Abstract
Development of vaccines against cytomegalovirus (CMV) is an important public health priority. We used a propagation-defective, single-cycle RNA replicon vector system derived from an attenuated strain of an alphavirus, Venezuelan equine encephalitis virus, to produce virus-like replicon particles (VRP) expressing various combinations of pp65, IE1, or gB proteins of human CMV. Protein expression in VRP-infected cells was highest with single-promoter replicons expressing pp65, IE1, a pp65/IE1 fusion protein, or the extracellular domain of gB and with double-promoter replicons expressing pp65 and IE1. Protein expression was lower with double- and triple-promoter replicons expressing gB, especially the full-length form of gB. BALB/c mice immunized with VRP expressing gB developed high titers of neutralizing antibody to CMV, and mice immunized with VRP expressing pp65, IE1, or a pp65/IE1 fusion protein developed robust antigen-specific T-cell responses as measured by gamma interferon enzyme-linked immunospot assay. Three overlapping immunodominant pp65 peptides contained a nine-amino-acid sequence (LGPISGHVL) that matches the consensus binding motif for a major histocompatibility complex H2-Dd T-cell epitope. These data provide the basis for further development and clinical evaluation of an alphavirus replicon vaccine for CMV expressing the pp65, IE1, and gB proteins.
Cytomegalovirus (CMV) is a betaherpesvirus that causes a life-long infection and can result in significant morbidity and mortality in individuals with impaired or immature immune systems. CMV disease is usually manifested as pneumonia, hepatitis, and an increased risk of graft failure in solid-organ and hematopoietic stem cell transplant recipients (17, 46), and congenital CMV infection is an important cause of congenital deafness and cognitive and motor functional impairment (3, 10). Existing drugs for the treatment or prevention of CMV disease are only partially effective, have a variety of side effects, and may fail because of drug resistance mutations (26, 45). An effective CMV vaccine would provide a great medical benefit and would also result in multibillion-dollar annual net savings in the cost of caring for persons with CMV disease (37).
Protective immunity to CMV involves both humoral and cellular mechanisms. Passive transfer of serum containing high titers of antibody to CMV reduces the risk of CMV disease in solid-organ transplant recipients (11, 35), adoptive transfer of cytotoxic T-lymphocyte clones specific for CMV antigens reconstitutes cellular immunity and prevents CMV viremia and CMV disease in bone marrow transplant recipients (41), and the risk of symptomatic CMV disease at birth and of neurological sequelae developing over the ensuing years is lower in infants born to mothers with preexisting immunity (12). Serum from CMV-seropositive individuals has virus-neutralizing activity in vitro, the principal target of which is the major CMV glycoprotein gB (5, 36). CMV-seropositive individuals also have a high frequency of CMV-specific CD8+ cytotoxic T-lymphocyte responses, the principal targets of which are a phosphoprotein with an apparent molecular mass of 65 kDa (pp65) and an immediate-early protein with a molecular mass of 72 kDa (IE1) (15). CMVs have strict species specificity, which has precluded the use of human CMV in animal models, but murine CMV and guinea pig CMV have been used to evaluate vaccine strategies in these host species. Protection against a CMV challenge had been demonstrated after immunization of mice with DNA vaccines containing genes homologous to pp65 or IE1 or immunization of guinea pigs with purified gB protein or with an alphavirus replicon vaccine expressing a pp65 homolog (4, 13, 25, 32).
As described below, we have used a propagation-defective, single-cycle RNA replicon vector system derived from an attenuated strain of an alphavirus, Venezuelan equine encephalitis (VEE) virus, to produce virus-like replicon particles (VRP) expressing human CMV pp65, IE1, or gB protein. These studies focused on the construction and in vitro and in vivo characterization of potential vaccine candidates to be composed of one or, at most, two VRP constructs to express the selected three CMV gene products. The constructs included single-, double-, and triple-promoter replicons that expressed the individual CMV proteins or a pp65/IE1 fusion protein. Although protein expression levels and VRP production yields varied among the constructs, all of the constructs tested in mice were immunogenic, inducing neutralizing antibody and antigen-specific T-cell responses, providing the basis for further development and clinical evaluation of an alphavirus replicon vaccine for CMV.
MATERIALS AND METHODS
Source of CMV genes and construction of replicon vector plasmids.
For isolation of CMV DNA, MRC-5 cells (CCL-171; ATCC, Manassas, VA) were grown in T75 flasks to ∼90% confluence in complete medium (minimum essential medium with Earle's salts [Invitrogen, Carlsbad, CA] supplemented with nonessential amino acids, l-glutamine, and 10% fetal bovine serum [FBS; HyClone, Logan, UT]) and then infected with CMV strain AD169 (ATCC VR538) or Towne (ATCC VR977). After 4 days of infection, cells were recovered by scraping and DNA was isolated with the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA).
The alphavirus replicon vector plasmids used in these studies were pERK and pERK3. The pERK replicon vector was derived from pVR21 (30) and modified to express a kanamycin resistance gene and to contain a multiple cloning site immediately downstream of the subgenomic 26S promoter. The pERK3 replicon vector plasmid was derived from pERK by adding an extended multiple cloning site to facilitate the insertion of multiple genes, each downstream of a 26S promoter, into the same replicon plasmid.
For recombinant plasmid construction, the genes coding for the CMV pp65, IE1, and gB proteins were amplified by PCR from viral DNA and cloned into alphavirus replicon vector plasmids. For each gene, the sense primer started with the G corresponding to the AUG start codon and the antisense primer contained a PacI restriction site downstream of the stop codon.
The pp65 gene was amplified from strain AD169 viral DNA with sense primer 5′-GGAGTCGCGCGGTCGCCGT and antisense primer 5′-GAGTTTAATTAAGCGCGTGCGGCGGGTGGCTCAACC (where the PacI restriction site is underlined and the stop codon is in bold). The amplicon containing the pp65 gene (GenBank accession no. EF531301) was digested with PacI and ligated into the pERK plasmid digested with EcoRV and PacI, and the recombinant plasmid was used to transform competent Escherichia coli cells (XL2-blue; Stratagene, La Jolla, CA).
