Abstract
We report the results of an evaluation of the LIAISON Treponema pallidum-specific assay, a one-step sandwich chemiluminescence immunoassay (CLIA), as a screening test and as a confirmatory test for the diagnosis of syphilis. The assay was compared with the CAPTIA Syphilis-G enzyme immunoassay (EIA) and with a testing algorithm that also included the rapid plasma reagin (RPR) and T. pallidum particle agglutination (PA) assays. As a screening test, the CLIA showed levels of agreement with the EIA and with the algorithm, respectively, of 94.1 and 100% for 51 samples from patients with primary or secondary syphilis, 93.2 and 98.7% for 999 samples sent to the laboratory for routine syphilis testing, 84.5 and 94.0% for 200 samples from human immunodeficiency virus-positive patients, 98.0 and 100% for 200 samples from pregnant patients, and 94.3 and 98.3% for 992 samples from apparently healthy adults. As a confirmatory test, the CLIA showed 99% agreement with the EIA for 204 RPR-positive samples. After resolution with further T. pallidum PA testing and the discarding of one sample of insufficient quantity, there was 100% agreement for the remaining 203 samples. For the total group of 2,645 samples, the overall relative sensitivity was 95.8% and the relative specificity was 99.1%. We conclude that the LIAISON CLIA demonstrated excellent sensitivity and specificity when evaluated as a confirmatory test and as a screening test for syphilis among various patient populations, including specific populations with reportedly increased rates of false-positive nontreponemal test results.
The spirochete Treponema pallidum subsp. pallidum is the etiologic agent of syphilis, an infectious disease which is acquired sexually or congenitally. Syphilis progresses through multiple clinical stages, with characteristic features including the painless and indurated chancre of primary syphilis, followed by spirochetemia with systemic symptoms and a rash in secondary syphilis. If untreated, the disease may then enter a latent phase for months, years, or even decades. Tertiary syphilis manifests years to decades after infection, classically affecting the cardiovascular or neurological system (11). The rate of primary and secondary syphilis reported in the United States decreased during the 1990s, with the rate reported in 2000 being the lowest since reporting began in 1941 (1). However, the rate of primary and secondary syphilis has subsequently increased each year since 2001, with increases noted particularly among men who have sex with men (1). Rates of syphilis remain higher in urban areas and are higher in the southern United States than in other regions of the country (1, 11).
Syphilis is infamous for its protean presentations and the resultant challenges in clinical diagnosis. Laboratory diagnosis is likewise challenging due to the lack of success in growing T. pallidum on artificial media, and the diagnosis is most often established by serologic testing (2, 4, 6, 8, 9, 11). The traditional approach to syphilis testing involves screening with a nontreponemal test, such as the rapid plasma reagin (RPR) test, followed by a confirmatory treponemal test with specificity for treponemal antigens (2, 6, 9). However, numerous conditions have been associated with false-positive nontreponemal test results, including other infections, pregnancy, connective-tissue diseases, malignancy, and narcotic addiction (6). Because these are relatively common conditions, false-positive results may actually exceed true-positive nontreponemal test results among low-risk populations (6). It has been suggested that a testing strategy using a treponemal test for screening may be useful, particularly in laboratories with a high volume of testing for syphilis (2, 4, 9, 10), since some treponemal tests have the advantage of being adaptable to an automated platform.
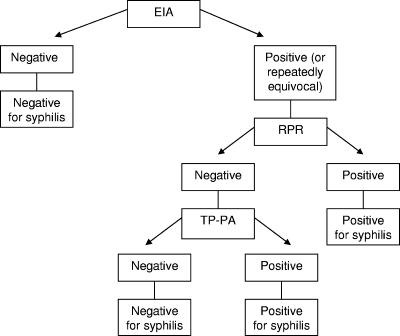
We report the results of an evaluation of the LIAISON T. pallidum-specific assay (Diasorin, Stillwater, MN), a one-step sandwich chemiluminescence immunoassay (CLIA), as a screening test and as a confirmatory test for the diagnosis of syphilis. The LIAISON CLIA was compared with the CAPTIA Syphilis-G enzyme immunoassay (EIA; Trinity Biotech, Bray, Ireland), another treponemal test which has been studied as both a screening and a confirmatory assay (5, 10, 12). The results of the CLIA were also compared with the results of testing according to an algorithm outlined in Fig. 1, including further testing with the RPR and T. pallidum particle agglutination (PA) assays, by using the approach previously described by Pope (9). The CLIA was evaluated as a screening test for various patient populations and was also evaluated as a confirmatory test for sera which were RPR positive.
