Abstract
Human African trypanosomiasis treatment is stage dependent, but the tests used for staging are controversial. Central nervous system involvement and its relationship with suramin treatment failure were assessed in 60 patients with parasitologically confirmed hemolymphatic-stage Trypanosoma brucei gambiense infection (white blood cell count of ≤5/μl and no trypanosomes in the cerebrospinal fluid [CSF]). The prognostic value of CSF interleukin-10, immunoglobulin M (IgM; as determined by nephelometry and the point-of-care LATEX/IgM test), total protein, and trypanosome-specific antibody was assessed. The IgM and interleukin-10 levels in serum were measured; and the presence of neurological signs, intrathecal IgM synthesis, and blood-CSF barrier dysfunction was determined. After suramin treatment, 14 of 60 patients had relapses (23%). Relapses were significantly correlated with intrathecal IgM synthesis (odds ratio [OR], 46; 95% confidence interval [CI], 8 to 260), a CSF IgM concentration of ≥1.9 mg/liter (OR, 11.7; 95% CI, 2.7 to 50), a CSF end titer by the LATEX/IgM assay of ≥2 (OR, 10.4; 95% CI, 2.5 to 44), and a CSF interleukin-10 concentration of >10 pg/ml (OR, 5; 95% CI, 1.3 to 20). The sensitivities of these markers for treatment failure ranged from 43 to 79%, and the specificities ranged from 74 to 93%. The results show that T. brucei gambiense-infected patients who have signs of neuroinflammation in CSF and who are treated with drugs recommended for use at the hemolymphatic stage are at risk of treatment failure. This highlights the need for the development and the evaluation of accurate point-of-care tests for the staging of human African trypanosomiasis.
Infection with the parasites Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense causes human African trypanosomiasis (HAT), or sleeping sickness. The disease progresses from a first hemolymphatic stage toward a second meningoencephalitic stage as the trypanosomes invade the brain (22). Brain invasion may occur after weeks in patients with the acute T. brucei rhodesiense disease form and after months or years in patients with chronic T. brucei gambiense infection. The drugs used for the treatment of the first hemolymphatic disease stage, pentamidine and suramin, are relatively safe but are ineffective against the second, meningoencephalitic stage. The latter stage is routinely treated with melarsoprol or intravenous eflornithine (21). Melarsoprol is a toxic drug that is associated with up to 5% of fatal encephalitic reactions and that is therefore not recommended for use for the treatment of the hemolymphatic stage. Since there are no specific clinical or biochemical signs in blood indicating the onset of the meningoencephalitic stage, staging and treatment choice are based on examination of the cerebrospinal fluid (CSF). White blood cell (WBC) counts >5/μl or the presence of trypanosomes in the CSF are routinely used as markers for central nervous system (CNS) involvement (22). Unfortunately, the limited specificities and sensitivities of these tests may lead to incorrect staging of the disease, with false-positive and false-negative interpretations and, subsequently, inefficient or unnecessarily toxic treatment.
Detection of intrathecal immunoglobulin M (IgM) synthesis is a specific and sensitive parameter for the detection of CNS involvement in cases of HAT caused by T. brucei gambiense (1), but the technology used to assess intrathecal synthesis is not suitable for use in the field. As a consequence of intrathecal synthesis, the levels of IgM and trypanosome-specific antibodies are increased in the CSF of patients with the meningoencephalitic stage (7, 13). Two point-of-care tests were developed on this basis: the LATEX/IgM assay, for the detection of IgM in the CSF of patients with sleeping sickness (10), and the LATEX/T. brucei gambiense assay, for the detection of trypanosome-specific antibodies (4). Both of these are self-contained tests that can be performed out of the laboratory, need a minimum of equipment, and give immediate results. Interleukin-10 (IL-10) has also been proposed as a potential marker for stage determination, because high serum and CSF IL-10 concentrations have been observed in patients with the meningoencephalitic stage, and those concentrations decline quickly after treatment (9, 14).
Intrathecal IgM synthesis, CSF IL-10 concentrations, CSF IgM concentrations, and the LATEX/IgM and the LATEX/T. brucei gambiense assays were assessed for their abilities to be used as markers of CNS involvement in patients with the hemolymphatic stage of T. brucei gambiense infection. Their relationship to treatment failure was studied.
(Parts of the results have been presented as a poster at the 28th Meeting of the International Scientific Council for Trypanosomiasis Research and Control, Addis Ababa, Ethiopia, 26 to 30 September 2005.)
