Abstract
Mycoplasma hyopneumoniae, the causative agent of porcine enzootic pneumonia, colonizes the respiratory cilia of affected swine, causing significant economic losses to swine production worldwide. Vaccination is the most cost-effective strategy for the control and prevention of this disease. The goal of this study was to design and evaluate a replication-defective recombinant adenovirus, rAdP97c, expressing the C-terminal portion of P97 adhesin (P97c), an important pathogenesis-associated protein of M. hyopneumoniae, as a new vaccine candidate against M. hyopneumoniae infection. P97c-specific immune responses were evaluated in BALB/c mice following intranasal and intramuscular inoculation with rAdP97c. Mice inoculated by both routes of immunization produced significant levels of specific immunoglobulin G (IgG) antibodies in the serum and in bronchoalveolar lavage fluids (BALs). Animals immunized intranasally also produced a significant level of P97c-specific IgA in BALs. Intramuscular inoculation of rAdP97c induced a systemic and mucosal Th1-type biased response, evidenced by the predominance of IgG2a in the serum and BALs, whereas intranasal inoculation resulted in a mixed Th1/Th2-type response (balanced levels of IgG1 and IgG2a) in both sytemic and mucosal compartments. P97c-specific antibodies were able to inhibit the growth of M. hyopneumoniae cells in vitro. These data suggest that rAdP97c vaccine may represent a new strategy for controlling infection by M. hyopneumoniae.
Mycoplasma hyopneumoniae is the etiological agent of enzootic porcine pneumonia (PEP), one of the most economically significant diseases in the swine industry worldwide. The disease is characterized by chronic nonproductive cough, retarded growth rate, and inefficient food conversion (29). Adherence of M. hyopneumoniae to the swine respiratory epithelial cells causes reduction of ciliary activity, ciliostasis, and loss of cilia (7), predisposing the swine to secondary infections. For example, it is now clear that M. hyopneumoniae potentiates and exacerbates the severity and duration of pneumonia caused by the porcine reproductive and respiratory syndrome virus (38). After colonizing, M. hyopneumoniae stimulates numerous changes, consisting of infiltrates, mononuclear cells (macrophages and lymphocytes) around bronchi and bronchioles, secretion of proinflammatory cytokines, and lymphoid hyperplasia of bronchus-associated lymphoid tissue (22, 26, 30). Traditionally, M. hyopneumoniae infection is controlled by the use of antibiotics. However, this practice does not prevent infection, and treatment cost is prohibitive. In addition to the use of antibiotics and animal management procedures, the prevention of PEP through vaccination is needed. The commonly used vaccines against M. hyopneumoniae are in the form of inactivated whole cells or bacterins. These vaccines are efficacious against M. hyopneumoniae challenge (8, 37) but do not prevent colonization by the pathogen or completely eliminate pneumonia (14). In addition, their preparation is very expensive, because the growth of M. hyopneumoniae in vitro requires a rich culture medium and is time-consuming (19).
To develop the next generation of M. hyopneumoniae vaccines, several research groups are pursuing different strategies, including subunit vaccines (6, 18) and utilization of bacterial or plasmid vectors expressing M. hyopneumoniae proteins (4, 5, 32). Some immunodominant antigens of M. hyopneumoniae have been identified. They include the cytosolic 36-kDa protein (P36), lipoproteins P65 and Mhp378 (17, 23, 35), and the P97 protein. The last is identified as a ciliary adhesion molecule on the basis that monoclonal antibodies against P97 inhibit adherence of M. hyopneumoniae to swine cilia in vitro (45). P97 contains two repeat regions, R1 and R2, located in its C-terminal portion (15). The cilium binding site is located in R1, and at least seven AAKPV/E repeats are required for functional binding (15, 24). R2, located downstream of R1, is involved in attachment of M. hyopneumoniae to the host extracellular matrix (16). P97 is typically well conserved among different strains of M. hyopneumoniae, and the lack of cytoadhesion of avirulent strains of M. hyopneumoniae is related to the absence of functional P97 adhesin (41). Therefore, P97 adhesin could represent an attractive target to develop effective vaccines against M. hyopneumoniae. However, when used as a recombinant vaccine, P97 did not protect swine from infection or reduce the severity of lung lesions caused by M. hyopneumoniae (18). On the other hand, Shimoji et al. (32) showed that intranasal immunization of pigs with an attenuated strain of Erysipelothrix rhusiopathiae YS-19 expressing the C-terminal portion of the P97 protein significantly reduced lung lesions caused by M. hyopneumoniae, despite the absence of antigen-specific antibody responses. This finding indicates that the P97 antigen can be protective if administered in a manner that increases its immunogenicity.
As M. hyopneumoniae infection is restricted to the swine respiratory tract, the ideal vaccine would be mucosally administered and able to stimulate a suitable mucosal immunity, including specific T helper (Th) response and immunoglobulin A (IgA), which can prevent the adherence of pathogens to mucosal cell surfaces (25). Replication-defective recombinant adenoviruses (rAds) are extensively used as antigen delivery vehicle vectors (11, 36). They display several attractive features, including (i) natural tropism for epithelial cells, (ii) efficient gene delivery to antigen-presenting cells, and (iii) high immunogenicity to induce both humoral and cellular immune responses to the transgene product, in some cases after a single inoculation (36).
The purpose of the present study was to construct a rAd expressing the C-terminal portion of M. hyopneumoniae P97 adhesin (rAdP97c) and to characterize the P97c-specific immune response induced in a murine model. Alternative routes of administration of rAdP97c and their effects on humoral immunity were evaluated.
MATERIALS AND METHODS
Cells, virus, and plasmids.
M. hyopneumoniae strain 25934 was obtained from the American Type Collection Culture (ATCC) (Manassas, VA). Escherichia coli DH5 and BL21(DE3)pLysS strains were used for plasmid DNA amplification and production of recombinant proteins, respectively, and were grown in Luria-Bertani medium at 37°C. Human embryonic kidney (HEK) 293 cells (ATCC CRC-1573) were used for the production of rAds, and they were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum and 2 mM l-glutamine at 37°C in a 5% CO2 incubator. The Ad used in this study was a replication-defective E1- and E3-deleted human serotype 5 (Ad5). The pAdPS-CMV5-Cuo-IRES-GFP (pAd) plasmid was used as an Ad5 transfer vector for the generation of rAds (28). Both pAd and Ad5 were obtained from B. Massie, Biotechnology Research Institute, National Research Council of Canada. The pGEX4T1 plasmid (Amersham Pharmacia Biotech, Baie d'Urfé, Québec, Canada) was used to express recombinant proteins in fusion with glutathione S-transferase (GST).