FIG. 1.
Testing algorithm, similar to that proposed by Pope (9). Further testing was performed if the CLIA result was positive and the EIA result was negative. TP-PA, T. pallidum PA test. (Adapted from reference 9 with permission of the publisher.)
MATERIALS AND METHODS
Evaluation of the LIAISON CLIA as a diagnostic screening test was performed using sera from several patient populations. Fifty-one samples from patients with primary or secondary syphilis, 999 samples sent to the laboratory for routine syphilis testing, 200 samples from human immunodeficiency virus (HIV)-positive patients, 200 samples from pregnant patients, and 992 samples from apparently healthy adults were obtained from commercial sample procurement agencies and tested with both the CLIA and the CAPTIA Syphilis-G EIA as described below. Technicians performing the tests were blinded to the results of all other tests for a given sample. All samples positive by one or both of the treponemal assays were also tested by the RPR assay. Samples positive by only one of the treponemal assays were tested with the T. pallidum PA assay, as were samples positive by both treponemal tests but negative by the RPR assay. No further testing was performed for samples negative by both the CLIA and the EIA. Based on the algorithm outlined in Fig. 1, an overall result of positive or negative was obtained for each sample and compared with the CLIA result.
To evaluate the performance of the LIAISON CLIA as a confirmatory test, 204 RPR-positive sera were tested with the CLIA, and results were compared to those obtained with the EIA. Further testing with the T. pallidum PA assay was performed in cases of discordant results.
The LIAISON T. pallidum-specific assay is a one-step sandwich qualitative CLIA performed on the LIAISON automated random-access analyzer. Paramagnetic microparticles are coated with a recombinant treponemal antigen (TpN17). A patient's serum, the coated microparticles, and an isoluminol-antigen conjugate are mixed and incubated. The isoluminol-antigen is the antigen linked to the isoluminol derivative and is the same antigen present on the solid phase. It is a recombinant antigen of T. pallidum (Tp17s), and it is used to bind the immunoglobulin G (IgG) and IgM antibodies in a sandwich manner. Specific antitreponemal antibodies present in the specimens bind to the paramagnetic particles and the antigen conjugate during the incubation. After the incubation, unbound components are washed away. Starter reagents are added, and a light signal is produced from a flash chemiluminescence reaction if antitreponemal antibodies are present. The light signal is measured by a photomultiplier as relative light units (RLU). Based on the signal generated for the sample compared with those generated for calibrators, an index value is determined. Thus, the index value for a patient's specimen is calculated by comparing the patient's light signal (RLU) to a working curve from the light signals (RLU) obtained from calibrators. The master curve is stored in the method file, and a working curve is calculated when the calibration is performed.
An index value of <0.9 is interpreted as negative, while a value of ≥1.1 is considered positive. Results between 0.9 and 1.1 are considered equivocal, and the test is repeated according to the manufacturer's instructions. The reagents are ready to use, and the calibrators are included in the integral. Samples and reagents can be continuously loaded while the instrument is in use and are recognized by a bar code reader. The sample area can accommodate 144 samples at a time, and the reagent area can accommodate up to 15 kit reagents. The onboard reagent area is refrigerated so that the integrals can be left on the instrument. The master curve is stored, and only a two-point calibration is required. The calibration is stable for 14 days.
For the CAPTIA Syphilis (T. pallidum)-G EIA (Trinity Biotech, Bray, Ireland), microtiter tray wells are coated with T. pallidum antigens. Patients’ specimens are added to the coated wells and incubated. Unbound components are washed away. Horseradish peroxidase monoclonal antibody conjugate is then added and reacts with any antibody bound onto the microtiter wells during incubation. Unbound components are again washed away, and tetramethylbenzidine enzyme substrate is added. The tetramethylbenzidine reacts with any horseradish peroxidase left on the microtiter wells. The reaction is stopped by adding sulfuric acid, and the assay result is measured spectrophotometrically. Any test resulting in an equivocal interpretation according to the manufacturer's instructions is then repeated in duplicate, and the sample is determined to be positive, negative, or equivocal based on the three total results.
The RPR test (Becton Dickinson Macro-Vue RPR 18-mm circle card test) is a macroscopic, nontreponemal flocculation card test. The antigen suspension contains choline chloride to eliminate the need to inactivate the serum with heat, EDTA to enhance the stability of the suspension, and finely divided charcoal particles as a visualizing agent. The RPR antigen suspension is mixed with the patient's specimen on a plastic-coated card. The RPR test measures IgM and IgG antibodies to lipoidal material. If antibodies are present, they combine with the charcoal particles and black clumps are seen on the white cards.