MATERIALS AND METHODS
Patients and samples.
Patients were consecutively enrolled during the routine screening and treatment procedures of the HAT control program at Bwamanda Hospital (Equateur Province, Democratic Republic of Congo) between February and April 1998. Patients with parasitologically confirmed T. brucei gambiense infection in the hemolymphatic stage (CSF WBC count of ≤5/μl and no trypanosomes in the CSF after double centrifugation) were eligible for this study. Patients with hemorrhagic CSF were excluded. All patients underwent complete clinical and neurological examinations for the detection of sleep disturbances, primitive and deep-tendon reflexes, mental state alterations, abnormal movements, convulsions, sensory neuropathy, and anomalies of tone and coordination. After pretreatment for coinfections (malaria, helminthiasis), they were treated with suramin (six intravenous injections of 20 mg/kg of body weight, with 3 days of rest between each injection). Serum and CSF samples were taken for routine diagnostic purposes before treatment; at 24 h posttreatment; and at 3, 6, 12, 18, and 24 months posttreatment, when the patients underwent full clinical, parasitological, and CSF examinations. The remaining volumes of serum and CSF were frozen until analysis. Treatment failure (or relapse) was defined as (i) the reappearance of trypanosomes in serum or CSF during follow-up, (ii) a combination of a CSF WBC count of >20/μl and a worsened general condition during follow-up, and/or (iii) a CSF WBC count of >20/μl after 24 months of follow-up. The increased cutoff of 20 WBCs/μl for a relapse was chosen to avoid unnecessary retreatment (2, 16). Patients with treatment failures were given rescue treatment with second-line regimens (2). Informed consent was obtained from each patient or accompanying relative prior to enrollment in the study. The study was approved by the National Ethical Committee of the Ministry of Health of the Democratic Republic of Congo.
Analyses.
IgM and albumin in CSF and serum were quantified on a nephelometer (Prospec; Dade-Behring). Blood-CSF barrier function was evaluated by using the CSF albumin/serum albumin quotient (QAlb), with an upper reference limit QAlb of [4 + (age/15)] × 10−3 (19). The intrathecal IgM fraction (IgMIF) was calculated by the equation {1 − [QLim(IgM)/QIgM]} × 100, where QIgM is the CSF IgM/serum IgM quotient, and QLim(IgM) is equal to 0.67 × [QAlb2 + (120 × 10−6)]1/2 − (7.1 × 10−3) (19). An IgMIF value of >0% was considered a positive result for intrathecal IgM synthesis.
For IgM quantification in CSF by the LATEX/IgM test (10), 20 μl of the LATEX/IgM test reagent was mixed with 20 μl of sample on a test card. The card was rocked, and the agglutination was scored after 5 min. The end titer of a sample (i.e., the highest dilution that yielded agglutination) was determined.
Specific antibodies in CSF were detected by mixing 15 μl of the LATEX/T. brucei gambiense test reagent with 30 μl of CSF on a test card (4). Patients whose undiluted CSF reacted by the LATEX/T. brucei gambiense test after 10 min of rocking were considered to have a positive result.
IL-10 was quantified in a sandwich enzyme-linked immunosorbent assay by using unlabeled and biotinylated rat anti-human IL-10 (BD Biosciences, Pharmingen) as the capture and the detection antibody. Serial twofold dilutions of serum (1:4 to 1:16) and CSF (1:2 to 1:8) were tested. Concentrations of 0 to 100 pg/ml recombinant human IL-10 (National Institute for Biological Standards and Control, United Kingdom) were included in each plate to establish a standard curve. Ultrasensitive streptavidin-peroxidase polymer (Sigma, Belgium) was used as the conjugate. The reaction was revealed by using 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (Boehringer, Germany), and the optical density was read at 415 nm (Multiskan RC, version 6.0; Labsystems). The detection limits were 6.7 and 13.4 pg/ml IL-10 for CSF and serum, respectively.
The total CSF protein concentration was determined in duplicate by the bicinchoninic acid protein assay by use of the microtiter plate protocol (Pierce, Rockford, IL) with bovine serum albumin as the standard. All these analyses were performed after completion of the follow-up.
Data analysis.