PCR amplification of the P97c gene and site-directed mutagenesis.
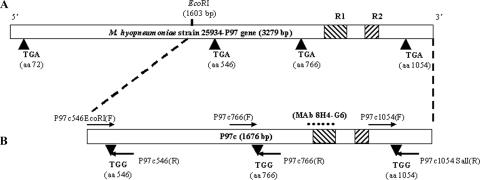
Genomic DNA from M. hyopneumoniae was prepared as previously described (3) and was used as a template for the amplification of a 1,676-bp fragment (containing the R1 and R2 regions), which was designated the C-terminal portion of the P97 gene (P97c). The oligonucleotide primers used for amplification were P97c546EcoRI(F) and P97c1054SalI(R) (Table 1). They were designed from the previously published sequence of the P97 adhesin gene (GenBank accession no. U50901). To express the full-length P97c protein in heterologous cells, mycoplasmal TGA codons (tryptophan) found in the P97c gene were replaced with the universal TGG (tryptophan) codons by site-directed mutagenesis using the overlapping extension-PCR method (Fig. 1). Primers each carrying the appropriate substitution used for site-directed mutagenesis are listed in Table 1. Amplification reactions were carried out using Vent DNA polymerase. After amplification, all products were sequenced to confirm that changes from A to G occurred at the third position of the targeted codons.
TABLE 1.
Oligonucleotide primer sequences
| Designationa | Nucleotide sequence (5′ to 3′)b | Descriptionc |
|---|---|---|
| P97c546EcoRI(F) | TGATGAATTCGGGGCCTTTAAATCCGTGCTTAATTCCTGGACAGGAAAAATTCAGC | Cloning and SDM |
| P97c546(R) | GTCTAATTTTGCCCAAGGAGCAAAATT | SDM |
| P97c766(F) | AATAATTTTGCTCCTTGGGCAAAATTAGAC | SDM |
| P97c766(R) | CAACCTCTGTTTTCCAATTTTTACCTTG | SDM |
| P97c1054(F) | CAAGGTAAAAATTGGAAAACAGAGGTTG | SDM |
| P97c1054(R) | TTGTCGACTTATTTAGATTCTGGTTCCTC | Cloning and SDM |
| pAd97cBglII(F) | GGAAGATCTGCCACCATGAATTCGGGGCCTTTAAATCC | Cloning |
| pAd97cBglII(R) | GGAAGATCTTTATTTAGATTCTGGTTCCTC | Cloning |
The numbers indicate the position of the nucleotide A (in TGA codons) replaced by the nucleotide G during site-directed mutagenesis. F, forward primer; R, reverse primer.
The underlined sequences represent EcoRI, SalI, or BglII restrictions sites. GCCACC, Kozak sequence.
SDM, site-directed mutagenesis.
FIG. 1.
(A) Schematic representation of the P97 adhesin gene of M. hyopneumoniae strain 25934 showing the R1 and R2 regions (hatched) and the positions of TGA codons. (B) Schematic representation of site-directed mutagenesis of TGA codons to TGG codons in the C-terminal portion of the P97 adhesin gene (P97c). The arrows indicate the orientations of the overlapping primers used. The region of the P97c protein recognized by MAb 8H4-G6 is indicated (square dot).
Cloning and purification of recombinant P97c.
The mutated P97c gene was digested with EcoRI and SalI and cloned into the pGEX4T1 plasmid. The resulting pGEX/P97c vector was transformed into E. coli BL21(DE3)pLysS. An overnight culture was diluted in 1 liter of LB medium supplemented with 100 μg/ml ampicillin and incubated at 37°C until late log phase (the A600 reached 0.6 to 0.8). Protein expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mM for 3 h. Cells were harvested and resuspended in ice-cold 0.1 M phosphate-buffered saline (PBS) (pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml lysozyme, and protease inhibitors. The cells were sonicated, and 1% Triton X-100 was added, followed by a 30-min incubation period at room temperature (RT). The GST-P97c protein was purified by affinity chromatography on glutathione-Sepharose 4B beads (Amersham Pharmacia) and either eluted with reduced glutathione (20 mM glutathione, 50 mM Tris-HCl [pH 8.0], 120 mM NaCl) or directly cleaved for 16 h at RT with 20 units of thrombin protease. The recombinant P97c protein (rP97c) was then dialyzed against PBS and decontaminated using a Detoxi-Gel endotoxin-removing column (Pierce, Rockford, IL). The protein concentration was determined using a Bio-Rad protein assay in conjunction with a bovine serum albumin standard curve (Bio-Rad, Mississauga, Ontario, Canada). The rP97c protein was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and confirmed by Western blot analysis with mouse monoclonal anti-P97c antibody (MAb 8H4-G6). This MAb was previously produced in our laboratory by intraperitoneally immunizing BALB/c mice with the recombinant GST-P97c fusion protein (unpublished data).
Construction of replication-defective rAds.
The mutated P97c gene was reamplified by PCR from the pGEX/P97c plasmid using the forward primer pAd97cBglII(F) (including a GCCACC Kozak consensus sequence and an ATG initiation codon) and the reverse primer pAd97cBglII(R). After digestion with BglII, the PCR product (1,676 bp) was inserted between the BglII and BamHI sites of the pAd plasmid under the control of the constitutive cytomegalovirus immediate-early promoter/enhancer, yielding the pAdP97c plasmid. The pAd vector was digested with BglII and BamHI to remove the gene encoding green fluorescent protein (GFP), in order to screen the positive clones by immunofluorescence analysis. rAd5 carrying P97c (rAdP97c) was generated using the transfection/infection method (10). pAdP97c was linearized by PacI digestion and transferred into HEK 293 cells using polyethylenimine as a transfection reagent (Polysciences Inc.). The next day, cells were infected with Ad5 at various multiplicities of infection and incubated for 4 days to maximize homologous recombination. The cells were lysed by freeze/thaw cycles, and supernatant was used to reinfect fresh HEK 293 cells for 6 h. After being overlaid with 1.25% agarose SeaPlaque-Dulbecco's modified Eagle's medium mixture, the cells were incubated until lysis plaques formed. The viral plaques were screened for P97c expression by immunofluorescence and Western blotting. One positive clone was plaque purified and amplified on HEK 293 cells. After freeze/thaw cycles, rAdP97c was purified by double cesium chloride centrifugation and dialyzed against PBS. The rAd5 expressing GFP (rAdGFP) was constructed in the same way. Viruses were titered by the 50% tissue culture infectious dose (TCID50) method (27) and stored at −80°C.