The T. pallidum PA assay (Furjirebio, Tokyo, Japan) is a passive agglutination procedure based on the agglutination of gel particles sensitized with the T. pallidum Nichols strain with antibodies found in the patient's specimen. Serum containing antibodies to treponemes reacts with the sensitized gel particles to form a smooth mat of agglutinated gel particles in a microtiter tray well. If antibodies are not present, the particles settle to the bottom of the microtiter tray well into a compact button.
The results of the CLIA were compared with those of the EIA and those obtained according to the testing algorithm outlined in Fig. 1. Overall levels of agreement and the levels of agreement for positive and negative results were then calculated. The relative sensitivity and specificity were calculated based on all 2,645 samples tested in this evaluation by using the result from the testing algorithm as the standard for comparison.
RESULTS
LIAISON CLIA as a screening test.
The results of testing 51 samples from patients with primary or secondary syphilis, 999 samples sent to the laboratory for routine syphilis testing, 200 samples from HIV-positive patients, 200 samples from pregnant patients, and 992 samples from apparently healthy adults are shown in Tables 1 and 2. Discordant CLIA and EIA findings included equivocal EIA results for 2 of the samples from patients with primary or secondary syphilis, 40 of the samples sent to the laboratory for routine syphilis testing, 4 of the samples from HIV-positive patients, 4 of the samples from pregnant women, and 19 of the samples from apparently healthy adults. Thus, 69, or 2.8%, of the 2,442 samples in the screening test portion of the evaluation had equivocal EIA results.
TABLE 1.
LIAISON CLIA as a screening test
| Sample population | No. of samples | No. of samples with indicated CLIA result
|
Comparison with EIA
|
Comparison with testing algorithm
|
||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Equivocal | No. of results in agreement with CLIA/no. of samples tested | % Agreement with CLIA (95% CI)a | No. of results in agreement with CLIA/no. of samples tested | % Agreement with CLIA (95% CI) | ||
| Patients with primary or secondary syphilis | 51 | 48 | 3 | 0 | 48/51 | 94.1 (83.8-98.8) | 51/51 | 100 (93.0-100) |
| Patients providing samples for routine testing | 999 | 41 | 957 | 1 | 931/999 | 93.2 (91.4-94.7) | 986/999 | 98.7 (97.8-99.3) |
| HIV-positive patients | 200 | 75 | 123 | 2 | 169/200 | 84.5 (78.7-89.2) | 188/200 | 94.0 (90.7-97.3) |
| Pregnant patients | 200 | 4 | 196 | 0 | 196/200 | 98.0 (95.0-99.5) | 200/200 | 100 (98.2-100) |
| Apparently healthy adults | 992 | 63 | 929 | 0 | 935/992 | 94.3 (92.6-95.6) | 975/992 | 98.3 (97.3-99.0) |
CI, confidence interval.
TABLE 2.
Agreement of CLIA results with those of the EIA and the testing algorithm for positive and negative samples
| Sample population | % Agreement between CLIA and EIA (no. of samples with indicated result by EIA)
|
% Agreement between CLIA and testing algorithm (no. of samples with indicated result by testing algorithm)
|
||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Patients with primary or secondary syphilis | 97.9 (48) | 100 (1) | 100 (48) | 100 (3) |
| Patients providing samples for routine testing | 55.0 (40) | 98.9 (919) | 91.4 (35) | 99.0 (964) |
| HIV-positive patients | 75.0 (92) | 96.2 (104) | 93.3 (75) | 94.4 (125) |
| Pregnant patients | 100 (4) | 100 (192) | 100 (4) | 100 (196) |
| Apparently healthy adults | 62.8 (86) | 99.3 (887) | 84.8 (66) | 99.2 (926) |
Among the samples sent for routine testing, seven of nine samples that were positive by the LIAISON CLIA and negative by the algorithm (EIA and RPR negative) were also positive by the T. pallidum PA procedure. This pattern was also seen among the samples from apparently healthy adults (two of seven) and HIV-positive patients (two of five) that were negative by the algorithm and positive by the CLIA. These 11 sets of apparently discrepant results may possibly represent early infections presenting with only IgM antibodies, as both the CLIA and the T. pallidum PA assay can detect both IgM and IgG while the EIA detects only IgG.
LIAISON CLIA as a confirmatory test.