The median values and the interquartile ranges were computed for each test. The median values for relapsing and nonrelapsing patients were compared by using the Kruskal-Wallis test. Continuous variables were dichotomized and compared by use of Fisher's exact test. Odds ratios (ORs) with a 95% confidence interval (CI) were calculated to assess the association with relapse. The sensitivities, specificities, and positive and negative predictive values were calculated with the 95% CIs. Receiver-operator characteristics curves were constructed to compare the sensitivity and the specificity of each parameter for the detection of a relapse over the whole spectrum of possible cutoff values. The area under the curve (AUC) was calculated to quantify the overall diagnostic accuracy (6, 23). The data were analyzed by using the SAS and Stata 8 software packages.
RESULTS
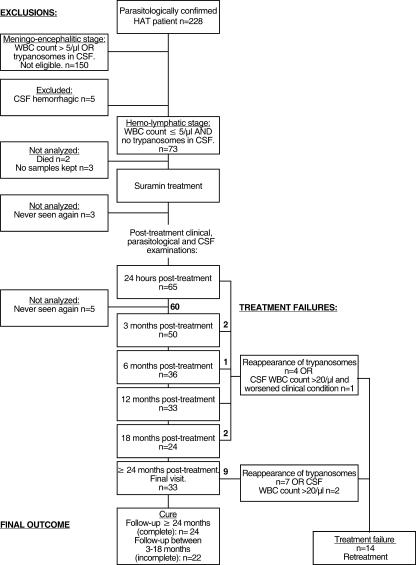
An overview of the study enrollment and follow-up process is shown in Fig. 1. Among the 73 patients enrolled in the study, 13 patients could not be included in the analysis for the following reasons. Two patients died of bronchopneumonia and pneumococcal meningitis before or during treatment. No pretreatment samples were kept for further analyses of three patients, three patients left the center before the 24-h follow-up time point, and five patients failed to attend the center for any other follow-up visits. This analysis reports on 60 patients, of whom 14 had relapses (23.3%). The follow-up periods (the last follow-up visits after treatment) were ≥24 months for 33 patients, 18 months for 8 patients, 12 months for 6 patients, 6 months for 5 patients, and 3 months for 8 patients. The male/female ratio was 1/2, and the mean age was 32.9 ± 10.1 years (range, 12 to 65 years).
FIG. 1.
Overview of study profile.
The pretreatment CSF and serum parameters are given in Table 1. Significant differences between cured and relapsed patients were observed for the intrathecal IgM fraction, the CSF IgM concentration, the LATEX/IgM test end titer, and the IL-10 concentration.
TABLE 1.
Pretreatment median values of CSF WBC count, IgMIF, CSF IgM concentration, CSF LATEX/IgM end titer, CSF IL-10 concentration, CSF total protein concentration, and serum IgM and IL-10 concentrations in cured and relapsed patients with hemolymphatic-stage sleeping sickness
| Parameter | Median value (interquartile range) for group
|
Pa | |
|---|---|---|---|
| Cured patients (n = 46) | Relapsed patients (n = 14) | ||
| CSF WBC count (no. of cells/μl) | 2 (1-3) | 3 (1-4) | 0.63 |
| IgMIF (%) | 0 (0-0)b | 50 (5-61)c | <0.0001 |
| CSF IgM concn (mg/liter) | 0.82 (0.48-1.79) | 3.33 (1.97-7.5) | 0.0005 |
| CSF LATEX/IgM test end titer | 0 (0-2) | 2 (2-8) | 0.0003 |
| CSF IL-10 concn (pg/ml) | 5.7 (1.9-7.4) | 8.3 (5.2-14.7) | 0.022 |
| CSF total protein (mg/liter) | 344 (291-401) | 318 (293-402) | 0.88 |
| Serum IgM concn (g/liter) | 4.53 (2.48-7.5)b | 5.13 (2.76-8.5)c | 0.59 |
| Serum IL-10 concn (pg/ml) | 90 (45-139)b | 124 (93-136)c | 0.14 |
Differences were tested by use of the Kruskal-Wallis test.
n = 44.
n = 13.
There was a significant association between the occurrence of relapse and the presence of intrathecal IgM synthesis before treatment (Table 2). The high diagnostic accuracy of IgMIF was reflected by the AUC of 0.86 (95% CI, 0.73 to 0.98). The presence of intrathecal IgM synthesis (IgMIF > 0%) had a sensitivity of 76.9% (95% CI, 66.3 to 87.6%) and a specificity of 93.2% (95% CI, 81.3 to 98.6%) for the detection of relapse. The positive and negative predictive values were 76.9% (95% CI, 66.3 to 87.6%) and 93.2% (95% CI, 81.3 to 98.6%), respectively.