Immunofluorescence analysis.
Indirect immunofluorescence assays were performed on HEK 293 cells seeded in 24-well plates and transfected with 2 μg of pAdP97c or infected with rAdP97c (multiplicity of infection, 100:1). Mock-infected cells were used as negative controls. After 48 h of incubation, the cells were washed with PBS-0.1% Tween 20 (PBST) and fixed with cold acetone-methanol (1:1). The plates were incubated with MAb 8H4-G6 for 1 h at 37°C. After the plates were washed, bound MAb was detected with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma-Aldrich, Oakville, Ontario, Canada) and visualized by fluorescence microscopy (Leica; Leitz, Wetzlar, Germany).
SDS-PAGE and Western blot analysis.
To determine the expression of rP97c in E. coli, proteins from transformed cells were subjected to 12% SDS-PAGE under reducing conditions and then visualized by Coomassie blue staining or transferred to nitrocellulose membranes (Bio-Rad). Cells transformed by pGEX-4T1 served as negative controls. To check for P97c expression in eukaryotic cells, HEK 293 cells were seeded in 60-mm dishes and transfected with pAdP97c or infected with rAdP97c as described above. Cells transfected with pAd or infected with rAdGFP or mock-infected cells were used as negative controls. The cells were lysed in a buffer containing 150 mM NaCl, 10 mM Tris [pH 7.8], 1% Triton X, 1% sodium deoxycholate, 0.1% SDS, and complete protease inhibitors. Cell lysates were separated on 12% SDS-PAGE and blotted onto a nitrocellulose membrane. For Western blot analysis, the membrane was blocked overnight at 4°C in 5% nonfat milk in PBST and then probed with MAb 8H4-G6 for 1 h at RT. After being washed with PBST, the membrane was treated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma). Immunoreactive protein bands were detected with 0.005% (wt/vol) 4-chloro-1-naphthol-0.015% (vol/vol) hydrogen peroxide in PBS.
Mice and immunization experiments.
Female BALB/c mice aged 7 to 8 weeks were obtained from Charles River Breeding Laboratory (Montreal, Quebec, Canada) and maintained under specific-pathogen-free conditions at the Experimental Biology Center of the Armand-Frappier Institute (Laval, Quebec, Canada). Immunization experiments were performed according to the Institutional Animal Care and Use Committee guidelines. Mice (n = 5 per group) were immunized either intranasally (i.n.) or intramuscularly (i.m.) with rAdP97c or rAdGFP (as a negative control). For i.n. immunization, mice were anesthetized with isofluorane and inoculated with 20 μl in each nare using a Finn pipette for a total of 5 × 107 TCID50 in PBS. For i.m. inoculation, a total volume of 100 μl of viruses (5 × 107 TCID50) was injected into each quadriceps (50 μl/quadriceps) using a 25-gauge needle. The mice received a booster at day 30 with the same dose. The mice were bled at days 14, 30, and 60 postinfection (p.i.). At day 60, the mice were sacrificed, and bronchoalveolar lavages were performed by infusion of 0.5 ml of PBS-5 mM EDTA into the lungs, using a 0.58-nm polyethylene catheter. Bronchoalveolar lavage fluids (BALs) were then filtered through a sterile 100-μm membrane to remove mucus and cell debris. Sera and BALs were heat inactivated at 56°C for 30 min, pooled by immunization group, and stored at −80°C until they were used.
Detection of P97c-specific antibodies.
P97c-specific antibody responses in immunized mice were assayed by Western blotting and enzyme-linked immunosorbent assay (ELISA). For Western blot analysis, rP97c was submitted to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked overnight at 4°C in 5% nonfat milk-PBST and cut into strips. Individual strips were probed with sera (diluted 1:200 in PBST) from rAdP97c-immunized mice. P97c-specific antibodies were detected with HRP-conjugated goat anti-mouse IgG and visualized as described above. Preimmune serum and sera from rAdGFP-immunized mice were used as negative controls. Levels of P97c-specific IgA and IgG were determined by indirect ELISA. Ninety-six-well plates (ICN Biomedicals) were coated with rP97c (0.5 μg/well, diluted in PBS). After overnight incubation at 4°C, the plates were washed three times with PBST and blocked for 2 h at RT with 100 μl of PBST plus 2% bovine serum albumin. The wells were washed, and 100 μl of sera (1:200 dilution in PBST) or BALs (1:50 dilution) was added to each well. Each sample was assayed in duplicate. The plates were incubated for 2 h at 37°C and then washed, and detection of bound immunoglobulins was performed by adding 100 μl of HRP-conjugated anti-mouse IgG (1:2,000) or IgA (1:1,000) (Sigma). After incubation for 1 h, the plates were washed four times, and 100 μl of 3,3′-5,5′-tetramethyl benzedine substrate (Sigma) was added to each well. The reaction was stopped by adding 30 μl of 2N HCl per well, and optical densities were read at 405 nm (OD405) with a spectrophotometer plate reader (Bio-Tek Instruments). In another set of experiments, P97c-specific IgG isotype responses were assayed using HRP-conjugated anti-mouse IgG1, IgG2a, IgG2b, or IgG3 as a secondary antibody (Boehringer Mannheim).
Growth inhibition assay.
M. hyopneumoniae cells were grown at 37°C for 3 days in Friis medium (12) supplemented with 20% (vol/vol) porcine serum, 5% yeast extract, 0.15 mg/ml bacitracin, 0.08 mg/ml thallium acetate. Cells were harvested by centrifugation at 20,000 × g for 25 min and resuspended in growth medium. Mycoplasma cells (approximately 1 × 104 color-changing units/100 μl) were seeded in triplicate in 96-well plates in the presence of sera or BALs (100-μl serial dilutions in PBS). Phenol red (40 μg/ml) was added to increase the sensitivity of the assay. Cells incubated with sera or BALs from mice immunized with rAdGFP or with medium without antibodies were used as negative controls. The change in color-changing units with phenol red as the indicator at OD560 was measured as an estimate of the growth of mycoplasmal cells (5).
