Abstract
The detection of urinary Histoplasma capsulatum polysaccharide antigen (HPA) by enzyme immunoassay (EIA) has proven useful for the presumptive diagnosis of histoplasmosis in AIDS patients. Assay limitations include (i) detection of a largely uncharacterized antigen and (ii) difficulty in reproducibly generating antibodies for use in the EIA. To improve antibody production for use in this test and to better understand the antigen being detected, we compared rabbit antibodies elicited using various immunization schedules, routes, and H. capsulatum-derived antigens. Antibodies were evaluated by EIA for their ability to detect purified H. capsulatum C antigen (C-Ag) and antigenuria. Reported as enzyme immunoassay (EI) units (the A450 with antigen divided by the A450 without antigen), results demonstrated that intravenous immunization of rabbits with whole, killed yeast-phase cells (yeast-i.v. regimen) produced antibodies giving the highest EI values in the C-Ag EIA (mean EI units ± standard deviation, 14.9 ± 0.6 versus 6.4 ± 0.4 for rabbits immunized with C-Ag versus 2.4 ± 0.3 for all other regimens combined). Yeast-i.v. antibodies were highly sensitive for the detection of antigenuria in patients with histoplasmosis, as shown by the following results: 12/12 patients compared to 10/12, 6/12, 3/12, and 3/12, respectively, for antibodies from rabbits immunized with (i) C-Ag; (ii) whole, killed yeast-phase cells administered subcutaneously and intramuscularly; (iii) yeast-phase culture filtrates; and (iv) HPA-positive urine. Rabbits immunized using the yeast-i.v. regimen also gave higher peak antibody titers than rabbits immunized by any other regimen (P < 0.03), and their antibodies were most comparable in reactivity to antibodies produced for use in the standard HPA-EIA test (P < 0.001). Therefore, rabbits immunized using the yeast-i.v. regimen produced the most sensitive antibodies with the highest titers for detection of C-Ag and antigenuria in histoplasmosis patients.
Histoplasma capsulatum is a thermally dimorphic fungus that is worldwide in distribution. Endemic to the Mississippi and Ohio River valleys of North America, H. capsulatum conidia are most often found in soil enriched with bird or bat guano (1, 7, 22). Disruption of contaminated soil causes aerosolization of fungal conidia which can then enter the body via inhalation (20). The resulting disease is usually self-limited in healthy individuals but can cause serious, disseminated disease in those with underlying immunosuppression (17, 30). A definitive diagnosis of histoplasmosis is obtained by positive culture from a clinical specimen or by histopathologic evidence of infection in tissues. However, recovery of H. capsulatum from clinical materials requires up to 4 weeks for growth to occur, and histopathologic testing, involving invasive procedures to obtain tissue, is insensitive and requires expertise for interpretation (36). Therefore, serologic tests to detect circulating anti-H. capsulatum antibodies are commonly relied upon as an aid to diagnosis.
Complement fixation and immunodiffusion are standard tests for the serologic diagnosis of histoplasmosis (21). Combined results from both tests help to improve the overall sensitivity and specificity for the diagnosis of histoplasmosis in immunocompetent patients (24). However, antibody titers are often negative or equivocal early in infection, and a second specimen, obtained 3 to 4 weeks later, is required for confirmation, thereby delaying diagnosis. Furthermore, immunosuppressed individuals, who are most at risk for the development of disseminated histoplasmosis, may be antibody deficient, leading to falsely negative serology results (27, 30, 43). For example, it has been reported that complement fixation titers of 1:32 or greater occurred in 83% (25 of 30) of nonimmunocompromised histoplasmosis patients but in only 50% (16 of 32) of immunocompromised patients (P < 0.05) (27). A diagnostic method that does not rely upon an antibody response is therefore especially valuable in such cases. Detection of H. capsulatum polysaccharide antigen (HPA) (13, 42) in body fluids, especially urine, has been useful in the presumptive diagnosis of H. capsulatum infections in patients with disseminated disease. For example, histoplasmosis antigenuria was detected in 92% of patients with disseminated disease, in 39% of patients with self-limited disease, and in 21% of patients with the chronic pulmonary form (31, 40).