TABLE 2.
Number of cured and relapsed patients after suramin treatment as a function of pretreatment test results, P value, and OR with 95% CI for association of test results with occurrence of relapse
| Parameter | No. of patients
|
Pa | OR (95% CI) | |
|---|---|---|---|---|
| Cured | Relapsed | |||
| Intrathecal IgM synthesis (n = 57): | ||||
| Absent | 41 | 3 | ||
| Present (IgMIF > 0%) | 3 | 10 | 0.0000015 | 46 (8.0-260) |
| CSF IgM concn (n = 60) of: | ||||
| <1.9 mg/liter | 35 | 3 | ||
| ≥1.9 mg/liter | 11 | 11 | 0.0004 | 11.7 (2.7-50) |
| CSF LATEX/IgM test end titer (n = 60) of: | ||||
| <2 | 34 | 3 | ||
| ≥2 | 12 | 11 | 0.001 | 10.4 (2.5-44) |
| <4 | 40 | 8 | ||
| ≥4 | 6 | 6 | 0.024 | 5.0 (1.3-20) |
| <8 | 41 | 8 | ||
| ≥8 | 5 | 6 | 0.014 | 6.2 (1.5-25) |
| CSF IL-10 concn (n = 60) of: | ||||
| ≤10 pg/ml | 40 | 8 | ||
| >10 pg/ml | 6 | 6 | 0.024 | 5.0 (1.3-20) |
Calculated by Fisher's exact test.
For the CSF IgM concentration, the AUC was 0.81 (95% CI, 0.69 to 0.92). The highest diagnostic accuracy was obtained by using a cutoff of 1.9 mg/liter, which resulted in a combination of a sensitivity of 78.6% (95% CI, 68.2 to 89.0%) with a specificity of 76.1% (95% CI, 65.3 to 86.9%). An IgM concentration in CSF higher than this cutoff was associated with relapse (Table 2). The positive and negative predictive values were 50.0% (95% CI, 28.2 to 71.8%) and 92.1% (95% CI, 78.6 to 98.3%), respectively.
CSF LATEX/IgM test end titers ≥2 were associated with relapses. The AUC for the CSF LATEX/IgM test end titer was 0.81 (95% CI, 0.68 to 0.93). A cutoff of the CSF LATEX/IgM test end titer ≥2 had a diagnostic sensitivity of 78.6% (95% CI, 49.2 to 95.3%), a specificity of 73.9% (95% CI, 58.9 to 85.7%), and positive and negative predictive values of 47.8% (95% CI, 26.8 to 69.4%) and 91.9% (95% CI, 78.1 to 98.3%), respectively.
IL-10 concentrations in CSF above 10 pg/ml were significantly associated with relapses. The AUC was 0.70 (95% CI, 0.52 to 0.88). The diagnostic accuracies for a relapse when the IL-10 concentration was >10 pg/ml were a sensitivity of 42.9% (95% CI, 30.3 to 55.4%) and a specificity of 87.0% (95% CI, 78.4 to 95.5%). The positive and negative predictive values were 50.0% (95% CI, 21.1 to 78.9%) and 83.3% (95% CI, 69.8 to 92.5%), respectively.
We observed no detectable relationship between the CSF total protein concentration, the serum IgM concentration, or the serum IL-10 concentration and relapse. The AUCs for these parameters were 0.49, 0.55, and 0.63, respectively. Neither the presence of a blood-CSF barrier dysfunction (in 25% of cured patients and 31% of relapsed patients) nor the positivity of CSF by the LATEX/T. brucei gambiense test (in 4% of cured patients and 14% of relapsed patients) was significantly associated with a relapse.
The most common neurological abnormalities were sleep disturbances (20/60 patients [30%]), the presence of primitive and deep-tendon reflexes (13% and 12% of patients, respectively), and confusion (6% of patients). However, none of those neurological signs was significantly associated with relapses in the patients studied.
DISCUSSION
We demonstrate that the presence of intrathecal IgM synthesis, elevated CSF IgM concentrations, and elevated CSF IL-10 concentrations are significantly associated with the failure of suramin treatment in patients with hemolymphatic-stage T. brucei gambiense infection.