For the confirmatory test portion of the evaluation, 204 samples known to be RPR positive were also tested with the CLIA to test the latter assay's ability to serve as a confirmatory treponemal test following a nontreponemal test. The CLIA yielded positive results for 201 samples, negative results for 2 samples, and equivocal results for 1 sample. As a confirmatory test, the CLIA agreed very well with the EIA (with results in agreement for 202 [99.0%] of 204 samples; 95% confidence interval, 97.3 to 100%). An additional sample which was equivocal by the EIA and positive by the CLIA was confirmed to be positive by the T. pallidum PA test. With the result resolved by the T. pallidum PA procedure taken into account, results were in agreement for 203 (100%) of 203 samples (95% confidence interval, 98.2 to 100%). One sample which was equivocal by the CLIA and positive by the EIA was of insufficient quantity for further testing (i.e., the result could not be resolved by the T. pallidum PA test, and the sample was excluded from the analysis).
Sensitivity and specificity.
When the results for all samples in this study were combined, sensitivity and specificity were calculated for the entire group of 2,645 samples. With the result according to the testing algorithm as the standard for comparison, the overall relative sensitivity of the assay was 95.8% and the overall relative specificity was 99.1%.
Precision.
The between-run precision of the LIAISON CLIA was studied using positive and negative controls run once a day for 19 days. The mean of the results for the positive control was 5.21 index units (standard deviation [SD], 0.27 index units; coefficient of variation [CV], 5.3%), and that for the negative control was 0.24 index units (SD, 0.03 index units; CV, 12.8%). The between-run precision of the CAPTIA Syphilis-G EIA was studied using positive and negative controls run once a day for 24 days. The mean of the results for the positive control was 4.49 index units (SD, 0.75 index units; CV, 16.6%), and that for the negative control was 0.24 index units (SD, 0.05 index units; CV, 20.2%).
DISCUSSION
Our evaluation of the LIAISON T. pallidum-specific assay as a screening test included testing samples from patients with medically diagnosed primary or secondary syphilis, samples sent for routine syphilis testing, and samples from apparently healthy adults. Also included were samples from pregnant patients and from HIV-positive patients, both of which represent populations with reportedly high rates of false-positive nontreponemal screening test results (8, 11). The rate of biological false-positive RPR results has been reported to be as high as 28% among screened pregnant women (8). The likelihood of biological false-positive RPR results among HIV-positive patients has been reported to be from 1 to 5.8% in some series (11). Dorigo-Zetsma et al. reported a high overall rate of biological false-positive RPR screening results, at 8.2%, but the rate of false positives was not significantly higher among their HIV-positive patients (3). In the present study, the LIAISON CLIA performed very well as a screening test for pregnant women. For samples from the HIV-positive group, there was a slightly lower rate of agreement (94.0%) with the algorithm results, particularly for negative samples (94.4%), than for samples from other groups.
As shown in Table 1, the results of our evaluation include a level of agreement from 84.5 to 98.0% with the CAPTIA Syphilis-G EIA findings. The diagnostic algorithm outlined in Fig. 1 was used to attempt to resolve discordant results, a significant number of which were due to equivocal EIA results, as noted above. Interestingly, this proportion (69, or 2.8%, of 2,442 equivocal results) is apparently identical to that determined by Reisner et al., who reported 2.8% equivocal EIA results for more than 10,000 total samples screened with the CAPTIA Syphilis-G EIA. They recommended using another treponemal test to resolve these equivocal results (10).
After resolving discordant cases according to the testing algorithm outlined in Fig. 1, the levels of agreement between the results of the CLIA and the results according to the algorithm ranged from 94.0 to 100% (Table 1), with the levels of agreement for positive and negative samples as shown in Table 2. The LIAISON assay has previously been reported to have 99.2% sensitivity and 99.9% specificity, based on a sample composed of blood donor sera, patients with known syphilis, and patients with conditions reported to cause false-positive nontreponemal test results (7). The overall relative sensitivity and specificity observed in the present study were 95.8 and 99.1%, respectively. We recognize, however, that these figures have been calculated in reference to tests that also have limitations.
Some discordant results may be explained by early infection, with only IgM present. This explanation is a possibility for seven of nine samples sent for routine testing, two of seven samples from apparently healthy adults, and two of five samples from HIV-positive patients that were positive by the CLIA and negative by the algorithm, as they were also positive by the T. pallidum PA test. These test results may potentially be seen in early infection, because the CLIA and the T. pallidum PA assay detect IgG and IgM while the EIA detects only IgG. Early syphilis may not be detected initially by serologic tests, as humoral antibodies usually do not appear until 1 to 4 weeks after infection (6). False-negative results in early primary syphilis are, therefore, a known limitation of all serologic tests due to this window of seronegativity (12).