The standard format of the HPA detection assay is a double antibody sandwich enzyme immunoassay (HPA-EIA) (13) modified from the original radioimmunoassay format (42). Antibodies raised for both the capture and detection of HPA were produced by immunizing rabbits subcutaneously with whole, killed H. capsulatum yeast-phase cells (in an adjuvant emulsion), followed by intravenous booster immunizations with live organisms (13, 42). Various modifications of the test format have been implemented over time (16, 42a), but the urinary antigen detected has never been purified to homogeneity or fully characterized. Studies to date have indicated that the antigen is primarily (>95%) carbohydrate by weight, is stable to boiling, and is not destroyed by pronase treatment (42). Unfortunately, the efficiency of antibody production against this antigen (42) in rabbits has been very poor; immunization of as many as 40 rabbits can be required to obtain a single rabbit with a sufficiently robust antibody response to be useful in the HPA-EIA.
This lack of a robust antibody response to the HPA antigen may be caused by any number of factors, including the following: (i) a poorly immunogenic antigen, (ii) an immunologically inaccessible antigen, (iii) an insufficient amount of specific antigen, (iv) ineffective or insufficient adjuvant to facilitate an adequate immune response, and/or (v) a nonoptimal immunization schedule and/or immunization route. Therefore, the present study was undertaken (i) to determine the best antigenic preparation to employ as well as the optimum immunization regimen to follow to reproducibly generate the maximum quantity of antibodies to detect H. capsulatum antigenuria and (ii) to obtain a better understanding of the nature of the antigen being detected. To accomplish these objectives, five different immunization regimens and several different H. capsulatum-derived antigen preparations were examined. Antibodies were then evaluated and compared to one another and to antibodies produced for use in the standard commercial HPA-EIA for their capacity to detect chromatographically purified H. capsulatum polysaccharide C antigen (C-Ag) and to detect antigenuria in histoplasmosis patients.
MATERIALS AND METHODS
Microorganism.
A clinical isolate of H. capsulatum (Thon strain) was kindly provided by L. Joseph Wheat, MiraVista Laboratories, Indianapolis, IN, and was used throughout this study to produce antigens for rabbit immunizations.
Chemicals.
All chemicals were obtained from Sigma-Aldrich, Co., St. Louis, MO, unless otherwise indicated.
Patient urine.
Banked patient urine samples (with direct identifiers removed) from 12 patients with histoplasmosis were obtained from L. Joseph Wheat (MiraVista Laboratories). Normal human urine was voluntarily obtained from healthy adult subjects at the Centers for Disease Control and Prevention (CDC).
Preparation of antigens for rabbit immunizations.
Rabbits were immunized by one of five different regimens using one of the four antigen preparations described below.
(i) Whole, killed yeast cells.
H. capsulatum yeast-phase cells were inoculated into Pine's liquid medium for yeast-phase H. capsulatum (23), and cultures were placed at 37°C on a platform shaker rotating at 150 rpm. After incubation for 72 h, thimerosal was added to the culture to a final concentration of 0.02%. Cultures were then incubated in the presence of thimerosal for an additional 48 h at 37°C with shaking. Yeast cells were harvested by centrifugation at 2,100 × g for 10 min and washed three times in 0.01 M phosphate-buffered saline (PBS; 8.1 mM Na2HPO4, 1.9 mM KH2PO4, 0.15 M NaCl), pH 7.2. Yeast cells were then suspended in PBS containing 0.01% (wt/vol) thimerosal to a final concentration of 30% (vol/vol) packed yeast cells. Suspensions were frozen at −20°C until used.
Immediately before use, yeast-phase H. capsulatum cells were thawed and diluted in PBS-thimerosal to a 0.2% (vol/vol) suspension of packed yeast cells for intravenous immunizations and to a 20% (vol/vol) suspension of packed yeast cells for subcutaneous immunizations. Sterility checks were performed by incubating 100-μl aliquots of the thimerosal-treated yeast cells on brain heart infusion agar containing 5% sheep erythrocytes (BBL-Becton Dickinson, Sparks, MD) and on Sabouraud dextrose agar (BBL) for 8 days at both 25°C and 37°C.