The IE1 gene open reading frame is encoded by exons 2, 3, and 4 and was constructed by amplification of the individual exons from AD169 viral DNA with primers that created an overlap, followed by denaturation and annealing of the three fragments and reamplification of the assembled complete open reading frame. The primer pairs used were 5′-GGAGTCCTCTGCCAAGAGAAAGATG and 5′-TGGCCTTGGTCACGGGTGTCTCGGGCCGTGGCACCTTGGAGGAAGGGCCCT for exon 2, 5′-GCCCGAGACACCCGTGACCA and 5′-CACTCGAACCTTAATCTGTTTGACGAGTTCTGCCAGGACATCT for exon 3, and 5′-TCAAACAGATTAAGGTTCGAGTGGACATGGT and 5′-GCCTTAATTAATTACTGGTCAGCCTTGCTTC (where the PacI restriction site is underlined and the stop codon is in bold) for exon 4. The amplicon containing the full-length IE1 gene (GenBank accession no. EF531302) was digested with PacI and ligated into the pERK3 plasmid digested with EcoRV and PacI, and the recombinant plasmid was used to transform Top 10 cells (Invitrogen).
The gB gene was amplified from Towne viral DNA with sense primer 5′-GGAATCCAGGATCTGGTGCC and antisense primer 5′-TCTCTTAATTAATTCAGACGTTCTCTTCTTCGTCGGAGTC (where the PacI restriction site is underlined and the stop codon is in bold). The amplicon containing the gB gene (GenBank accession no. EF531303) was digested with PacI and ligated into the pERK plasmid digested with EcoRV and PacI, and the recombinant plasmid was used to transform XL2-blue cells. DNA sequencing identified a single nucleotide difference from the published sequence that resulted in an Ile-156→Val change. A truncated form of the gB gene (TrgB), encoding the extracellular portion of gB, was amplified from cloned gB DNA with the sense primer used to amplify the full-length clone and antisense primer 5′-TGACTTAATTAATCACTGCTTATACGAATTGAACTCGCGC. The antisense primer introduced a stop codon (in bold) immediately following the Gln-692 codon (7), followed by a PacI restriction site (underlined). The amplicon containing the gene for TrgB (GenBank accession no. EF531305) was digested with PacI and ligated into the pERK3 plasmid digested with EcoRV and PacI, and the recombinant plasmid was used to transform Top 10 cells.
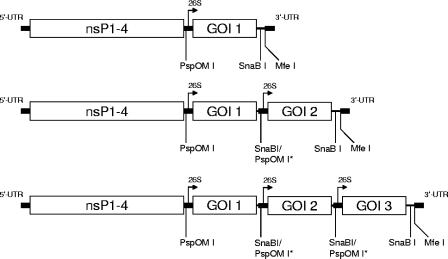
Replicon vectors expressing two or three CMV genes were constructed from single-promoter replicon plasmids. To create a double-promoter replicon vector expressing two CMV genes from two subgenomic promoters, each gene cassette was isolated after digestion with PspOMI and MfeI (Fig. 1). The PspOMI site was filled in with T4 DNA polymerase (New England BioLabs, Ipswich, MA), followed by DNA purification and digestion with MfeI. A replicon plasmid containing the first CMV gene was digested with SnaBI and MfeI and ligated with the second gene cassette to produce a double-promoter expression construct (Fig. 1). A third CMV gene under the control of a third subgenomic promoter was constructed in a similar fashion.
FIG. 1.
Schematic representation of replicon cDNAs designed to express one, two, or three CMV proteins. UTR, untranslated region; nsP, nonstructural protein; 26S, subgenomic promoter; GOI, gene of interest. Key restriction enzyme sites are indicated. PspOMI sites that were filled in with T4 DNA polymerase are indicated as PspOMI*.
A pp65/IE1 fusion gene was constructed by amplification of the genes for pp65 and IE1 from the pp65-IE1 double-promoter replicon plasmid with primers that created an overlap, followed by denaturation and annealing of the two fragments and reamplification of the assembled fusion gene. The sense primer for the pp65 portion (primer 1, 5′-GCAAACGCAAATCAGCA) was located at nucleotide 1303 of the pp65 gene, just upstream of a NotI sequence in the pp65 gene. The antisense primer for the pp65 portion (5′-CATCGCCTCGACGCCCAAAAAGCACCGAGGTGAGTCCTCTGCCAAGAGAAAGATGGACCCT) contained the 3′ end of the pp65 gene without the stop codon (in bold) and the 5′ end of the IE1 gene without the start codon. The sense primer for the IE1 portion (5′-GAGTCCTCTGCCAAGAGAAAGAT) contained the 5′ end of the IE1 gene without the start codon. The antisense primer for IE1 (primer 2, 5′-TAAGAGCCGCGAGCGATCCT) was located downstream of the NotI sequence in the vector. Following initial DNA amplification, these pp65 and IE1 amplicons were combined, annealed, and reamplified with the 5′ primer used to amplify pp65 (primer 1) and the 3′ primer used to amplify IE-1 (primer 2) to create an in-frame fusion gene. The amplicon containing the pp65/IE1 fusion gene (GenBank accession no. EF531304) was digested with NotI, ligated into the pp65-IE1 double-promoter replicon plasmid that had been digested with NotI (resulting in excision of 5′ sequences from the pp65 gene, the second promoter, the entire IE1 gene, and downstream vector sequences), and treated with calf intestine alkaline phosphatase (New England BioLabs), and the recombinant plasmid was used to transform Top 10 cells.
For expression of proteins used to generate antibody reagents, replicon plasmids expressing pp65, IE1, or TrgB, each with a C-terminal His6 tag, were engineered in the pERK vector, with primers modified to include six histidine codons before the stop codon.
All recombinant plasmid clones were analyzed by restriction endonuclease digestion and DNA sequence analysis of the region containing the CMV gene to verify authenticity.
Antibody reagents.
Purified, His6-tagged pp65, IE1, and TrgB were produced for use as immunogens to generate antigen-specific antibodies. The proteins were expressed in baby hamster kidney cells (CCL-10; ATCC) after electroporation with replicon RNA transcribed in vitro from replicon plasmids with a T7 RiboMax RNA transcription kit (Promega, Madison, WI) and purified with an RNeasy kit (QIAGEN). Cells were incubated overnight in complete medium for pp65 and IE1 or OptiPRO SFM (Invitrogen) for TrgB. For a description of the protein purification method used, see the supplemental material. As assessed by Coomassie blue (pp65) or silver (IE1 and gB) staining of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels, the purity of the His-tagged proteins was approximately 95%.