Statistical analysis.
Statistical differences were determined by using a two-tailed Student t test. Data are expressed as the means ± the standard deviations of the means. A P value of <0.05 was considered significant.
Nucleotide sequence accession number.
The GenBank accession number for the C-terminal portion of the P97 gene (P97c) is AY512905.
RESULTS
Construction of a rAd expressing P97c.
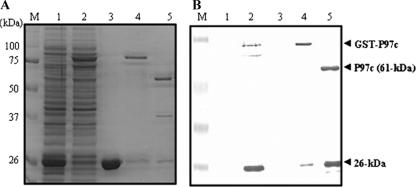
In order to ensure full-length expression of P97c in heterologous cells, the TGA codons (which encode a tryptophan rather than a translation stop in mycoplasmas) within the DNA sequence of P97c were converted to TGG codons by site-directed mutagenesis (Fig. 1). The mutated P97c gene was cloned into the pGEX-4T1 plasmid and expressed as a fusion protein with GST in E. coli. The expected mass of the P97c protein is 61 kDa. After purification, the SDS-PAGE analysis revealed a prominent band of 87 kDa corresponding to the GST-P97c fusion protein (Fig. 2A, lane 4). After thrombin cleavage, the expected protein band of 61 kDa was observed, as well as two other bands of approximately 26 and 35 kDa (Fig. 2A, lane 5). Western blotting using an anti-P97c MAb (MAb 8H4-G6) revealed the expected 61-kDa band, together with the product of 26 kDa, but not 36 kDa (Fig. 2B, lane 5). No immunoreactive bands were detected with controls (Fig. 2B, lanes 1 and 3). These results suggest that the epitope recognized by this MAb is located in the 26-kDa polypeptide, as indicated in Fig. 1. A previous study reported that the P97 cilium adhesin is cleaved at multiple sites during its expression in host cells, generating a family of peptides (9).
FIG. 2.
Expression of P97c in E. coli. (A) SDS-PAGE detection of IPTG (isopropyl-β-d-thiogalactopyranoside)-induced expression of P97c in BL21(DE3)pLys. The pGEX/P9c plasmid was transformed into the BL21(DE3)pLys strain of E. coli and induced by 1 mM IPTG for 3 h at 37°C. Proteins were separated on 12% SDS-PAGE, followed by Coomassie blue staining. (B) Western blot analysis using an anti-P97c MAb (MAb 8H4-G6). Lane 1, E. coli transformed with pGEX-4T1; lane 2, E. coli transformed with pGEX/P97c; lane 3, purified GST protein; lane 4, purified GST-P97c protein; lane 5, purified P97c protein after thrombin cleavage. M, molecular mass marker (in kDa).
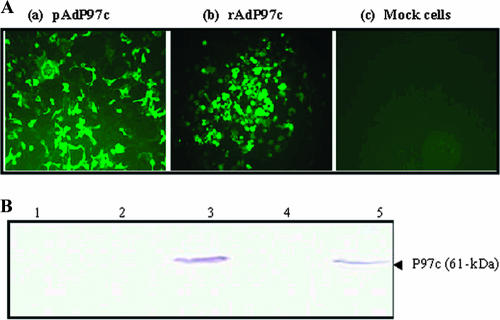
Next, the mutated P97c gene was subcloned into the Ad5 transfer vector. The resulting plasmid (pAdP97c) was used to generate rAd5 carrying the P97c gene (rAdP97c) by homologous recombination in HEK 293 cells. To assess whether P97c protein could be expressed in mammalian cells, cells were transfected with pAdP97c or infected with rAdP97c. Indirect immunofluorescence analysis performed with MAb 8H4-G6 demonstrated that the protein was expressed by both constructs. A strong immunofluorescence signal was detected in cells transfected with pAdP97c or infected with rAdP97c (Fig. 3A, a and b). As expected, no fluorescence signal was visible in mock-infected cells (Fig. 3A, c). The cell lysates were assayed by Western blotting using the same MAb. A band of 61 kDa, corresponding to the expected size of P97c, was detected in the lysates from cells transfected with pAdP97c or infected with rAdP97c (Fig. 3B, lanes 3 and 5, respectively), but not from controls (Fig. 3B, lanes 1, 2, and 4). However, the P97c protein seemed not to be cleaved in HEK 293 cells, since the 26-kDa product was not detected.
FIG. 3.
Expression of the P97c gene in 293 cells. (A) Indirect immunofluorescence analysis. HEK 293 cells were transfected with pAdP97c (a), infected with rAdP97c (b), or mock infected (c) and probed with an anti-P97c MAb (MAb 8H4-G6). (B) Western blot analysis. Proteins were separated on 12% SDS-PAGE, transferred onto nitrocellulose membranes, and probed with MAb 8H4-G6. Lane 1, mock-infected cells; lane 2, cells transfected with original transfer vector; lane 3, cells transfected with pAdP97c; lane 4, cells infected with rAdGFP; lane 5, cells infected with rAdP97c. The arrow indicates the expected mass of the P97c protein.
Systemic antibody responses elicited by rAdP97c.
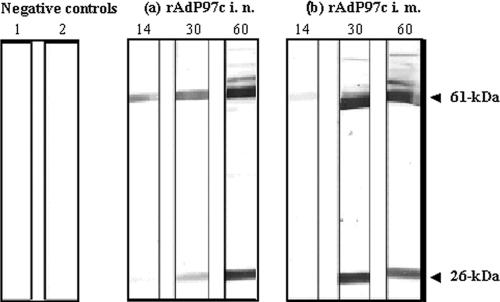
The ability of rAdP97c to stimulate an antibody response to P97c was determined after i.n. or i.m. immunization of BALB/c mice at day 0 and boost at day 30. Mice inoculated with rAdGFP were used as negative controls. First, preimmune serum and sera collected at days 14, 30, and 60 were analyzed for the presence of P97c-specific antibodies by Western blotting using the purified rP97c as the antigen. As expected, P97c-specific antibodies were not detected in the preimmune serum and serum from mice immunized with rAdGFP (Fig. 4, lanes 1 and 2, respectively). P97c-specific IgG antibodies were detectable in sera of mice immunized with rAdP97c by either the i.n. or the i.m. route (Fig. 4, a and b, respectively). Sera collected at days 14, 30, and 60 p.i. immunoreacted with the 61-kDa protein (P97c). In addition, sera collected at days 30 and 60 also immunoreacted with the cleaved product of 26 kDa, but not of 35 kDa, suggesting that the immunogenic epitopes of P97c are located in the 26-kDa fragment (containing the R1 region), as indicated in Fig. 1.