(ii) C-Ag.
Culture filtrates from mycelial-phase growth of H. capsulatum (45) were purified chromatographically using a CM-Sepharose column as described previously (44). The resulting C-Ag preparation contained no detectable H or M antigens as determined by immunodiffusion assay against H. capsulatum reference antisera (24). The C-Ag preparation contained >95% carbohydrate, as measured by the micro phenol-sulfuric acid procedure (12), and <1% protein, as determined by the Bradford assay (4). The final C-Ag preparation contained 10 mg of carbohydrate per ml in 0.01 M borate-buffered saline (9.5 mM H3BO3, 0.5 mM Na2B4O7, and 0.15 M NaCl, pH 8.0).
(iii) HPA-positive urine.
HPA-positive urine from a patient with proven disseminated histoplasmosis was kindly provided by L. Joseph Wheat. Urine had been autoclaved and concentrated 20-fold before receipt. Analysis of the concentrated, HPA-positive urine by the phenol-sulfuric acid and Bradford assays demonstrated that the final carbohydrate and protein concentrations for this antigen preparation were 19.9 mg/ml and 5.67 mg/ml, respectively.
(iv) Yeast-phase culture filtrate antigen.
Pine's liquid medium for yeast-phase H. capsulatum (23) was inoculated with H. capsulatum yeast-phase cells and incubated at 37°C for 7 days on a rotating platform (150 rpm). Culture supernatants were harvested by centrifugation at 10,000 × g for 20 min and were concentrated 10-fold by pressure ultrafiltration using a YM-10 membrane (Amicon, Inc., Danvers, MA). The resulting 10-fold concentrate was further concentrated 2.6-fold by using a centrifugal filtration unit (Centricon instrument with a 10,000-molecular-weight cutoff; Millipore Corp., Billerica, MA). The 26-fold concentrated yeast-phase culture filtrate antigen demonstrated final carbohydrate and protein concentrations of 20 mg/ml and 1.5 mg/ml, respectively.
Immunization regimens.
Female, New Zealand White rabbits, 2 to 3 kg in weight, were used in the following experiments. Rabbits were fed and watered ad libitum, and all Institutional Animal Care and Use Committee recommendations were followed. Twenty rabbits were divided into five groups such that each group was subjected to one of the following immunization regimens: protocol 1, intravenous injection followed by subcutaneous booster injections with whole, killed H. capsulatum yeast-phase cells (yeast-i.v. group); protocol 2, subcutaneous and intramuscular injection followed by subcutaneous booster injections with H. capsulatum-derived C-Ag (C-Ag group); protocol 3, subcutaneous and intramuscular injection followed by subcutaneous booster injections with whole, killed H. capsulatum yeast-phase cells (yeast-s.c. group); protocol 4, subcutaneous and intramuscular injection followed by subcutaneous booster injections with concentrated HPA-positive patient urine (H. capsulatum antigen-positive urine group); and protocol 5, subcutaneous and intramuscular injection followed by subcutaneous booster injections with concentrated H. capsulatum yeast-phase culture filtrate antigen (culture filtrate group). One rabbit immunized using protocol 3 died during the immunization process, prior to the generation of a significant antibody response, and was eliminated from further evaluation.
(i) Protocol 1.
Four rabbits were injected intravenously with 1 ml each of whole, killed H. capsulatum yeast-phase cells on days 0, 1, 4, 8, 10, 15, 18, 23, 29, and 32. After day 56, rabbits were boosted every 4 to 6 weeks by injecting a total of 1 ml of whole, killed H. capsulatum yeast-phase cells subcutaneously at four dorsal sites. Rabbits were bled from the central ear artery on day 0 (before immunization) and on days 14, 28, 42, 49, and 56 and then weekly beginning 7 to 10 days after each booster injection.
(ii) Protocols 2 through 5.