Goats were immunized at Pocono Rabbit Farms and Laboratories (Canadensis, PA) with purified, His6-tagged pp65, IE1, or TrgB protein mixed with complete Freund's adjuvant (first inoculation) and incomplete Freund's adjuvant (subsequent inoculations) at approximately 2-week intervals. Starting 6 weeks after the priming immunization, serum was obtained every 1 to 2 weeks and tested by indirect immunofluorescence and Western blot assays with VRP-infected cells and cell lysates, respectively. After immunized animals were shown to have high titers of anti-pp65, anti-IE1, or anti-gB antibodies, final bleeds were performed to obtain hyperimmune serum specific for one of the three CMV proteins.
VRP production.
VRP were prepared by a modification of previously described methods (30). Briefly, helper RNAs, coding for the VEE virus capsid and envelope proteins, and replicon RNA, coding for the CMV proteins, were separately transcribed in vitro with T7 RiboMax RNA transcription kits, purified with RNeasy kits, and coelectroporated into CHO K1 cells (CCL-61; ATCC) or Vero cells (BioReliance, Manassas, VA) with a BTX ECM-820 electroporation unit (Harvard Apparatus, Holliston, MA). VRP were harvested 20 to 24 h after electroporation by elution from cells with a buffer containing 0.5 M NaCl and 20 mM Na phosphate, pH 7.2. For Western blot analysis, VRP were used without further purification. For silver staining of SDS-PAGE gels, VRP were purified by capture on a HiTrap Heparin HP column (GE Healthcare, Piscataway, NJ) and elution with a linear 0 to 1 M NaCl gradient. The VRP concentration, expressed in infectious units (IU) per milliliter, was determined by an antigen-specific immunofluorescence assay in which serial dilutions of VRP were added to a monolayer of Vero cells, cultured overnight, and reacted with goat antibody specific for gB, pp65, or IE1, followed by fluorescein isothiocyanate-labeled anti-goat antibody to detect cells expressing the CMV protein.
Protein expression in VRP-infected cells.
Vero cells seeded in 48-well tissue culture plates (1 × 105 to 2 × 105 cells/well) were infected with VRP at a multiplicity of infection of 10 IU/cell and incubated in OptiPro SFM for 18 to 22 h. Cell monolayers were washed three times with cold phosphate-buffered saline, lysed in 150 μl of extraction buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.5% SDS, 1× Complete Protease Inhibitor [Roche, Indianapolis, IN]), and normalized by protein content with the bicinchoninic acid protein assay kit (Pierce, Rockford IL), and 1 μg of total protein per lane was resolved by 4 to 12% gradient SDS-PAGE (Invitrogen). Proteins were visualized by silver staining as recommended by the manufacturer (Invitrogen) or by Western blot analysis after transfer onto polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). For Western analysis, immunoblots were probed with a murine monoclonal antibody specific for pp65 (Rumbaugh-Goodwin Institute, Plantation, FL) or goat polyclonal antibodies specific for IE1 or gB, followed by alkaline phosphatase-conjugated species-specific secondary antibody (Sigma, St. Louis, MO, or Invitrogen), followed by color development with an alkaline phosphatase conjugate substrate kit (Bio-Rad). In some experiments, cell lysates were treated with peptide-N-glycosidase F (PNGase F; Sigma) to cleave N-linked oligosaccharides from gB prior to SDS-PAGE and Western blot analysis.
Animal immunization.
Six- to 7-week-old female BALB/c mice were obtained from Charles River Laboratories, Raleigh, NC, and acclimated for 1 week prior to any procedure. Mice were housed in microisolator cages at Integrated Laboratory Systems (Research Triangle Park, NC) or Piedmont Research Center (Morrisville, NC) and fed standard rodent chow. Groups of mice were immunized at weeks 0 and 3 and boosted at various times thereafter, under light isoflurane anesthesia, by subcutaneous injection in both rear footpads with 1 × 106 IU of VRP in diluent (phosphate-buffered saline containing 5% sucrose [Mallinckrodt, Paris, KY] and either 1% [vol/vol] human serum albumin [Instituto Grifols, Barcelona, Spain] or 0.1% normal mouse serum [BioMeda, Foster City, CA]). Footpad injections (20 μl per site) were performed with a 30-gauge needle and a 0.1-ml repeating syringe (Hamilton, Reno, NV). Control animals were inoculated with diluent alone or with VRP expressing an irrelevant antigen. Serum samples were obtained by retroorbital bleeding before immunization, 20 days after the first immunization and 7 days after subsequent immunizations. Spleens were harvested at least 7 days after the last immunization.
CMV neutralization assay.
A virus neutralization assay was developed with a mink lung cell line, FV-ELVIRA-CMV (Diagnostic Hybrids Inc., Athens, OH), that had been transformed with DNA containing a Sindbis virus replicon expressing a β-galactosidase gene (SinRep/LacZ) under the control of an early CMV promoter (18). Human CMV carries out an abortive infection in mink lung cells but is able to induce the SinRep/LacZ replicon to produce β-galactosidase, which can be detected by histochemical staining or a colorimetric assay (see the supplemental material for details of the methods used). To determine the serum neutralizing-antibody titer, a standard amount of Towne strain CMV was mixed with ELVIRA overlay medium (Diagnostic Hybrids) containing 10% guinea pig complement (Cedarlane Laboratories, Hornby, Ontario, Canada), added to an equal volume of serial dilutions of heat-inactivated (56°C, 30 min) test serum in minimum essential medium with Earle's salts with 10% heat-inactivated FBS supplemented with nonessential amino acids and penicillin-streptomycin, and incubated overnight at 2 to 8°C. The serum-CMV-complement mixtures were added to monolayers of FV-ELVIRA-CMV cells and cultured overnight at 37°C in 5% CO2, and responses to infection were assessed by β-galactosidase expression in the colorimetric assay. The endpoint titer was the last serum dilution at which there was at least a 50% reduction in β-galactosidase expression.
Peptides.