FIG. 4.
Western blot analysis for P97c-specific antibody responses. The recombinant P97c protein was submitted to 12% SDS-PAGE and transferred onto a nitrocellulose membrane. Individual strips were incubated with serum (diluted 1:200). Lane 1, preimmune serum; lane 2, serum from rAdGFP-immunized mice; a and b, sera collected at days 14, 30, and 60 postimmunization from mice immunized with rAdP97c via the i.n. or i.m. route, respectively. These sera reacted with the expected protein corresponding to P97c (61 kDa) and also with the product of 26 kDa.
Levels of P97c-specific antibodies induced by rAdP97c were further determined by indirect ELISA. As shown in Table 2, mice immunized (i.n. and i.m.) with rAdP97c produced significant levels of specific IgG as early as day 14 p.i. compared with the control groups (P < 0.05). There was no considerable difference between the i.n. and i.m. routes for the induction of specific antibodies at days 14 and 30. In contrast, we found that at day 60, the P97c-specific IgG level was two times higher in i.m.-inoculated animals than i.n.-immunized mice.
TABLE 2.
ELISA for P97c-specific antibody responses of BALB/c mice immunized with rAdP97ca
| Group | Mean OD405 ± SD (P)
|
|||||
|---|---|---|---|---|---|---|
| Serum
|
BAL
|
|||||
| IgG on:
|
IgA on day 60 | IgG on day 60 | IgA on day 60 | |||
| Day 14 | Day 30 | Day 60 | ||||
| rAdGFP i.n. | 0.068 ± 0.003 | 0.071 ± 0.0049 | 0.066 ± 0.0063 | 0.075 ± 0.009 | 0.087 ± 0.009 | 0.071 ± 0.002 |
| rAdGFP i.m. | 0.065 ± 0.002 | 0.071 ± 0.0063 | 0.058 ± 0.0014 | 0.058 ± 0.0014 | 0.081 ± 0.015 | 0.067 ± 0.001 |
| rAdP97c i.n. | 0.249 ± 0.003 (0.008)* | 0.36 ± 0.029 (0.026)* | 0.668 ± 0.046 (0.019)* | 0.106 ± 0.018 (0.054) | 0.781 ± 0.028 (0.012)* | 0.378 ± 0.037 (0.029)* |
| rAdP97c i.m. | 0.231 ± 0.005 (0.004)* | 0.487 ± 0.022 (0.0084)* | 1.138 ± 0.108 (0.0229)* | 0.106 ± 0.018 (0.054) | 0.587 ± 0.012 (0.01)* | 0.096 ± 0.022 (0.154) |
Groups of five mice were immunized with rAdP9c or rAdGFP (negative control) by the i.n. or i.m. route. Samples were collected at days 14, 30, and 60 and pooled by group. Sera were diluted 1:200 and BALs 1:50 and then analyzed for P97c-specific antibodies by ELISA in duplicate. *, significant difference (P < 0.05) compared with rAdGFP groups.
Mucosal antibody responses elicited by rAdP97c.
To determine levels of mucosal IgG and IgA responses against P97c induced in mice following immunization with rAdP97c, BALs were collected at day 60 p.i. and examined by indirect ELISA (Table 1). Significant levels of P97c-specific IgG were detected in BALs of mice inoculated with rAdP97c by the i.n. and i.m. routes (P < 0.05). There was no considerable difference between the routes for the induction of local IgG. Immunization using the i.m. route did not induce P97c-specific IgA in BALs. In contrast, mice immunized i.n. gave a significant IgA response. This result indicates that P97c-specific IgA antibodies detected in BALs were locally produced in the respiratory tract.
P97c-specific IgG isotypes.
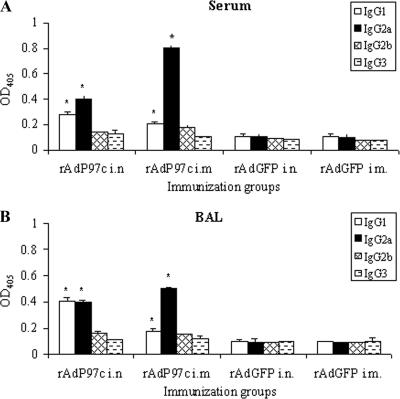
The P97c-specific IgG isotypes in sera and BALs were measured at day 60 p.i. in order to determine which Th subset responses were elicited. In mice, IgG1 is indicative of a Th2-type response, whereas IgG2a is predominantly produced during a Th1-type response (34). In sera of mice immunized with rAdP97c i.n. and i.m., IgG1 and IgG2a were significantly detected (P < 0.05); levels of IgG2b and IgG3 did not rise significantly above those of negative controls (Fig. 5A). Mice immunized i.m. produced more IgG2a than IgG1 (IgG2a/IgG1 ratio = 4), whereas those immunized i.n. had approximately equal levels of IgG2a and IgG1, with a ratio of 1.4. Analysis of P97c-specific IgG isotypes in BALs also revealed that IgG1 and IgG2a were predominantly induced (Fig. 5B). Both i.n. and i.m. inoculation of rAdP97c generated profiles similar to those observed in the sera. These results suggest that the Th-type response to P97c stimulated in mice by rAdP97c is modulated by the route of immunization. Immunization by the i.m. route seems to favor a Th1-type response against P97c, while the i.n. route induces rather a mixed Th1/Th2-type response in both systemic and mucosal compartments.
FIG. 5.
Specific IgG isotype responses in mice immunized with rAd97c. Mice (five per group) were immunized with rAdP97c or with rAdGFP (negative control). Pools of sera (A) and BALs (B) collected at day 60 p.i. were examined for P97c-specific IgG isotypes by ELISA in duplicate. The data represent the mean OD405 plus the standard deviations. *, significant difference (P < 0.05) compared with negative controls.
