult horses results in mild increases in serum IgG(T) concentrations, along with marked increases in serum IgGa and IgGb concentrations (31). Only a significant increase in serum IgGb concentrations was noted in infected adult horses in the present study. Foals showed marked increases in serum IgGa and IgGb levels following infection with R. equi, resulting in concentrations in serum that were significantly higher than those of adult horses. In a previous study, endogenous IgGb production could not be detected in foals until day 63 of age (39). The present study clearly shows that much younger foals can mount a considerable IgGb response provided the right stimulus. In agreement with the current study, previous studies have also shown that foals naturally exposed to R. equi produce mainly IgGa but also IgGb (25, 43). In mice and humans, IgG subisotypes are indirect indicators of T-cell responses, reflecting the role of Th1 and Th2 cytokines in class switching by B cells. The direct association between IgG subisotypes and the cytokine profile has not been established in horses. In the present study, IgGc and IgG(T) were associated with a Th2 (IL-4) cytokine profile, whereas IgGa was more associated with a Th1 (IFN-γ) response. New terminology for equine IgG subisotypes (IgG1 to IgG7) has been proposed based on the complete map of the Ig heavy chain constant gene region (47). However, the terminology has not been widely used in recent publications (9, 11, 20, 26, 48). The new nomenclature has been difficult to implement mainly because commercially available reagents are still sold based on the original nomenclature. For example, IgGa (original nomenclature) is composed of both IgG1 and IgG2, IgGc is composed of both IgG6 and IgG7, and IgG(T) is composed of IgG3 and IgG5 (46). Therefore, the use of the new nomenclature while reagents labeled based on the old terminology were being used would be very confusing. As a result of the lack of specific reagents for each IgG subisotype, the original nomenclature was used in the present publication.
In conclusion, the present study shows that foals have decreased lymphoproliferative responses to R. equi antigens compared to adult horses. However, their peculiar susceptibility to infection with R. equi cannot be explained by generalized IFN-γ deficiency or inappropriate polarization of the immune response toward the Th2 phenotype. Further work is required to identify the fundamental host basis of the susceptibility of foals to R. equi pneumonia.
Acknowledgments
This project was supported by the Florida Department of Regulation Pari-Mutuel Wagering Trust Fund. We thank the Florida Thoroughbred Breeders' and Owners' Association for support of the equine research breeding herd.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19:157-171. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B. 2005. Neonatal T cell function. J. Pediatr. Gastroenterol. Nutr. 40(Suppl. 1):S5-S7. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 4.Ainsworth, D. M., G. Grunig, M. B. Matychak, J. Young, B. Wagner, H. N. Erb, and D. F. Antczak. 2003. Recurrent airway obstruction (RAO) in horses is characterized by IFN-gamma and IL-8 production in bronchoalveolar lavage cells. Vet. Immunol. Immunopathol. 96:83-91. [DOI] [PubMed] [Google Scholar]
- 5.Arlotti, M., G. Zoboli, G. L. Moscatelli, G. Magnani, R. Maserati, V. Borghi, M. Andreoni, M. Libanore, L. Bonazzi, A. Piscina, and R. Ciammarughi. 1996. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand. J. Infect. Dis. 28:463-467. [DOI] [PubMed] [Google Scholar]
- 6.Balson, G. A., G. D. Smith, and J. A. Yager. 1997. Immunophenotypic analysis of foal bronchoalveolar lavage lymphocytes. Vet. Microbiol. 56:237-246. [DOI] [PubMed] [Google Scholar]
- 7.Barrios, C., C. Brandt, M. Berney, P. H. Lambert, and C. A. Siegrist. 1996. Partial correction of the TH2/TH1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur. J. Immunol. 26:2666-2670. [DOI] [PubMed] [Google Scholar]
- 8.Breathnach, C. C., T. Sturgill-Wright, J. L. Stiltner, A. A. Adams, D. P. Lunn, and D. W. Horohov. 2006. Foals are interferon gamma-deficient at birth. Vet. Immunol. Immunopathol. 112:199-209. [DOI] [PubMed] [Google Scholar]
- 9.Cauchard, J., S. Taouji, C. Sevin, F. Duquesne, M. Bernabe, C. Laugier, and J. J. Ballet. 2006. Immunogenicity of synthetic Rhodococcus equi virulence-associated protein peptides in neonate foals. Int. J. Med. Microbiol. 296:389-396. [DOI] [PubMed] [Google Scholar]