Synthetic peptides spanning the CMV pp65 or IE1 protein (15-mers overlapping by 11 amino acids) were obtained as a gift from Skip Maino, Becton Dickinson, San Jose, CA (pp65) or purchased from SynPep, Dublin, CA (IE1) and had a purity of >90% as determined by high-performance liquid chromatography. Lyophilized peptides were resuspended in 100% dimethyl sulfoxide at 100 mg/ml, stored at −80°C, and diluted in R-10 medium (RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 0.01 M HEPES, 2 mM glutamine, and 10% heat-inactivated FBS) for use. Dimethyl sulfoxide concentrations in all assay mixtures were ≤0.1% (vol/vol).
ELISPOT assay.
A gamma interferon enzyme-linked immunospot (IFN-γ ELISPOT) assay was used to quantify the number of antigen-specific cytokine-secreting T cells in animals immunized with VRP expressing pp65 or IE1 (see the supplemental material for details of the methods used). The mean number of spot-forming cells (SFC) from duplicate wells, after subtraction of counts from cells cultured with no peptide, was determined for each animal. A response was considered positive if this value was greater than 20 SFC/106 splenic lymphocytes. In five experiments (73 values), the mean number of SFC from the no-peptide control wells was 0.23 ± 0.84 SFC/106 splenic lymphocytes (range, 0 to 4).
For some experiments, splenic lymphocytes were separated into CD8-enriched and CD8-depleted populations with a negative-selection CD8+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA) and compared in IFN-γ ELISPOT assays with unseparated bulk splenic lymphocyte preparations.
Epitope mapping was performed with pools of pp65 and IE1 peptides as described by Kern et al. (19).
Statistical analysis.
Statistical analysis was performed with Microsoft Excel with Analysis ToolPack installed. Groups were compared by single-factor analysis of variance to detect differences in immune responses, and multiple comparisons were performed with the Tukey test (47).
RESULTS
VRP production.
VRP expressing the CMV pp65, IE1, and gB (full-length and truncated) proteins were produced from single-, double-, and triple-promoter replicons (Table 1). The VRP yield was lower for replicons expressing full-length gB compared with replicons containing a truncated gene expressing the extracellular portion of gB (TrgB) and was especially low with triple-promoter constructs expressing full-length gB (data not shown).
TABLE 1.
VRP vaccines produced with alphavirus replicons expressing CMV genes
| VRP designationa | CMV gene 1 | CMV gene 2 | CMV gene 3 |
|---|---|---|---|
| pp65 | pp65 | ||
| IE1 | IE1 | ||
| gB | gB | ||
| TrgB | TrgB | ||
| pp65+IE1 dp | pp65 | IE1 | |
| pp65+gB dp | pp65 | gB | |
| IE1+pp65 dp | IE1 | pp65 | |
| IE1+gB dp | IE1 | gB | |
| pp65/IE1 fusion | pp65/IE1 | ||
| pp65/IE1 fusion+gB dp | pp65/IE1 | gB | |
| pp65/IE1 fusion+TrgB dp | pp65/IE1 | TrgB | |
| pp65+gB IE1+tp | pp65 | gB | IE1 |
| IE1+gB+pp65 tp | IE1 | gB | pp65 |
| IE1+pp65+gB tp | IE1 | pp65 | gB |
| pp65+IE1+gB tp | pp65 | IE1 | gB |
| pp65+IE1+TrgB tp | pp65 | IE1 | TrgB |
TrgB, truncated gB; dp, double promoter; tp, triple promoter.
Protein expression from VRP-infected cells.
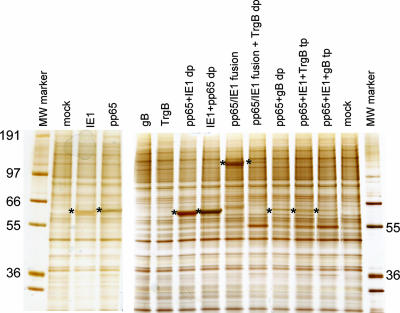
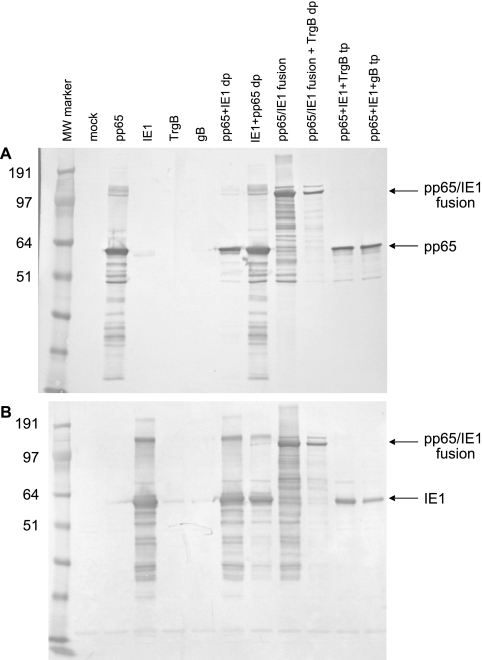
SDS-PAGE analysis of proteins extracted from Vero cells infected with VRP for 18 to 22 h showed high-level expression of pp65, IE1, or a pp65/IE1 fusion protein, as assessed by silver staining (Fig. 2) or Western blot analysis (Fig. 3). Expression of these two proteins was generally similar when they were expressed alone or from a double-promoter replicon but was reduced when they were expressed from triple-promoter replicons expressing full-length gB or TrgB. Expression of the pp65/IE1 fusion protein was reduced when it was expressed from a double-promoter replicon in which a gene encoding TrgB was expressed from the second promoter.
FIG. 2.
Analysis of protein expression in Vero cells infected with VRP expressing CMV antigens by SDS-PAGE and silver staining. VRP designations are as in Table 1. The positions of pp65, IE1, and the pp65/IE1 fusion protein are indicated by asterisks. The values on the left and right are molecular weights (MW) in thousands.
FIG. 3.
Western blot analysis of protein expression in Vero cells infected with VRP expressing pp65 or IE1. (A) Immunoblots probed with a murine monoclonal antibody specific for pp65. (B) Immunoblots probed with polyclonal goat antibodies specific for IE1. VRP designations are as in Table 1. The values on the left are molecular weights (MW) in thousands.