Intrathecal IgM synthesis, detected as the IgMIF, was previously shown to be the most sensitive marker for CNS involvement in T. brucei gambiense-infected patients (12). It was present in 22% of patients in this study, which corroborates earlier reports of intrathecal IgM synthesis in up to 14% of patients with first-stage infections (1, 12). Previously, we also showed that the presence of intrathecal IgM synthesis in patients with early second-stage infections treated with pentamidine is associated with the risk for treatment failure (11). We now demonstrate a similar association in patients with first-stage infections treated with suramin. Detection of intrathecal IgM synthesis had the best sensitivity and the best specificity for the detection of a relapse and high positive and negative predictive values.
Unfortunately, in areas where HAT is endemic, IgMIF determination is not possible in rural health centers that are not equipped with the ability to quantitate albumin and IgM. The high IgM concentrations in the CSF of sleeping sickness patients originate both from an increased blood-derived fraction and from intrathecal synthesis and are detectable by the LATEX/IgM test (10, 12). Indeed, IgMIF, the total CSF IgM concentration, and the CSF LATEX/IgM test end titer were correlated (data not shown). Elevated CSF IgM concentrations (≥1.9 mg/liter) and CSF LATEX/IgM test end titers ≥2 were associated with treatment failures and had acceptable sensitivities of 79% and specificities of 74 to 76% for the detection of relapses. The LATEX/IgM test end titer cutoff of ≥2 is lower than the cutoffs of 4 and 8 proposed before (10, 11). The serum and CSF IgM concentrations in these patients with first-stage infections were also lower than those reported earlier (12). Such unexplained variations show that the proposal of cutoffs for IgM levels in CSF for disease staging in patients with HAT is a complex issue. The absolute value of CSF IgM depends on the serum IgM concentration and barrier function (12); in contrast, IgMIF takes these aspects into account (19). The lower cutoff in the present group might therefore be a consequence of the lower blood-derived IgM concentrations, due to lower blood IgM concentrations and a lower frequency of blood-CSF barrier dysfunction. Despite this shortcoming, among all the tests examined, the LATEX/IgM test is the only test suitable for field use. The LATEX/IgM test or other point-of-care tests for the detection of IgM in CSF merit further validation.
Although its AUC, sensitivity, and specificity were inferior to those of the IgM-associated parameters, a relationship between the CSF IL-10 concentration and treatment failure was demonstrated for the first time. The observed IL-10 concentrations overlap those reported previously for patients with first-stage T. brucei gambiense and T. brucei rhodesiense infections (9, 14). None of our patients showed higher IL-10 concentrations in CSF than in serum (data not shown), which does not exclude the intrathecal origin of this cytokine in CSF (15).
In contrast to our findings for patients with the early second stage (11), no association between trypanosome-specific antibodies in CSF and treatment failure was observed. The absence of any detectable relationship between the total CSF protein concentration and relapse confirms earlier findings (11) and the limited impact of CSF total protein concentration determination for disease staging (12). The irrelevance of clinical signs and symptoms for the staging of HAT was corroborated (3). The absence of a detectable relationship between the serum IL-10 or IgM level and relapse is not surprising, as trypanosomiasis induces a strong immune stimulation in the hemolymphatic compartment.
Although pentamidine is the first-line treatment for T. brucei gambiense-infected patients in the hemolymphatic stage, the patients in this study were treated with suramin because of a shortage in the supply of pentamidine. The published cure rates achieved with suramin for T. brucei rhodesiense- and T. brucei gambiense-infected patients are about 95% (18). The observed relapse rate of 23% achieved with suramin therefore seems high but corresponds to the failure rates of between 25 and 35% reported for suramin treatment of patients with first-stage T. brucei gambiense infections in the Democratic Republic of Congo in the 1950s (17, 18). Due to the unusual treatment regimen and its relatively high failure rate, the results should be interpreted with care, especially when they are extrapolated to patients treated with pentamidine. However, our findings correspond to those of an earlier study carried out with pentamidine-treated patients (11). In addition, the relatively high positive predictive values of about 75% for intrathecal IgM synthesis and 50% for the CSF IgM concentration and the LATEX/IgM test are influenced by the high relapse rate found in this study. Relapse rates below 10% would result in lower positive predictive values.