- 10.Chirino-Trejo, J. M., J. F. Prescott, and J. A. Yager. 1987. Protection of foals against experimental Rhodococcus equi pneumonia by oral immunization. Can. J. Vet. Res. 51:444-447. [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha, C. W., T. C. McGuire, L. S. Kappmeyer, S. A. Hines, A. M. Lopez, O. A. Dellagostin, and D. P. Knowles. 2006. Development of specific immunoglobulin Ga (IgGa) and IgGb antibodies correlates with control of parasitemia in Babesia equi infection. Clin. Vaccine Immunol. 13:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donisi, A., M. G. Suardi, S. Casari, M. Longo, G. P. Cadeo, and G. Carosi. 1996. Rhodococcus equi infection in HIV-infected patients. AIDS 10:359-362. [DOI] [PubMed] [Google Scholar]
- 13.Flaminio, M. J., B. R. Rush, E. G. Davis, K. Hennessy, W. Shuman, and M. J. Wilkerson. 2000. Characterization of peripheral blood and pulmonary leukocyte function in healthy foals. Vet. Immunol. Immunopathol. 73:267-285. [DOI] [PubMed] [Google Scholar]
- 14.Flaminio, M. J., B. R. Rush, and W. Shuman. 1999. Peripheral blood lymphocyte subpopulations and immunoglobulin concentrations in healthy foals and foals with Rhodococcus equi pneumonia. J. Vet. Intern. Med. 13:206-212. [DOI] [PubMed] [Google Scholar]
- 15.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of TH1 and TH2 immunity in neonatal mice. Science 271:1728-1730. [DOI] [PubMed] [Google Scholar]
- 16.Garton, N. J., M. Gilleron, T. Brando, H. H. Dan, S. Giguère, G. Puzo, J. F. Prescott, and I. C. Sutcliffe. 2002. A novel lipoarabinomannan from the equine pathogen Rhodococcus equi. Structure and effect on macrophage cytokine production. J. Biol. Chem. 277:31722-31733. [DOI] [PubMed] [Google Scholar]
- 17.Giguère, S., J. M. Gaskin, C. Miller, and J. L. Bowman. 2002. Evaluation of a commercially available hyperimmune plasma product for prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J. Am. Vet. Med. Assoc. 220:59-63. [DOI] [PubMed] [Google Scholar]
- 18.Giguère, S., M. K. Hondalus, J. A. Yager, P. Darrah, D. M. Mosser, and J. F. Prescott. 1999. Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi. Infect. Immun. 67:3548-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giguère, S., B. N. Wilkie, and J. F. Prescott. 1999. Modulation of cytokine response of pneumonic foals by virulent Rhodococcus equi. Infect. Immun. 67:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman, L. B., B. Wagner, M. J. Flaminio, K. H. Sussman, S. M. Metzger, R. Holland, and N. Osterrieder. 2006. Comparison of the efficacy of inactivated combination and modified-live virus vaccines against challenge infection with neuropathogenic equine herpesvirus type 1 (EHV-1). Vaccine 24:3636-3645. [DOI] [PubMed] [Google Scholar]
- 21.Harvey, R. L., and J. C. Sunstrum. 1991. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev. Infect. Dis. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 22.Hines, M. T., K. M. Paasch, D. C. Alperin, G. H. Palmer, N. C. Westhoff, and S. A. Hines. 2001. Immunity to Rhodococcus equi: antigen-specific recall responses in the lungs of adult horses. Vet. Immunol. Immunopathol. 79:101-114. [DOI] [PubMed] [Google Scholar]
- 23.Hines, S. A., D. M. Stone, M. T. Hines, D. C. Alperin, D. P. Knowles, L. K. Norton, M. J. Hamilton, W. C. Davis, and T. C. McGuire. 2003. Clearance of virulent but not avirulent Rhodococcus equi from the lungs of adult horses is associated with intracytoplasmic gamma interferon production by CD4+ and CD8+ T lymphocytes. Clin. Diagn. Lab. Immunol. 10:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper-McGrevy, K. E., S. Giguère, B. N. Wilkie, and J. F. Prescott. 2001. Evaluation of equine immunoglobulin specific for Rhodococcus equi virulence-associated proteins A and C for use in protecting foals against Rhodococcus equi-induced pneumonia. Am. J. Vet. Res. 62:1307-1313. [DOI] [PubMed] [Google Scholar]
- 25.Hooper-McGrevy, K. E., B. N. Wilkie, and J. F. Prescott. 2003. Immunoglobulin G subisotype responses of pneumonic and healthy, exposed foals and adult horses to Rhodococcus equi virulence-associated proteins. Clin. Diagn. Lab. Immunol. 10:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper-McGrevy, K. E., B. N. Wilkie, and J. F. Prescott. 2005. Virulence-associated protein-specific serum immunoglobulin G-isotype expression in young foals protected against Rhodococcus equi pneumonia by oral immunization with virulent R. equi. Vaccine 23:5760-5767. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz, M. L., N. D. Cohen, S. Takai, T. Becu, M. K. Chaffin, K. K. Chu, K. G. Magdesian, and R. J. Martens. 2001. Application of Sartwell's model (lognormal distribution of incubation periods) to age at onset and age at death of foals with Rhodococcus equi pneumonia as evidence of perinatal infection. J. Vet. Intern. Med. 15:171-175. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, J. A., J. F. Prescott, and R. J. Markham. 1983. The pathology of experimental Corynebacterium equi infection in foals following intrabronchial challenge. Vet. Pathol. 20:440-449. [DOI] [PubMed] [Google Scholar]