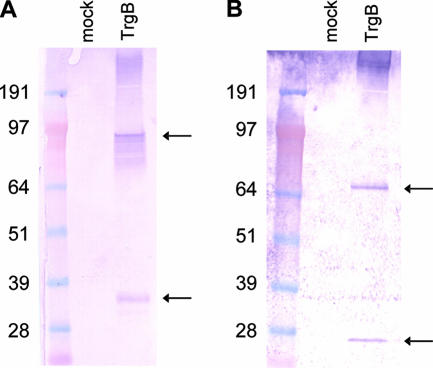
SDS-PAGE analysis of proteins extracted from Vero cells infected with VRP for 18 to 22 h showed high-level expression of TrgB (Fig. 4) and lower levels of expression of full-length gB (data not shown). Under reducing conditions, TrgB was cleaved into two immunoreactive fragments, presumably at the consensus furin cleavage site (39), and treatment with PNGase F resulted in faster migration of more tightly focused bands, consistent with N-linked glycosylation of both fragments (Fig. 4). Expression of TrgB was reduced when it was expressed from a double-promoter replicon in which pp65 or a pp65/IE1 fusion protein was expressed from the first promoter (data not shown).
FIG. 4.
Western blot analysis of protein expression in Vero cells infected with VRP expressing TrgB. Immunoblots were prepared under reducing conditions with cell lysates from mock-infected cells (lane 1 in each panel) or VRP-infected cells (lane 2 in each panel) either with no glycosidase treatment (A) or after treatment with PNGase F (B) and probed with polyclonal goat antibodies specific for gB. The positions of two immunoreactive cleavage fragments are indicated by the arrows. The values on the left are molecular weights in thousands.
Antibody response to VRP expressing gB.
In preliminary experiments, colorimetric and microscopic assays of cells infected with serial dilutions of CMV demonstrated a good correlation (R2 = 0.9996) between the two assays (see Fig. S1A in the supplemental material). The colorimetric assay was subsequently used to measure neutralization of CMV infection by sera from mice immunized with either TrgB VRP or full-length gB VRP. For a representative example with individual mouse sera, see Fig. S1B in the supplemental material; results from groups of mice immunized with different VRP are shown in Table 2. Within each group of mice, mean reciprocal titers were higher after the third dose than after the second dose, and the highest titers were achieved in mice immunized with VRP expressing the extracellular form of gB from a single-promoter replicon. For comparison, serum samples from two CMV-seropositive humans were tested in this assay and had titers of 320 and 640.
TABLE 2.
CMV neutralization by serum from mice immunized with VRP expressing CMV gB
| VRP vaccine | CMV neutralization titer (mean ± SEM)a
|
||
|---|---|---|---|
| Day −1 | Day 28 | Day 63 | |
| gB | <40 | 93 ± 13 | 400 ± 107 |
| TrgB | <40 | 253 ± 43b | 553 ± 67c |
| pp65+gB dp | <40 | 73 ± 7 | 240 ± 36 |
| pp65+IE1+gB tp | <40 | 60 ± 9 | 200 ± 40c |
| pp65+IE1+TrgB tp | <40 | 140 ± 41 | 373 ± 89 |
| pp65/IE1 fusion+TrgB dp | <40 | 133 ± 17 | 267 ± 79 |
Mice (six per group) were immunized with the indicated vaccines at days 0, 21, and 56, and serum was obtained on days 28 and 63. The neutralization titer is the reciprocal of the serum dilution that resulted in a 50% reduction in CMV infectivity. VRP designations are as in Table 1. The titers in this assay were 320 and 640 for serum samples from two CMV-seropositive humans.
At day 28, the antibody response in the group immunized with TrgB VRP was significantly different (P < 0.05) from those of all other groups, but other differences between the groups were not statistically significant.
At day 63, the antibody response in groups immunized with TrgB VRP or pp65+IE1+gB tp VRP were significantly different (P < 0.05) from one another, but other differences between the groups were not statistically significant.
Cellular immune response to VRP expressing pp65 or IE1.
In initial experiments, splenic lymphocytes from mice immunized with VRP expressing pp65 or IE1 from a single-promoter replicon were evaluated by IFN-γ ELISPOT assay with pools of overlapping peptides that span the pp65 protein (138 peptides) or the IE1 protein (120 peptides). Splenic lymphocytes collected 30 days after administration of the third dose to mice immunized on days 1, 22, and 98 showed robust T-cell responses, with mean numbers of SFC per 106 splenic lymphocytes of 1,490 for pp65 and 363 for IE1. Mean responses in wells containing cells treated with concanavalin A or an irrelevant peptide were 638 and 0.6 SFC/106 splenic lymphocytes, respectively.
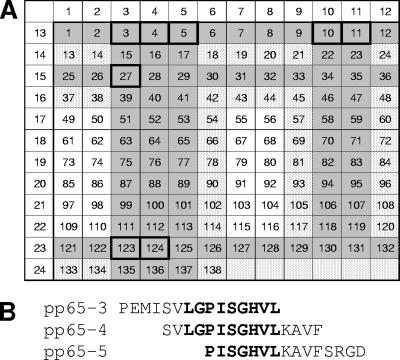
To facilitate the identification of potential T-cell epitopes in pp65, the 138 overlapping peptides were distributed among 24 pools arranged in a 12 by 12 matrix such that each peptide was contained in exactly two pools (Fig. 5A). In a first experiment in which the 24 pools were combined into 12 larger pools, 7 of 12 pools induced a positive IFN-γ ELISPOT response. In a second experiment in which 14 pools representing the 7 larger pools were tested, 7 of the 14 pools induced a positive IFN-γ ELISPOT response (see Table S1 in the supplemental material). When the 14 peptides identified by the intersection of these seven pools in the matrix (Fig. 5A) were tested individually, eight peptides representing at least four T-cell epitopes induced a positive IFN-γ ELISPOT response (Table 3). A dominant response was observed with peptides 3, 4, and 5, which contain a nine-amino-acid sequence (Fig. 5B) that matches the consensus binding motif for a major histocompatibility complex (MHC) H2-Dd T-cell epitope predicted by SYFPEITHI algorithms (http://www.syfpeithi.de/scripts/MHCServer.dll/home.htm) (31). Studies with a pool of the three peptides that contain the immunodominant pp65 epitope demonstrated positive IFN-γ ELISPOT responses in both the CD8-enriched and CD8-depleted populations (see Fig. S2 in the supplemental material).