We cannot exclude the possibility that our study is slightly biased, as 22/60 (37%) of the patients had incomplete follow-up (no treatment failure and a last follow-up visit at between 3 and 18 months posttreatment). This rate of compliance is comparable to the rates reported in several clinical trials (5, 8, 20). The assumption that such patients with unknown outcomes are cured is in line with that used in other reports (20). The removal of patients with unknown outcomes from the statistical analysis had no substantial influence on the results: intrathecal IgM synthesis, elevated CSF IgM concentrations of >1.9 mg/liter, CSF LATEX/IgM test end titers ≥2, and CSF IL-10 concentrations >10 pg/ml remained significantly associated with treatment failure (data not shown).
Our findings lead us to question the current staging for HAT. IgM and IL-10 in CSF are typical components of the neuroinflammatory response in patients with the meningoencephalitic stage of T. brucei gambiense infection. Although the exact biological role and the significance of elevated IgM and IL-10 concentrations in CSF remain to be elucidated, our results illustrate the lack of accuracy of the present staging tools, leading to misclassification and the inappropriate treatment of cases. The urgent need for improved staging tools, preferentially point-of-care tests, is apparent. Prospective studies are needed to validate assays for CSF IgM and CSF IL-10 concentrations as alternative staging tests and to assess how they can help clinicians improve their treatment decisions.
Acknowledgments
Financial support was received from the Belgian Ministry of Foreign Affairs, Directorate General for Development Co-Operation.
Logistic, medical, and laboratory support was provided by the Centre de Développement Intégral-Bwamanda (CDI-Bwamanda, NGO); Medische Missie Samenwerking (MEMISA Belgium, NGO); the staff of Bwamanda hospital, in particular, the late sister J. Verbunt; and the Programme National de Lutte contre la Trypanosomiase Humaine Africaine (PNLTHA, Ministry of Health, R.D. Congo). We also thank A. Nangouma (PNLTHA, Central African Republic) for help with transport of the samples, N. Bebronne (ITM, Antwerp, Belgium), and K. Walther (University Göttingen) for help with analysis of the samples.
We do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Bisser, S., V. Lejon, P. M. Preux, B. Bouteille, A. Stanghellini, M. O. Jauberteau, P. Büscher, and M. Dumas. 2002. Blood-cerebrospinal fluid barrier and intrathecal immunoglobulins compared to field diagnosis of central nervous system involvement in sleeping sickness. J. Neurol. Sci. 193:127-135. [DOI] [PubMed] [Google Scholar]
- 2.Bisser, S., F. X. N′Siesi, V. Lejon, P.-M. Preux, S. Van Nieuwenhove, M. M. C. Bilengue, and P. Büscher. 2007. Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness. J. Infect. Dis. 195:322-329. [DOI] [PubMed] [Google Scholar]
- 3.Boa, Y. F., M. A. Traore, F. Doua, M. T. Kouassi-Traore, B. E. Kouassi, and C. Giordano. 1988. Les différents tableaux cliniques actuels de la trypanosomiase humaine Africaine à T. b. gambiense. Analyse de 300 dossiers du foyer de Daloa, Côte-d'Ivoire. Bull. Soc. Pathol. Exot. Fil. 81:427-444. [PubMed] [Google Scholar]
- 4.Büscher, P., V. Lejon, E. Magnus, and N. Van Meirvenne. 1999. Improved latex agglutination test for detection of antibodies in serum and cerebrospinal fluid of Trypanosoma brucei gambiense infected patients. Acta Trop. 73:11-20. [DOI] [PubMed] [Google Scholar]
- 5.Cross, P., F. Doua, and S. Jaffar. 2006. The risk factors for relapse among patients with African trypanosomiasis in Daloa, Cote d'Ivoire. Trop. Doct. 36:90-93. [DOI] [PubMed] [Google Scholar]
- 6.DeLong, E. R., D. M. DeLong, and D. L. Clarke-Pearson. 1988. Comparing the areas under two or more correlated receiver operating curves: a non-parametric approach. Biometrics 44:837-845. [PubMed] [Google Scholar]