- 29.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1995. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect. Immun. 63:3037-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1996. Transfer of a CD4+ Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lung. Infect. Immun. 64:1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, A. M., M. T. Hines, G. H. Palmer, D. C. Alperin, and S. A. Hines. 2002. Identification of pulmonary T-lymphocyte and serum antibody isotype responses associated with protection against Rhodococcus equi. Clin. Diagn. Lab. Immunol. 9:1270-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 33.Martens, R. J., Martens, J. G., and R. A. Fiske. 1989. Rhodococcus equi foal pneumonia: pathogenesis and immunoprophylaxis. Proc. Am. Assoc. Equine Pract. 35:189-213. [Google Scholar]
- 34.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. USA 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ota, M. O., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, M. J. Newport, P. Aaby, H. Whittle, P. H. Lambert, K. P. McAdam, C. A. Siegrist, and A. Marchant. 2002. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168:919-925. [DOI] [PubMed] [Google Scholar]
- 36.Patton, K. M., T. C. McGuire, D. G. Fraser, and S. A. Hines. 2004. Rhodococcus equi-infected macrophages are recognized and killed by CD8+ T lymphocytes in a major histocompatibility complex class I-unrestricted fashion. Infect. Immun. 72:7073-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pongracz, J., S. Parnell, G. Anderson, J. P. Jaffrezou, and E. Jenkinson. 2003. Con A activates an Akt/PKB dependent survival mechanism to modulate TCR induced cell death in double positive thymocytes. Mol. Immunol. 39:1013-1023. [DOI] [PubMed] [Google Scholar]
- 38.Sarzotti, M., D. S. Robbins, and P. M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 271:1726-1728. [DOI] [PubMed] [Google Scholar]
- 39.Sheoran, A. S., J. F. Timoney, M. A. Holmes, S. S. Karzenski, and M. V. Crisman. 2000. Immunoglobulin isotypes in sera and nasal mucosal secretions and their neonatal transfer and distribution in horses. Am. J. Vet. Res. 61:1099-1105. [DOI] [PubMed] [Google Scholar]
- 40.Takai, S., T. Anzai, K. Yamaguchi, S. Kakizaki, J. Takahagi, Y. Sato, F. Takehara, Y. Tamada, S. Matsukura, A. Tani, M. Kato, N. Seno, Y. Sasaki, S. Tsubaki, and M. Kamada. 1994. Prevalence of virulence plasmids in environmental isolates of Rhodococcus equi from horse-breeding farms in Hokkaido. J. Equine Sci. 5:21-25. [Google Scholar]
- 41.Takai, S., N. Fukunaga, S. Ochiai, T. Sakai, Y. Sasaki, and S. Tsubaki. 1996. Isolation of virulent and intermediately virulent Rhodococcus equi from soil and sand on parks and yards in Japan. J. Vet. Med. Sci. 58:669-672. [DOI] [PubMed] [Google Scholar]
- 42.Takai, S., S. A. Hines, T. Sekizaki, V. M. Nicholson, D. A. Alperin, M. Osaki, D. Takamatsu, M. Nakamura, K. Suzuki, N. Ogino, T. Kakuda, H. Dan, and J. F. Prescott. 2000. DNA Sequence and comparison of virulence plasmids from Rhodococcus equi ATCC 33701 and 103. Infect. Immun. 68:6840-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takai, S., I. Nakata, N. Fukii, Y. Kimura, Y. Sasaki, T. Kakuda, S. Tsubaki, T. Kondo, and T. Sugiura. 2002. Isotype-specific antibody responses to Rhodococcus equi in foals on a horse-breeding farm with a persistent incidence of R. equi infection. J. Equine Sci. 13:63-70. [Google Scholar]
- 44.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 45.Wada, R., M. Kamada, T. Anzai, A. Nakanishi, T. Kanemaru, S. Takai, and S. Tsubaki. 1997. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet. Microbiol. 56:301-312. [DOI] [PubMed] [Google Scholar]
- 46.Wagner, B. 2006. Immunoglobulins and immunoglobulin genes of the horse. Dev. Comp. Immunol. 30:155-164. [DOI] [PubMed] [Google Scholar]
- 47.Wagner, B., D. C. Miller, T. L. Lear, and D. F. Antczak. 2004. The complete map of the Ig heavy chain constant gene region reveals evidence for seven IgG isotypes and for IgD in the horse. J. Immunol. 173:3230-3242. [DOI] [PubMed] [Google Scholar]
- 48.Wagner, B., W. H. Miller, E. E. Morgan, J. M. Hillegas, H. N. Erb, W. Leibold, and D. F. Antczak. 2006. IgE and IgG antibodies in skin allergy of the horse. Vet. Res. 37:813-825. [DOI] [PubMed] [Google Scholar]
- 49.Witonsky, S., R. M. Gogal, Jr., V. Buechner-Maxwell, and S. A. Ahmed. 2003. Immunologic analysis of blood samples obtained from horses and stored for twenty-four hours. Am. J. Vet. Res. 64:1003-1009. [DOI] [PubMed] [Google Scholar]
- 50.Zink, M. C., and J. A. Johnson. 1984. Cellular constituents of clinically normal foal bronchoalveolar lavage fluid during postnatal maturation. Am. J. Vet. Res. 45:893-897. [PubMed] [Google Scholar]