Abstract
CBA/N mice were immunized with PspA in a poly(ethylene oxide) matrix to examine its ability to deliver the antigen and modulate the immune response. All mice receiving PspA in the matrix survived a lethal pneumococcal challenge and had serum anti-PspA antibody levels statistically higher than mice receiving PspA alone (P < 0.009).
Streptococcus pneumoniae, the pneumococcus, is a major cause of morbidity and mortality worldwide (16). This bacterial pathogen is considered the most common cause of community-acquired pneumonia and meningitis and is among the most common causes of sinusitis, chronic bronchitis, and otitis media (7, 10). Multidrug-resistant pneumococci continue to be a major concern for the effective treatment of respiratory tract infections (1). In addition, pneumococcal infections are among the major causes of death due to infections in the United States (12). This is especially true among elderly individuals and those with underlying chronic disease (6, 8, 11).
Pneumococcal surface proteins that are important in physiology or pathogenesis represent potential vaccine components as well as targets for new classes of therapeutic agents (16). Pneumococcal surface protein A, PspA, is one of the best characterized of these proteins. PspA elicits protective immunity in mice against pneumococcal challenge (2), and antibodies from humans vaccinated with PspA passively protect mice against infection (3).
We used this well-characterized mouse model of protective immune response to examine the ability of a poly(ethylene oxide) matrix to deliver antigen and modulate the immune response. The matrix represents a novel delivery system that can augment the immune response and does not require an injection. This system will likely lead to broader acceptance of vaccines. As proof of concept, the matrix containing PspA was implanted subcutaneously in mice for direct comparison to subcutaneous injection of the antigen.
Bacterial strains and growth conditions.
S. pneumoniae WU2, which was used to challenge mice, was grown in Todd-Hewitt broth with 0.5% yeast extract at 37°C. Serial dilutions were performed to determine the CFU of the pneumococci by plating on blood agar and incubating at 37°C under reduced-oxygen conditions.
Statistical analysis.
Data were compared by analysis of variance with Tukey's correction for multiple comparisons and Student's unpaired t test (GraphPad InStat). P values of <0.05 were considered significant.
Immunization with PspA incorporated into a poly(ethylene oxide) matrix.
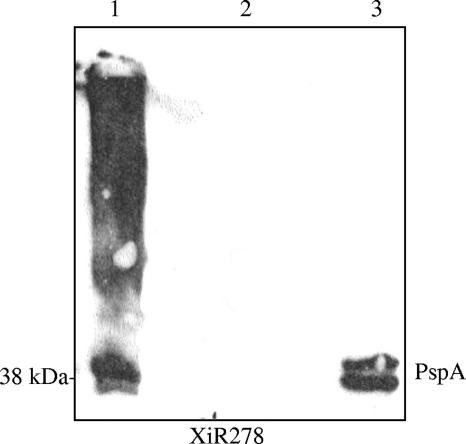
Recombinant PspA (rPspA) used in this study was derived from the nonencapsulated strain Rx1 and purified as previously described (21). rPspA/Rx1 was incorporated into hot-melt extrusion matrices composed of poly(ethylene oxide). This process has been shown to be a viable technique for delivery of drugs (20). A volume of polymer slurry was mixed with 500 μg of rPspA/Rx1 such that a 5-mm disk of the final material would contain approximately 30 μg of rPspA/Rx1 using an extruder that produced a single sheet or film of polymerized matrix. During the extrusion process, the film reached a maximum temperature of 90°C for approximately 30 seconds. PspA has been shown to be a very stable protein that can tolerate exposure to heat (14). The films were processed into 5-mm-diameter disks. The disks were evaluated postextrusion for PspA content, physical uniformity, and dissolution rate. The disks containing PspA and bovine serum albumin (BSA) were dissolved in water for 30 minutes and then loaded on the gel. Western blot analysis of the rPspA incorporation in the matrix was performed as previously described (18). The matrix was proven not to inhibit PspA interaction with an anti-PspA monoclonal antibody. By Western blot analysis, PspA is detected in the matrix as a diffused pattern due to the incorporation in the poly(ethylene oxide) matrix, which did not retard the monoclonal antibody's accessibility to the protein (Fig. 1).
FIG. 1.
Immunoblot analysis of PspA incorporated in the matrix. The disks containing PspA (lane 1) and BSA (lane 2) dissolved in water were reacted with XiR278 (anti-PspA monoclonal antibody) in the Western blot. The lane with PspA in the disk shows a diffused pattern due to the incorporation in the poly(ethylene oxide) matrix. Purified rPspA/Rx1 (5 μg) in lane 3 served as a positive control.