Growth inhibition of M. hyopneumoniae.
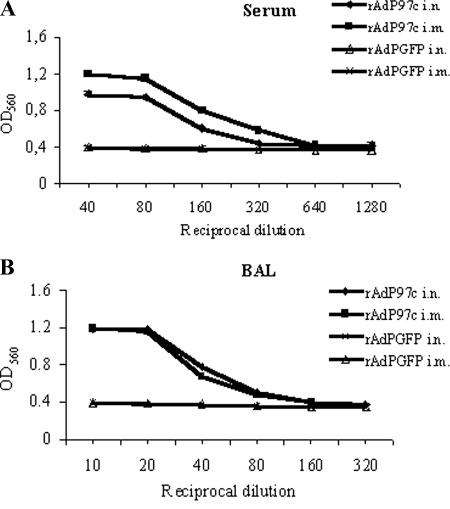
We examined whether P97c-specific antibodies induced by rAdP97c were capable of interfering with the growth of M. hyopneumoniae. The growth of M. hyopneumoniae can be monitored in vitro by the color and OD change of the indicator (phenol red) in the growth medium (5, 44). As the mycoplasmal cells grow, the red color of phenol red gradually turns yellow, resulting in a decrease in the OD. We performed growth inhibition assays by incubation of M. hyopneumoniae cells in the presence of medium without antibodies (as a negative control) or with serial dilutions of sera or BALs of immunized mice collected at day 60 p.i. The OD decreased from 1.22 (at day zero) to 0.4 (after 3 days of incubation) as M. hyopneumoniae cells grew in the culture medium without antibodies, indicating cell growth during the incubation period (data not shown). In contrast, in the presence of sera of mice immunized (i.n. and i.m.) with rAdP97c, the inhibitory effect was observed. The best inhibition was obtained with dilutions of 1:40 and 1:80 (Fig. 6A). BALs of mice inoculated (i.n and i.m.) with rAdP97c also exhibited inhibitory activity, and the best inhibition was achieved with dilutions ranging from 1:10 to 1:40 (Fig. 6B). As expected, the growth of cells was not affected by the samples from mice immunized (i.n and i.m.) with rAdGFP. The results indicate that the growth of M. hyopneumoniae was inhibited by P97c-specific antibodies in the sera and BALs and that the inhibitory effect was concentration dependent.
FIG. 6.
Growth inhibition of M. hyopneumoniae. Mycoplasma cells were cultured in the presence of serial dilutions of sera (A) or BALs (B) from mice immunized with rAdP97c or with negative control rAdGFP. The growth inhibition was determined by measuring the OD560. The results are means of triplicate experiments ± standard deviations.
DISCUSSION
The most effective strategy to prevent diseases caused by bacteria may be to block interactions between microbial adhesins and their host cell receptors. Many studies have demonstrated the efficiency of adhesin-based vaccines in blocking attachment in vitro, as well as in vivo (42). As a more successful example, vaccination with the FimH adhesin of uropathogenic E. coli reduced in vivo colonization of the bladder mucosa by the pathogen up to 99% in the murine cystitis model (20). However, pure adhesins generally elicit poor immune responses and need to be administered together with an immunostimulative carrier molecule or vector (6, 42). The initial event in M. hyopneumoniae pathogenesis is its adherence to the cilia of the respiratory tract epithelial cells (46), which is mediated by specific regions of adhesins, such as the R1 motif located in the C-terminal portion of P97 (15, 24). Therefore, vaccines inducing immune responses to the R1 region could prevent colonization of pigs by the microorganism. Replication-defective rAds display a natural tropism for the respiratory epithelial cells and are known to be efficient in eliciting both humoral and cellular immunity to the expressed antigens (36).
In the present study, a rAd vector carrying the C-terminal portion of the P97 adhesin (rAdP97c) was constructed and evaluated for its potential to induce P97c-specific immune responses in mice. First, we demonstrated that the P97c protein was sufficiently expressed in vitro in cells infected with rAdP97c and could be detected by immunoblotting. To our knowledge, this is the first report of the construction of a rAd expressing a mycoplasmal antigen. P97c-specific immune responses induced by rAdP97c were examined in BALB/c mice. We found that when inoculated by either the i.n. or i.m. route, rAdP97c stimulated the production of systemic and mucosal antibodies to P97c, likely directed to the fragment containing the R1 repeat region. Significant P97c-specific IgG responses were induced after a single inoculation and were considerably enhanced by a booster immunization. Interestingly, inoculation of rAdP97c by the i.n. route (the natural route of M. hyopneumoniae infection) elicited a suitable mucosal immune response, as evidenced by the presence of P97c-specific IgA in BALs. IgA is produced primarily by plasma cells in the mucosa and is actively secreted, whereas IgG can appear in mucosal secretions as a transudate from serum (2). Passive diffusion could account for a portion of IgG detected in BALs after i.m. inoculation. Our results are in agreement with previous studies showing that mucosal immunization with rAd results in both local and systemic immune responses, while parenteral immunization preferentially induces a systemic immune response (21, 43).
The rAdP97c-induced antisera and BALs were shown to be able to inhibit the growth of M. hyopneumoniae cells. The inhibition activity was likely related to the presence of IgG, since despite their IgA levels, BALs from i.n.-immunized mice did not demonstrate a higher inhibition than those from mice immunized by the i.m. route (Fig. 6A). In addition, the inhibition effects of samples were proportional to their IgG titers. The mechanism by which antibodies inhibit the growth of M. hyopneumoniae remains unknown, and thus, we cannot rule out the possibility that P97c-specific IgA could interfere with the pathogen in vivo and specifically during the adherence process.
Conceicao et al. (6) have shown that immunization of mice with pure recombinant R1 of P97 adhesin did not induce specific systemic and mucosal antibodies to R1. In contrast, immunization with the R1 region fused to the B subunit of the heat-labile enterotoxin B subunit of E. coli (rLTBR1) produced high levels of specific antibody and cellular responses. Other systems have been developed in which the R1 region is expressed by an attenuated strain of Salmonella enterica serovar Typhimurium aroA (4) or E. rhusiopathiae YS-19 (32). Mice immunized with these vectors failed to elicit a humoral immune response to the antigen, indicating that these live strategies are less effective than rAdP97c for the induction of antibody responses against the P97 adhesin. Our results also demonstrate that rAdP97c displays the ability to enhance the immunogenicity of the R1 antigen.