The evaluation of the CLIA as a confirmatory test showed 99.0% agreement with the CAPTIA EIA results and 100% agreement with the results of further testing with the T. pallidum PA assay after a single sample of insufficient quantity was excluded from the analysis. However, this group of samples seems to include too few samples negative for syphilis, that is, samples with false-positive RPR test results, to thoroughly evaluate the ability of the assay to resolve false-positive nontreponemal test results. Previously published data suggest that this assay is useful in situations associated with false-positive RPR results, such as pregnancy and the presence of other infections or autoimmune diseases (7).
Laboratories with high volumes of syphilis testing may benefit from the use of an automated screening test. Although reagent costs tend to be higher for a treponemal test than for a nontreponemal test, the use of an automated immunoassay generally decreases the amount of technical time required (9, 10). For example, one group reported that the technical time required to perform an EIA screening procedure was approximately half that necessary for the manual RPR test (10). Depending on the volume of tests performed and the availability of technicians trained in performing and interpreting the manual tests, an automated immunoassay may be a more efficient screening test. The automated platform and one-step method of the LIAISON CLIA are well-suited to providing a rapid diagnosis in laboratories with high test volumes, with the first results available within about 40 min and the capability of providing up to 180 results per hour.
The appropriate use of the LIAISON T. pallidum-specific assay requires the consideration of the clinical context in which the test is performed. The CLIA, like other treponemal assays, does not differentiate recent versus remote or previously treated infections, and therefore, clinical history is important in determining the significance of the test result. A limitation of this study is that, although the test performed well for patients with primary or secondary syphilis, patients with manifestations of tertiary syphilis were not included, nor did our evaluation address the diagnosis of congenital syphilis. However, these populations were included in a previous study by Marangoni et al. showing excellent sensitivity (7).
In summary, the LIAISON CLIA can be used as an automated method for the qualitative detection of treponemal antibodies in human serum. It may have a role either as a diagnostic screening test or as a confirmatory test following a nontreponemal screening test. As with all other tests for syphilis, its interpretation must be combined with clinical findings and results from other tests for optimal use.
Acknowledgments
We thank Mary Hawkins, Joyce Johnson, Kim Greer, and Ginger Jordan for their work and their technical expertise. We also thank Carolyn Chaffin for her administrative expertise.
This work was supported by Diasorin, Inc.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2006. Sexually transmitted disease surveillance, 2005. U.S. Department of Health and Human Services, Atlanta, GA.
- 2.Centers for Disease Control and Prevention. 2006. The syphilis elimination technical appendix. U.S. Department of Health and Human Services, Atlanta, GA.
- 3.Dorigo-Zetsma, J. W., D. Belewu, H. Meless, E. Sanders, R. A. Coutinho, A. Schaap, and D. Wolday. 2004. Performance of routine syphilis serology in the Ethiopian cohort on HIV/AIDS. Sex. Transm. Infect. 80:96-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egglestone, S. I., and A. J. L. Turner. 2000. Serological diagnosis of syphilis. Commun. Dis. Public Health 3:158-162. [PubMed] [Google Scholar]
- 5.Hooper, N. E., D. C. Malloy, and S. Passen. 1994. Evaluation of a Treponema pallidum enzyme immunoassay as a screening test for syphilis. Clin. Diagn. Lab. Immunol. 1:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen, S. A., B. M. Steiner, and A. H. Rudolph. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marangoni, A., V. Sambri, S. Accardo, F. Cavrini, A. D'Antuono, A. Moroni, E. Storni, and R. Cevenini. 2005. Evaluation of LIAISON Treponema Screen, a novel recombinant antigen-based chemiluminescence immunoassay for laboratory diagnosis of syphilis. Clin. Diagn. Lab. Immunol. 12:1231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeling, R. W., and H. Ye. 2004. Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bull. W. H. O. 82:439-446. [PMC free article] [PubMed] [Google Scholar]
- 9.Pope, V. 2004. Use of treponemal tests to screen for syphilis. Infect. Med. 21:399-404. [Google Scholar]
- 10.Reisner, B. S., L. M. Mann, C. A. Tholcken, R. T. Waite, and G. L. Woods. 1997. Use of the Treponema pallidum-specific Captia Syphilis IgG assay in conjunction with the rapid plasma reagin to test for syphilis. J. Clin. Microbiol. 35:1141-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh, A. E., and B. Romanowski. 1999. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin. Microbiol. Rev. 12:187-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young, H., A. Moyes, A. McMillan, and J. Patterson. 1992. Enzyme immunoassay for anti-treponemal IgG: screening or confirmatory test? J. Clin. Pathol. 45:37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]