CBA/N (CBA/CAHN-BTK XID/J) mice (Jackson Laboratory, Bar Harbor, ME) were immunized with the laminated-film systems containing poly(ethylene oxide) incorporated with PspA. An 8-mm incision was made in the skin of anesthetized mice in the inguinal area, and the 5-mm-diameter disk with 30 μg of rPspA/Rx1 in the matrix was inserted under the skin. A group of mice were given 30 μg of BSA in the matrix as a control. Other groups of mice were surgically manipulated and then injected with either 30 μg of rPspA/Rx1 in lactated Ringer's solution or 30 μg of rPspA/Rx1 with 30 μg of alum. At 14 days, the procedure was repeated on the opposite side of the inguinal area. At 21 days, the mice were bled and then challenged intravenously with 10 times the 100% lethal dose of WU2.
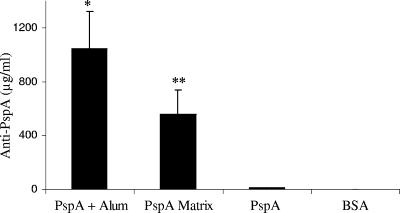
All of the mice that received PspA with alum or in the matrix survived a lethal pneumococcal challenge compared to the control mice, none of whom survived (Table 1). Serum PspA-specific immunoglobulin levels were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (2). ELISA results revealed that the level of serum anti-PspA antibodies for mice immunized with PspA in the matrix was 554.69 ± 183.23 μg/ml, which was not statistically different from the anti-PspA level in mice immunized with PspA on alum, 1,042.22 ± 281.81 μg/ml (Fig. 2). Interestingly, the level of anti-PspA antibodies elicited by PspA in the matrix was statistically higher than the level of anti-PspA antibodies in mice receiving PspA without adjuvant, which elicited 10.99 ± 4.26 μg/ml (P < 0.009). Our data demonstrated that the delivery of PspA via the laminated film results in a significant increase in antibody responses.
TABLE 1.
Survival of mice following challenge with S. pneumoniae WU2a
| Antigen | No. of mice alive/no. dead | % Survival |
|---|---|---|
| PspA (lactated Ringer's) | 4:1 | 80 |
| PspA (alum) | 5:0 | 100 |
| PspA (matrix) | 5:0 | 100 |
| BSA | 0:5 | 0 |
CBA/N mice were immunized on days 0 and 14 with 30 μg of PspA/Rx1 in lactated Ringer's solution, alum, or the matrix. BSA represents the control group. Mice (n = 5) were challenged with 10 times the 100% lethal dose of WU2. P was <0.0001 for the survival of mice immunized with PspA in the matrix, on alum, and in lactated Ringer's solution versus BSA-immunized mice.
FIG. 2.
Anti-PspA responses of CBA/N mice immunized with PspA. Sera collected on day 21 were assayed for anti-PspA antibodies. Mice that were immunized with the matrix containing PspA had significantly higher antibody responses than mice receiving PspA injected subcutaneously. *, P < 0.006 for mice immunized with PspA on alum (1,042.22 ± 281.81 μg/ml) versus PspA; **, P < 0.009 for mice immunized with PspA in matrix (554.69 ± 183.23 μg/ml) versus PspA (10.99 ± 4.26 μg/ml).
Cross-reactivity.
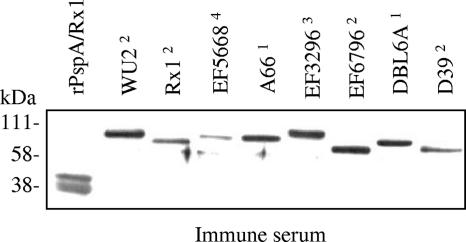
Western blot analysis of the cross-reactivity of the serum from mice immunized with PspA in the matrix was performed as previously described (18). The immune sera from mice immunized with PspA in the matrix reacted with PspA in cell lysates of different pneumococcal strains and recombinant PspA (Fig. 3). The data demonstrate that the quality of antibody elicited by PspA incorporated in the matrix is equivalent to that of antibodies elicited by immunization with PspA on alum.
FIG. 3.
Immunoblot of pneumococcal cell lysates reacted with immune serum from CBA/N mice immunized with PspA in matrix. Five micrograms of total protein of whole-cell lysates was loaded into each well. Purified rPspA/Rx1 (5 μg) served as a positive control. The superscript numbers represent the PspA clade of the pneumococcal strain. The blot represents PspA family 1, clades 1 and 2, and PspA family 2, clades 3 to 5.
Current research has focused on developing alternative dosage systems capable of intimate contact with the mucosa and able to release the drug for a prolonged period of time. Hot-melt extrusion technology is being used to produce thin films containing different drugs for transdermal, transmucosal, and wound care applications (19). The advantages of this system are that the dissolution rate, size, and shape of the final product and the amount of the incorporated vaccine antigen can be varied. Also, the film is water soluble, which allows the amount of vaccine material released from the disk to be accurately determined.