- 7.Greenwood, B. M., and H. C. Whittle. 1973. Cerebrospinal-fluid IgM in patients with sleeping-sickness. Lancet ii:525-527. [DOI] [PubMed] [Google Scholar]
- 8.Legros, D., S. Evans, F. Maiso, J. C. K. Enyaru, and D. Mbulamberi. 1999. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans. R. Soc. Trop. Med. Hyg. 93:439-442. [DOI] [PubMed] [Google Scholar]
- 9.Lejon, V., J. Lardon, G. Kenis, L. Pinoges, D. Legros, S. Bisser, X. N'Siesi, E. Bosmans, and P. Büscher. 2002. Interleukin-6, IL-8 and IL-10 in serum and CSF of T. b. gambiense sleeping sickness patients before and after treatment. Trans. R. Soc. Trop. Med. Hyg. 96:329-333. [DOI] [PubMed] [Google Scholar]
- 10.Lejon, V., D. Legros, M. Richer, J. A. Ruiz, V. Jamonneau, F. Doua, N. Djé, F. X. N'Siesi, S. Bisser, E. Magnus, I. Wouters, J. Konings, T. Vervoort, F. Sultan, and P. Büscher. 2002. IgM quantification in the cerebrospinal fluid of sleeping sickness patients by a latex card agglutination test. Trop. Med. Int. Health 7:685-692. [DOI] [PubMed] [Google Scholar]
- 11.Lejon, V., D. Legros, A. Savignoni, M. Gastellu Etchegorry, D. Mbulamberi, and P. Büscher. 2003. Neuro-inflammatory risk factors for treatment failure in “early second stage” sleeping sickness patients treated with pentamidine. J. Neuroimmunol. 144:132-138. [DOI] [PubMed] [Google Scholar]
- 12.Lejon, V., H. Reiber, D. Legros, N. Djé, E. Magnus, I. Wouters, C. Sindic, and P. Büscher. 2003. Intrathecal immune response pattern for improved diagnosis of central nervous system involvement in trypanosomiasis. J. Infect. Dis. 187:1475-1483. [DOI] [PubMed] [Google Scholar]
- 13.Lucasse, C. 1964. Fluorescent antibody test as applied to cerebrospinal fluid in human sleeping sickness. Bull. Soc. Pathol. Exot. Fil. 57:283-292. [PubMed] [Google Scholar]
- 14.MacLean, L., M. Odiit, and J. M. Sternberg. 2001. Nitric oxide and cytokine synthesis in human African trypanosomiasis. J. Infect. Dis. 184:1086-1090. [DOI] [PubMed] [Google Scholar]
- 15.MacLean, L., M. Odiit, and J. M. Sternberg. 2006. Intrathecal cytokine responses in Trypanosoma brucei rhodesiense sleeping sickness patients. Trans. R. Soc. Trop. Med. Hyg. 100:270-275. [DOI] [PubMed] [Google Scholar]
- 16.Miézan, T. W., N. N. Djé, F. Doua, and F. Boa. 2002. Trypanosomose humaine africaine en Côte d'Ivoire: caractéristiques biologiques après traitement. A propos de 812 cas traités dans le foyer de Daloa (Côte d'Ivoire). Bull. Soc. Pathol. Exot. Fil. 95:362-365. [PubMed] [Google Scholar]
- 17.Neujean, G. 1950. Contribution a l'étude des liquides rachidiens et céphaliques dans la maladie du sommeil a “Trypanosoma gambiense”. Ann. Soc. Belg. Med. Trop. 30:1125-1387. [PubMed] [Google Scholar]
- 18.Pépin, J., and F. Milord. 1994. The treatment of human African trypanosomiasis. Adv. Parasitol. 33:1-47. [DOI] [PubMed] [Google Scholar]
- 19.Reiber, H., and J. B. Peter. 2001. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 184:101-122. [DOI] [PubMed] [Google Scholar]
- 20.Schmid, C., M. Richer, M. M. C. Bilengue, T. Josenando, F. Chappuis, C. R. Manthelot, A. Nangouma, F. Doua, P. N. Asumu, P. P. Simarro, and C. Burri. 2005. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: confirmation from a multinational study (Impamel II). J. Infect. Dis. 191:1922-1931. [DOI] [PubMed] [Google Scholar]
- 21.Van Nieuwenhove, S. 1999. Present strategies in the treatment of human African trypanosomiasis, p. 253-281. In M. Dumas, B. Bouteille, and A. Buguet (ed.), Progress in human African trypanosomiasis, sleeping sickness. Springer, Paris, France.
- 22.WHO. 1998. Control and surveillance of African trypanosomiasis. WHO Tech. Rep. Ser. 881:1-113. [PubMed] [Google Scholar]
- 23.Zhou, X.-H., N. A. Obuchowski, and D. K. McClish. 2002. Statistical methods in diagnostic medicine, p. 1-464. John Wiley & Sons, Inc., New York, NY.