The induction of P97c-specific antibodies in BALs could be important, since local IgA can prevent attachment of mycoplasmas to the ciliated epithelium and IgG can participate in opsonization and phagocytosis (31, 40). However, the relationship between locally secreted antibody responses and protection against mycoplasma infections is still controversial. In humans, Mycoplasma pulmonis-specific IgA responses have been found to correlate better with protection than serum (33). Similarly, Avikan and Ley (1) found a positive correlation between Mycoplasma gallisepticum-specific IgA levels in tracheal secretions and decreased lesions caused by the microorganism. In contrast, Djordjevic et al. (8) reported that locally secreted antibodies appeared to play a limited role in recovery from M. hyopneumoniae infection, since the pathogen can survive despite vigorous local antibody responses in the host.
It is suggested that an effective immunity against M. hyopneumoniae requires humoral and cell-mediated immune responses (14, 37). Both immune responses are driven by activation of CD4+ Th cells. Differentiation of naive CD4+ Th cells into Th1 and Th2 cells determines whether humoral or cell-mediated immunity will be predominant (13, 34). CD4+ Th1 cells are involved in the cell-mediated immune response and activate B cells to produce opsonizing antibodies, such as IgG2a, whereas CD4+ Th2 cells favor humoral immunity and secretion of IgG1 and IgA. Here, we have demonstrated that rAdP97c is capable of inducing both Th1 and Th 2 responses to P97c, as evidenced by the presence of IgG2a and IgG1 in sera and BALs of immunized mice. After i.m. immunization of mice with rAdP97c, there is a clear predominance of P97c-specific IgG2a in sera and BALs, suggesting a Th1-biased immune response. Of note, i.n. inoculation stimulated a mixed Th1/Th2-type response in both systemic and mucosal compartments. However, many factors, such as the vector, the delivery route, and the antigen, may modulate immune responses (13, 39).
In summary, the data presented in this study demonstrate that vaccination of mice with rAdP97c leads to the elicitation of both systemic immune responses to the encoded antigen, suggesting that this strategy may represent a new approach to the design of vaccines against M. hyopneumoniae. However, the immune response of mice should not be extrapolated, and the effectiveness of rAdP97c for the control of PEP requires further studies in pigs.
Acknowledgments
This study was supported by the Conseil de Recherches en Pêche et Agroalimentaire du Québec (grant 4600).
We thank Veronika Von Messling for critical review of the manuscript.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Avikan, A. L., and D. H. Ley. 1993. Protective immune response to Mycoplasma gallisepticum demonstrated in respiratory-tract washings from M. gallisepticum-infected chickens. Avian Dis. 37:697-705. [PubMed] [Google Scholar]
- 2.Brandtzaeg, P., and I. N. Farstad. 1998. The human mucosal B-cell system, p. 439-468. In J. Mestecky, P. L. Ogra, and P. Bland (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, CA.
- 3.Caron, J., N. Sawyer, B. Ben Abdel Moumen, K. Cheikh Saad Bouh, and S. Dea. 2000. Species-specific monoclonal antibodies to Escherichia coli-expressed p36 cytosolic protein of Mycoplasma hyopneumoniae. Clin. Diagn. Lab. Immunol. 7:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, A. Y., S. R. Fry, J. Forbes-Faulkner, G. Daggard, and T. K. Mukkur. 2006. Evaluation of the immunogenicity of the P97R1 adhesin of Mycoplasma hyopneumoniae as a mucosal vaccine in mice. J. Med. Microbiol. 55:923-929. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y. L., S. N. Wang, W. J. Yang, Y. J. Chen, H. H. Lin, and D. Shiuan. 2003. Expression and immunogenicity of Mycoplasma hyopneumoniae heat shock protein antigen P42 by DNA vaccination. Infect. Immun. 71:1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conceicao, F. R., A. N. Moreira, and O. A. Dellagostin. 2006. A recombinant chimera composed of R1 repeat region of Mycoplasma hyopneumoniae P97 adhesin with Escherichia coli heat-labile enterotoxin B subunit elicits immune response in mice. Vaccine 24:5734-5743. [DOI] [PubMed] [Google Scholar]
- 7.DeBey, M. C., and R. F. Ross. 1994. Ciliostasis and loss of cilia induced by Mycoplasma hyopneumoniae in porcine tracheal organ cultures. Infect. Immun. 62:5312-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djordjevic, S. P., G. J. Eamens, L. F. Romalis, P. J. Nicholls, V. Taylor, and J. Chin. 1997. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust. Vet. J. 75:504-511. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic, S. P., S. J. Cordwell, M. A. Djordjevic, J. Wilton, and F. C. Minion. 2004. Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesin. Infect. Immun. 72:2791-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elahi, S. M., W. Oualikene, L. Naghi, M. O'Connor-McCourt, and B. Massie. 2002. Adenovirus-based libraries: efficient generation of recombinant adenoviruses by positive selection with the adenovirus protease. Gene Ther. 18:1238-1240. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, T. B., P. M. Alves, J. G. Aunins, and M. J. Carrondo. 2005. Use of adenoviral vectors as veterinary vaccines. Gene Ther. 12:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis, N. F. 1973. The pathogenicity of Mycoplasma flocculare. Acta Vet. Scand. 14:344-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad, D., S. Liljeqvist, S. Stahl, P. Perlmann, K. Berzins, and N. Ahlborg. 1998. Differential induction of immunoglobulin G subclasses by immunization with DNA vectors containing or lacking a signal sequence. Immunol. Lett. 61:201-204. [DOI] [PubMed] [Google Scholar]