In this study, we investigated the ability of a matrix containing PspA delivered subcutaneously to elicit protection against fatal pneumococcal challenge. Our results demonstrated that films implanted subcutaneously in mice can elicit protective immune responses. Immunization with PspA in the lactated Ringer's solution demonstrated the ability to elicit a specific immune response. Previous studies in our laboratory have established that minimal levels of anti-PspA antibodies are sufficient for protection against pneumococcal challenge (2). Immunization of mice using PspA with adjuvant has repeatedly been demonstrated to be very effective in eliciting protection (3-5, 9, 13, 15, 17). The poly(ethylene oxide) matrix used both serves as a potential delivery system and offers adjuvant characteristics. This study demonstrates the adjuvant effects of the matrix on the immune response and the resultant protection from a lethal pneumococcal challenge. This shows that PspA in the matrix is recognized by the immune system and that the immune response is similar to that in mice immunized with PspA on alum and is not a local response.
The laminated-film system has been used to study the prophylaxis and treatment of oral candidiasis by incorporating clotrimazole in the film system and the delivery of dental analgesia such as lidocaine (19, 20). To our knowledge, this study is the first to investigate the efficacy of pneumococcal immunization via a laminated-film system. Further development of this immunization model would allow for the evaluation of the mucosal immune response against carriage and systemic pneumococcal infections. Results from these studies will advance the development of an effective transmucosal delivery system against bacterial infections.
Acknowledgments
We thank Lisa Quin and Chinwendu Onwubiko for critical analysis of the manuscript.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Ball, P. 1999. New fluoroquinolones: real and potential roles. Curr. Infect. Dis. Rep. 1:470-479. [DOI] [PubMed] [Google Scholar]
- 2.Bosarge, J. R., J. M. Watt, D. O. McDaniel, E. Swiatlo, and L. S. McDaniel. 2001. Genetic immunization with the region encoding the α-helical domain of PspA elicits protective immunity against Streptococcus pneumoniae. Infect. Immun. 69:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. White, K. Prellner, A. Hermansson, P. C. Aerts, H. Van Dijk, and M. J. Crain. 1997. PspA and PspC: their potential for use as pneumococcal vaccines. Microb. Drug Resist. 3:401-408. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858-867. [DOI] [PubMed] [Google Scholar]
- 6.Cassiere, H. A., and M. S. Niederman. 1998. Community-acquired pneumonia. Dis. Mon. 44:613-675. [DOI] [PubMed] [Google Scholar]
- 7.Fedson, D. S., and J. A. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 8.Gant, V., and S. Parton. 2000. Community-acquired pneumonia. Curr. Opin. Pulm. Med. 6:226-233. [DOI] [PubMed] [Google Scholar]
- 9.He, X., and L. S. McDaniel. 2007. The genetic background of Streptococcus pneumoniae affects protection in mice immunized with PspA. FEMS Microbiol. Lett. 269:189-195. [DOI] [PubMed] [Google Scholar]
- 10.Klein, D. L. 1999. Pneumococcal disease and the role of conjugate vaccines. Microb. Drug Resist. 5:147-157. [DOI] [PubMed] [Google Scholar]
- 11.Lynch, J. P., III, and F. J. Martinez. 1998. Community-acquired pneumonia. Curr. Opin. Pulm. Med. 4:162-172. [PubMed] [Google Scholar]
- 12.Mandell, L. A., J. G. Bartlett, S. F. Dowell, T. M. File, Jr., D. M. Musher, and C. Whitney. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel, L. S., B. A. Ralph, D. O. McDaniel, and D. E. Briles. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323-337. [DOI] [PubMed] [Google Scholar]
- 14.McDaniel, L. S., G. Scott, K. Widenhofer, J. M. Carroll, and D. E. Briles. 1986. Analysis of a surface protein of Streptococcus pneumoniae recognised by protective monoclonal antibodies. Microb. Pathog. 1:519-531. [DOI] [PubMed] [Google Scholar]
- 15.McDaniel, L. S., J. S. Sheffield, P. Delucchi, and D. E. Briles. 1991. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 59:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel, L. S., and E. Swiatlo. 2004. Pneumococcal disease: pathogenesis, treatment, and prevention. Infect. Dis. Clin. Pract. 12:93-98. [Google Scholar]
- 17.McDaniel, L. S., J. Yother, M. Vijayakumar, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, Q. C., J. R. Bosarge, L. R. Quin, and L. S. McDaniel. 2006. Enhanced protective immunity against pneumococcal infection with PspA DNA and protein. Vaccine 24:5755-5761. [DOI] [PubMed] [Google Scholar]
- 19.Repka, M. A., K. Gutta, S. Prodduturi, M. Munjal, and S. P. Stodghill. 2005. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 59:189-196. [DOI] [PubMed] [Google Scholar]
- 20.Repka, M. A., S. Prodduturi, and S. P. Stodghill. 2003. Production and characterization of hot-melt extruded films containing clotrimazole. Drug Dev. Ind. Pharm. 29:757-765. [DOI] [PubMed] [Google Scholar]
- 21.Thornton, J., and L. S. McDaniel. 2005. THP-1 monocytes up-regulate intercellular adhesion molecule 1 in response to pneumolysin from Streptococcus pneumoniae. Infect. Immun. 73:6493-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]