FIG. 5.
Identification of immunodominant epitopes in pp65 and IE1. (A) Matrix arrangement of overlapping pp65 peptides. The shaded cells indicate peptides contained in single pools that stimulated T-cell responses, and the stippled cells indicate peptides contained in pairs of pools that stimulated T-cell responses. The individual peptides confirmed to induce T-cell responses are boxed. (B) Sequence of the pp65 peptides containing an immunodominant epitope. The putative H2-Dd T-cell epitope is in bold.
TABLE 3.
IFN-γ ELISPOT responses to individual peptides by splenic lymphocytes from BALB/c mice immunized with VRP expressing CMV pp65
| Peptide | Sequence | Responsea |
|---|---|---|
| 3 | PEMISVLGPISGHVL | 1,521 |
| 4 | SVLGPISGHVLKAVF | 1,871 |
| 5 | PISGHVLKAVFSRGD | 778 |
| 10 | HETRLLQTGIHVRVS | 76 |
| 11 | LLQTGIHVRVSQPSL | 69 |
| 27 | SQEPMSIYVYALPLK | 21 |
| 123 | AGILARNLVPMVATV | 44 |
| 124 | ARNLVPMVATVQGQN | 23 |
IFN-γ ELISPOT response (number of SFC/106 splenic lymphocytes, average of duplicates) by pooled splenic lymphocytes from groups of six mice immunized at days 0, 21, and 98 and sacrificed at day 189. The mean ± standard deviation response to the other six peptides tested was −3.6 ± 3.1 (range, −8 to 3). The response of cells alone with no peptide was 15.
To facilitate the identification of potential T-cell epitopes in IE1, the 120 overlapping peptides were distributed among 22 pools arranged in an 11 by 11 matrix such that each peptide was contained in exactly two pools. In a first experiment, 17 of the 24 pools induced a positive IFN-γ ELISPOT response (data not shown). When the 84 peptides identified by the intersection of these 17 pools in the matrix were tested individually, 16 peptides representing at least eight T-cell epitopes induced a positive IFN-γ ELISPOT response (see Table S2 in the supplemental material). None of these peptides contained a sequence that matches the consensus binding motif for an MHC H2-Kd or H2-Dd T-cell epitope predicted by the SYFPEITHI algorithms.
Subsequent studies used peptide pools containing immunodominant and subdominant pp65 and IE1 peptides to evaluate the cellular immune response in mice immunized with VRP expressing pp65 or IE1, alone or in combination with other CMV antigens. All of the constructs tested induced a robust cellular immune response to the immunizing antigen (Table 4).
TABLE 4.
IFN-γ ELISPOT responses to immunodominant and subdominant peptide pools by splenic lymphocytes from BALB/c mice immunized with VRP expressing CMV pp65 and IE1
| VRP vaccineb | pp65 responsea
|
IE1 responsea
|
||
|---|---|---|---|---|
| Dominant | Subdominant | Dominant | Subdominant | |
| pp65 | 1,134 ± 108 | 214 ± 32 | −0.7 ± 1.9 | −2.0 ± 2.8 |
| IE1 | −1 ± 0.1 | −1 ± 0.4 | 124 ± 26 | 330 ± 47 |
| pp65+IE1 dp | 1,736 ± 212 | 103 ± 16 | 96 ± 6 | 228 ± 35 |
| pp65+gB dp | 976 ± 109 | 119 ± 32 | 6 ± 2.4 | NTc |
| IE1+pp65 dp | 1,471 ± 168 | 214 ± 34 | 98 ± 11 | 165 ± 18 |
| pp65+IE1+gB tp | 1,039 ± 105 | 107 ± 23 | 42 ± 24 | 68 ± 33 |
| pp65+IE1+TrgB tp | 1,063 ± 100 | 105 ± 26 | 81 ± 20 | 139 ± 29 |
| pp65/IE1 fusion | 1,255 ± 144 | 134 ± 25 | 81 ± 19 | 149 ± 25 |
| pp65/IE1 fusion+TrgB dp | 640 ± 63 | 51 ± 16 | 29 ± 14 | 31 ± 17 |
IFN-γ ELISPOT response (mean ± standard deviation number of SFC/106 splenic lymphocytes) by splenic lymphocytes from individual mice (six per group) immunized at days 0, 21, and 56 and sacrificed at day 63. The dominant pools contained pp65 peptides 3, 4, and 5 or IE1 peptides 47 to 50, and the subdominant pools contained pp65 peptides 10, 11, 27, 123, and 124 or IE1 peptides 15, 38, 39, 53, 54, 86, 87, 103, 104, 119, and 120. The mean responses of cells alone with no peptide for the nine groups of animals were 7, 3, 7, 3, 3, 71, 10, 3, and 52, respectively.
VRP designations are as in Table 1.
NT, not tested.
Cellular immune responses were also measured 4 or 7 months after administration of a third dose of VRP. A mouse immunized with IE1 VRP at weeks 0, 3, and 26 and tested with a single immunodominant IE1 peptide at week 42 had an IFN-γ ELISPOT response of 199 SFC/106 splenic lymphocytes. A mouse immunized with pp65 VRP at weeks 0, 3, and 14 and tested with a single immunodominant pp65 peptide at week 42 had an IFN-γ ELISPOT response of 1,134 SFC/106 splenic lymphocytes.
DISCUSSION
The present study provides the first preclinical evaluation of novel alphavirus replicon vaccines for CMV. VRP containing CMV genes expressed high levels of CMV proteins in cells infected in vitro and induced robust antibody responses to gB and cellular immune responses to pp65 and IE1 in mice immunized in vivo.
Alphavirus replicon vaccines can be constructed to express multiple foreign proteins with multiple RNA promoters, and the level of foreign protein expression from multiple-promoter replicons can vary, depending on the specific combination of proteins being expressed. For example, in vitro expression of pp65 and IE1 in VRP-infected cells was similar in single- and double-promoter replicons when these two proteins were expressed together but expression of pp65 and IE1 was reduced when they were expressed with gB in triple-promoter replicons (Fig. 2 and 3). Expression of full-length gB or TrgB was also reduced when it was expressed with pp65 and IE1 in double- or triple-promoter replicons. However, the in vivo level of expression with all of the constructs tested was sufficient to induce robust humoral and cellular immune responses (Tables 2 and 4).