- 14.Haesebrouck, F., F. Pasmans, K. Chiers, D. Maes, R. Ducatelle, and A. Decostere. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100:255-268. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, T., and F. C. Minion. 1998. Identification of the cilium binding epitope of the Mycoplasma hyopneumoniae P97 adhesin. Infect. Immun. 66:4762-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins, C., J. L. Wilton, F. C. Minion, L. Falconer, M. J. Walker, and S. P. Djordjevic. 2006. Two domains within the Mycoplasma hyopneumoniae cilium adhesin bind heparin. Infect. Immun. 74:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, M. F., M. B. Heidari, S. J. Stull, M. A. McIntosh, and K. S. Wise. 1990. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae p65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect. Immun. 58:2637-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King, K. W., D. H. Faulds, E. L. Rosey, and R. J. Yancey, Jr. 1997. Characterization of the gene encoding Mhp1 from Mycoplasma hyopneumoniae and examination of Mhp1's vaccine potential. Vaccine 15:25-35. [DOI] [PubMed] [Google Scholar]
- 19.Kobisch, M., and N. F. Friis. 1996. Swine mycoplasmoses. Res. Sci. Tech. Off. Int. Epiz. 15:1569-1605. [DOI] [PubMed] [Google Scholar]
- 20.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 21.Lemiale, F., W. P. Kong, L. M. Akyurek, X. Ling, Y. Huang, B. K. Chakrabarti, M. Eckhaus, and G. J. Nabel. 2003. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 77:10078-10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzo, H., O. Quesada, P. Assuncao, A. Castro, and F. Rodriguez. 2006. Cytokine expression in porcine lungs experimentally infected with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 109:199-207. [DOI] [PubMed] [Google Scholar]
- 23.Meens, J., M. Selke, and G. F. Gerlach. 2006. Identification and immunological characterization of conserved Mycoplasma hyopneumoniae lipoproteins Mhp378 and Mhp651. Vet. Microbiol. 25:85-95. [DOI] [PubMed] [Google Scholar]
- 24.Minion, F. C., C. Adams, and T. Hsu. 2000. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 68:3056-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neutra, M. R., and P. A. Kozlowski. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148-158. [DOI] [PubMed] [Google Scholar]
- 26.Okada. M., T. Asai, M. Ono, T. Sakano, and S. Sato. 2000. Cytological and immunological changes in bronchoalveolar lavage fluid and histological observation of lung lesions in pigs immunized with Mycoplasma hyopneumoniae inactivated vaccine prepared from broth culture supernate. Vaccine 18:2825-2831. [DOI] [PubMed] [Google Scholar]
- 27.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romieu-Mourez, R., M. Solis, A. Nardin, D. Goubau, V. Baron-Bodo, R. Lin, B. Massie, M. Salcedo, and J. Hiscott. 2006. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res. 66:10576-10585. [DOI] [PubMed] [Google Scholar]
- 29.Ross, R. F. 1992. Mycoplasmal diseases, p. 537-551. In A. D. Leman, B. Straw, W. Mengeling, S. D'Allaire, and D. Taylor (ed.), Diseases of swine, 7th ed. Iowa State University Press, Ames.
- 30.Sarradell, J., M. Andrada, A. S. Ramirez, A. Fernandez, J. C. Gomez-Villamandos, A. Jover, H. Lorenzo, P. Herraez, and F. Rodriguez. 2003. A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet. Pathol. 40:395-404. [DOI] [PubMed] [Google Scholar]
- 31.Sheldrake, R. F., L. F. Romalis, and M. M. Saunders. 1993. Serum and mucosal antibody responses against Mycoplasma hyopneumoniae following intraperitoneal vaccination and challenge of pigs with M. hyopneumoniae. Res. Vet. Sci. 55:371-376. [DOI] [PubMed] [Google Scholar]
- 32.Shimoji, Y., E. Oishi, Y. Muneta, H. Nosaka, and Y. Mori. 2003. Vaccine efficacy of the attenuated Erysipelothrix rhusiopathaie YS-19 expressing a recombinant protein of Mycoplasma hyopneumoniae P97 adhesin against mycoplasmal pneumonia of swine. Vaccine 21:532-537. [DOI] [PubMed] [Google Scholar]
- 33.Simecka, J. W. 2005. Immune responses following mycoplasma infection, p. 485-534. In A. Blanchard and G. Browing (ed.), Mycoplasma: molecular biology, pathogenicity and strategies for control. CRC Press, Oxford, United Kingdom.
- 34.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 35.Strasser, M., J. Frey, G. Bestetti, M. Kobisch, and J. Nicolet. 1991. Cloning and expression of a species-specific early immunogenic 36-kilodalton protein of Mycoplasma hyopneumoniae in Escherichia coli. Infect. Immun. 59:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatsis, N., and H. C. Ertl. 2004. Adenovirus as vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thacker, E. L., B. J. Thacker, M. Kuhn, P. A. Hawkins, and W. R. Waters. 2000. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 61:1384-1389. [DOI] [PubMed] [Google Scholar]
- 38.Thacker, E. L., P. G. Halbur, R. F. Ross, R. Thanawongnuwech, and B. J. Thacker. 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37:620-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vercammen, M., T. Scorza, K. Huygen, J. De Braekeleer, R. Diet, D. Jacobs, E. Saman, and H. Verscheren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker, J., R. Lee, N. Mathy, S. Doughty, and J. Conlon. 1996. Restricted B-cell responses to microbial challenge of the respiratory tract. Vet. Immunol. Immunopathol. 54:197-204. [DOI] [PubMed] [Google Scholar]
- 41.Wilton, J. L., A. L. Scarman, M. J. Walker, and S. P. Djordjevic. 1998. Reiterated repeat region variability in the ciliary adhesin gene of Mycoplasma hyopneumoniae. Microbiology 144:1931-1943. [DOI] [PubMed] [Google Scholar]
- 42.Wizemann, T. M., J. E. Adamou, and S. Langermann. 1999. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 5:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang, Z., Y. Li, G. Gao, J. M. Wilson, and H. C. Ertl. 2003. Mucosally delivered E1-deleted adenoviral vaccine carriers induce transgene product-specific antibody responses in neonatal mice. J. Immunol. 171:4287-4293. [DOI] [PubMed] [Google Scholar]
- 44.Yang, W. F., J. F. Lai, K. C. Peng, H. J. Chiang, C. N. Weng, and D. Shiuan. 2005. Epitope mapping of Mycoplasma hyopneumoniae using phage displayed peptide libraries and the immune responses of the selected phagotopes. J. Immunol. Methods. 304:15-29. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielinski, G. C., and R. F. Ross. 1993. Adherence of Mycoplasma hyopneumoniae to porcine ciliated respiratory tract cells. Am. J. Vet. Res. 54:1262-1269. [PubMed] [Google Scholar]