Neutralizing antibodies are considered to be important in protection against most viral pathogens, and two antibody-binding sites on the extracellular portion of gB have been identified as the principal target of neutralizing antibodies in serum from persons naturally infected with CMV: AD-1, located between amino acid residues 552 and 635, and AD-2, located between amino acid residues 50 and 78 (24, 40). When constructing the gB replicon plasmid, a PCR-generated mutation resulted in an amino acid substitution at position 155, a conservative substitution that is not in AD-1 or AD-2. Immunization of mice with VRP expressing either full-length gB or TrgB induced antibodies that neutralized CMV, and the magnitude of the neutralization titers was similar to that of the neutralization titers in serum from CMV-infected humans (Table 2).
Cellular immune responses are also important in protection against CMV. Although recent studies indicate that T-cell epitopes from a large number of CMV proteins are recognized by peripheral blood mononuclear cells from naturally infected individuals, pp65 and IE1 are two of the three proteins recognized by CD8+ T cells from the majority of CMV-infected individuals (38), and evidence from preclinical and clinical studies indicates that pp65 and IE1 are principal targets of protective cellular immune responses (6, 13, 25, 32, 41). Immunization of mice with VRP expressing pp65 or IE1, separately or as an in-frame fusion protein, induced robust cellular immune responses as measured by IFN-γ ELISPOT assay. Epitope mapping with overlapping peptides identified an immunodominant peptide in pp65 that matches the consensus binding motif for an MHC H2-Dd T-cell epitope and also identified three other regions of pp65 and eight regions of IE1 that were recognized by T cells from immunized mice but did not contain a consensus binding motif for an MHC H2-Kd or H2-Dd T-cell epitope. The immunodominant pp65 peptides apparently contained epitopes recognized by both class I and class II MHC molecules, since these peptides were recognized by both CD8-enriched and CD8-depleted splenic lymphocytes, and it is possible that the failure to detect a consensus binding motif for a class I MHC molecule with the other pp65 peptides and the IE1 peptides indicates that these other peptides are recognized by class II MHC molecules. Alternatively, these peptides may be examples of those that do not fit the binding motif but nonetheless are capable of interacting with sufficient strength to trigger a CD8 T-cell response (23). The cellular immune responses to both pp65 and IE1 were long lasting, as evidenced by the detection of memory T cells in IFN-γ ELISPOT assays 4 to 7 months after the last immunization.
Several alphavirus replicons have been evaluated as potential vaccine vector systems (33). The use of VRP based on VEE virus is especially attractive because they express heterologous proteins to high levels (30), target expression to dendritic cells (22), and are capable of inducing both humoral and cellular immune responses to the vectored gene products (1, 2, 8, 9, 16, 20, 21, 28-30, 34, 42-44). In previously published studies, alphavirus replicon vaccines derived from VEE virus were significantly more immunogenic than alphavirus replicon vaccines derived from Sindbis virus (27), and ex vivo infection of peripheral blood mononuclear cells from rhesus macaques is significantly more efficient with VRP derived from VEE virus than with VRP derived from Sindbis virus (14). In addition, unlike other vaccines based on viral vectors, anti-vector immunity does not appear to prevent the ability of VRP vaccines derived from VEE virus to induce potent immune responses. For example, titers of antibody to influenza virus HA and protection from influenza virus challenge after immunization with VRP expressing HA are similar in naive mice and in mice that had received two prior immunizations with VRP expressing Lassa fever virus nucleoprotein (30). Results from the present study also demonstrated enhancement of CMV-neutralizing antibody titers after administration of a booster dose (Table 2).
The choice of which alphavirus replicon vaccines are appropriate for further development and advancement toward clinical trials is based on a combination of factors, including expression levels in vitro, the ability to induce immune responses in vivo, and ease of manufacturing. Results from the present study indicate that a bivalent alphavirus replicon vaccine with one component expressing a pp65/IE1 fusion protein and the other expressing the extracellular domain of CMV gB has characteristics that make it suitable for further development and clinical evaluation.
Supplementary Material
Acknowledgments
We thank Robert Reid for technical assistance and Vernon Maino for providing pp65 peptides.
We were all employees of AlphaVax, Inc., when this research was conducted, and all of us, except Bryan Rivers, own stock in the company.
Studies involving the use of animals complied with all relevant federal guidelines and institutional policies.
Footnotes
Published ahead of print on 18 April 2007.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. David Wilson, I. K. Liu, and N. James MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. [DOI] [PubMed] [Google Scholar]
- 2.Balasuriya, U. B., H. W. Heidner, J. F. Hedges, J. C. Williams, N. L. Davis, R. E. Johnston, and N. J. MacLachlan. 2000. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:10623-10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. [DOI] [PubMed] [Google Scholar]
- 4.Bourne, N., M. R. Schleiss, F. J. Bravo, and D. I. Bernstein. 2001. Preconception immunization with a cytomegalovirus (CMV) glycoprotein vaccine improves pregnancy outcome in a guinea pig model of congenital CMV infection. J. Infect. Dis. 183:59-64. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. J., L. Vugler, and E. B. Stephens. 1988. Induction of complement-dependent and -independent neutralizing antibodies by recombinant-derived human cytomegalovirus gp55-116 (gB). J. Virol. 62:3309-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H. D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson, C., W. J. Britt, and T. Compton. 1997. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology 239:198-205. [DOI] [PubMed] [Google Scholar]
- 8.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, N. L., A. West, E. Reap, G. MacDonald, M. Collier, S. Dryga, M. Maughan, M. Connell, C. Walker, K. McGrath, C. Cecil, L. H. Ping, J. Frelinger, R. Olmsted, P. Keith, R. Swanstrom, C. Williamson, P. Johnson, D. Montefiori, and R. E. Johnston. 2002. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life 53:209-211. [DOI] [PubMed] [Google Scholar]
- 10.Demmler, G. J. 1994. Congenital cytomegalovirus infection. Semin. Pediatr. Neurol. 1:36-42. [PubMed] [Google Scholar]
- 11.Falagas, M. E., D. R. Snydman, R. Ruthazer, J. Griffith, B. G. Werner, R. Freeman, and R. Rohrer. 1997. Cytomegalovirus immune globulin (CMVIG) prophylaxis is associated with increased survival after orthotopic liver transplantation. Clin. Transplant. 11:432-437. [PubMed] [Google Scholar]
- 12.Fowler, K. B., S. Stagno, R. F. Pass, W. J. Britt, T. J. Boll, and C. A. Alford. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663-667. [DOI] [PubMed] [Google Scholar]
- 13.González Armas, J. C., C. S. Morello, L. D. Cranmer, and D. H. Spector. 1996. DNA immunization confers protection against murine cytomegalovirus infection. J. Virol. 70:7921-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, S., F. Zhou, C. E. Greer, H. Legg, T. Tang, P. Luciw, J. zur Megede, S. W. Barnett, J. J. Donnelly, D. T. O'Hagan, J. M. Polo, and M. Vajdy. 2006. Antibody responses against HIV in rhesus macaques following combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. AIDS Res. Hum. Retrovir. 22:993-997. [DOI] [PubMed] [Google Scholar]
- 15.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meri, S. Plotkin, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 16.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 17.Hibberd, P. L., and D. R. Snydman. 1995. Cytomegalovirus infection in organ transplant recipients. Infect. Dis. Clin. N. Am. 9:863-877. [PubMed] [Google Scholar]
- 18.Ivanova, L., S. Schlesinger, and P. D. Olivo. 1999. Regulated expression of a Sindbis virus replicon by herpesvirus promoters. J. Virol. 73:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kern, F., T. Bunde, N. Faulhaber, F. Kiecker, E. Khatamzas, I. M. Rudawski, A. Pruss, J. W. Gratama, R. Volkmer-Engert, R. Ewert, P. Reinke, H. D. Volk, and L. J. Picker. 2002. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J. Infect. Dis. 185:1709-1716. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. S., B. K. Dyas, S. S. Nystrom, C. M. Lind, J. F. Smith, and R. G. Ulrich. 2002. Immune protection against staphylococcal enterotoxin-induced toxic shock by vaccination with a Venezuelan equine encephalitis virus replicon. J. Infect. Dis. 185:1192-1196. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. S., P. Pushko, M. D. Parker, M. T. Dertzbaugh, L. A. Smith, and J. F. Smith. 2001. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infect. Immun. 69:5709-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985-2993. [PubMed] [Google Scholar]
- 24.Meyer, H., V. A. Sundqvist, L. Pereira, and M. Mach. 1992. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J. Gen. Virol. 73:2375-2383. [DOI] [PubMed] [Google Scholar]
- 25.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65). J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paya, C. V. 2001. Prevention of cytomegalovirus disease in recipients of solid-organ transplants. Clin. Infect. Dis. 32:596-603. [DOI] [PubMed] [Google Scholar]
- 27.Perri, S., C. E. Greer, K. Thudium, B. Doe, H. Legg, H. Liu, R. E. Romero, Z. Tang, Q. Bin, T. W. Dubensky, Jr., M. Vajdy, G. R. Otten, and J. M. Polo. 2003. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 77:10394-10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pushko, P., M. Bray, G. V. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and J. F. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 29.Pushko, P., J. Geisbert, M. Parker, P. Jahrling, and J. Smith. 2001. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J. Virol. 75:11677-11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 31.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 32.Schleiss, M. R., J. C. Lacayo, Y. Belkaid, A. McGregor, G. Stroup, J. Rayner, K. Alterson, J. D. Chulay, and J. F. Smith. 2007. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J. Infect. Dis. 195:789-798. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger, S. 2001. Alphavirus vectors: development and potential therapeutic applications. Expert Opin. Biol. Ther. 1:177-191. [DOI] [PubMed] [Google Scholar]
- 34.Schultz-Cherry, S., J. K. Dybing, N. L. Davis, C. Williamson, D. L. Suarez, R. Johnston, and M. L. Perdue. 2000. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology 278:55-59. [DOI] [PubMed] [Google Scholar]
- 35.Snydman, D. R., B. G. Werner, B. Heinze-Lacey, V. P. Berardi, N. L. Tilney, R. L. Kirkman, E. L. Milford, S. I. Cho, H. L. Bush, A. S. Levey, et al. 1987. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N. Engl. J. Med. 317:1049-1054. [DOI] [PubMed] [Google Scholar]
- 36.Speckner, A., D. Glykofrydes, M. Ohlin, and M. Mach. 1999. Antigenic domain 1 of human cytomegalovirus glycoprotein B induces a multitude of different antibodies which, when combined, results in incomplete virus neutralization. J. Gen. Virol. 80:2183-2191. [DOI] [PubMed] [Google Scholar]
- 37.Stratton, J. R., J. S. Durch, and R. S. Lawrence. 2000. Vaccines for the 21st century: a tool for decisionmaking. National Academy Press, Washington, DC. [PubMed]
- 38.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vey, M., W. Schafer, B. Reis, R. Ohuchi, W. Britt, W. Garten, H. D. Klenk, and K. Radsak. 1995. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology 206:746-749. [DOI] [PubMed] [Google Scholar]
- 40.Wagner, B., B. Kropff, H. Kalbacher, W. Britt, V. A. Sundqvist, L. Ostberg, and M. Mach. 1992. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J. Virol. 66:5290-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, J. A., M. Bray, R. Bakken, and M. K. Hart. 2001. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology 286:384-390. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, J. A., and M. K. Hart. 2001. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 75:2660-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson, J. A., M. Hevey, R. Bakken, S. Guest, M. Bray, A. L. Schmaljohn, and M. K. Hart. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664-1666. [DOI] [PubMed] [Google Scholar]
- 45.Zaia, J. A. 2002. Prevention of cytomegalovirus disease in hematopoietic stem cell transplantation. Clin. Infect. Dis. 35:999-1004. [DOI] [PubMed] [Google Scholar]
- 46.Zaia, J. A., and S. J. Forman. 1995. Cytomegalovirus infection in the bone marrow transplant recipient. Infect. Dis. Clin. N. Am. 9:879-900. [PubMed] [Google Scholar]
- 47.Zar, J. H. 1999. Biostatistical analysis, 4th ed., p. 210-214. Prentice Hall, Upper Saddle River, NJ.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.