All antigen preparations for immunization protocols 2 through 5 were made by emulsifying equal volumes of the given antigen with an adjuvant (TiterMax USA, Inc., Norcross, GA). Emulsification was facilitated by repeated passage of the antigen-adjuvant mixture through a double-hubbed syringe. Initially, each rabbit received 0.2 ml of the emulsion in four different inoculation sites: subcutaneously over each shoulder and intramuscularly in each hind quarter. After day 56, rabbits were boosted subcutaneously every 4 to 6 weeks at the original immunization sites, but antigen was suspended in saline rather than in adjuvant. Each rabbit was bled on day 0 (before immunization) and on days 14, 28, 42, 49, and 56 and then weekly beginning 7 to 10 days after each booster injection.
Indirect EIA to screen rabbit antisera.
Chromatographically purified C-Ag (44) was diluted to 0.1 μg/ml in PBS containing 0.01% (wt/vol) sodium azide. One hundred microliters of this solution was then added to each well of a flat-bottom microtiter plate (Immulon 2 HB; Thermo Electron Corp., Milford, MA). Plates were covered with plastic film and held at 4°C for 48 to 72 h. Microtiter plate wells were then washed three times with distilled water, and 100 μl of PBS containing 0.025% (wt/vol) sodium azide was added to each well. Covered plates were stored at 4°C for up to 7 days before use. Immediately before use, plates were washed three times with PBS containing 0.05% (vol/vol) Tween 20 (PBS-T), and any remaining fluid was removed by vigorous inversion onto paper towels.
Antiserum from each of the immunized rabbits was serially diluted from 1:1,000 to 1:512,000 in PBS-T containing 0.1% (wt/vol) bovine serum albumin (BSA). Serially diluted antiserum was then added to the wells of the microtiter plates. Plates were covered with plastic film and incubated for 1 h at ambient temperature. Microtiter plate wells were then washed three times with PBS-T (without BSA) and vigorously inverted onto paper towels to remove any remaining fluid. One hundred microliters of horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) (H+L; Bio-Rad Laboratories, Hercules, CA), diluted 1:2,000 in PBS-T, was then added to each well. Plates were covered with plastic film and incubated for 1 h at ambient temperature. The wells were washed four times with PBS-T before addition of 100 μl of a colorimetric substrate mixture (50:50 [vol/vol] mixture of 3,3′,5, 5′-tetramethylbenzidine [TMB] colorimetric substrate and H2O2, supplied in a reagent kit; KPL, Inc., Gaithersburg, MD). Plates were incubated for 30 min, and results were obtained spectrophotometrically at A650 (SpectraMax 250; Molecular Devices Corp., Sunnyvale, CA).
Antibody purification.
Purification of rabbit Igs was initially performed using standard ammonium sulfate precipitation methods (19). Briefly, equal volumes of rabbit antiserum and 70% saturated ammonium sulfate solution were combined and incubated at room temperature for 4 h with continuous gentle stirring. The preparation was then centrifuged at 10,000 × g for 5 min, and the pellet was resuspended in distilled water to the original volume of antiserum. An equal volume of 70% saturated ammonium sulfate was added with continuous gentle stirring. The mixture was centrifuged at 10,000 × g for 5 min, and the ammonium sulfate precipitation procedure was repeated once again. The sample was centrifuged as before, and the pellet was resuspended to one-half of the original volume of antiserum using distilled water. The ammonium sulfate-precipitated antibodies were then dialyzed overnight at 4°C against 1,000 volumes of PBS (Slide-A-Lyzers; Pierce Chemical Co., Rockford, IL). The dialyzed sample was assayed for protein content using a bicinchoninic acid protein assay reagent kit (Pierce). All antibody preparations were suspended to a final concentration of 10 mg/ml and were stored at −20°C until used.
Dot blot enzyme immunoassay.
One microliter of each of the following was pipetted onto strips cut from nitrocellulose membrane (Optitran, 0.45-μm porosity; Schleicher & Schuell BioScience, Inc., Keene, NH) to produce discrete dots: C-Ag (1.6, 8, and 40 μg/ml), normal human urine, and PBS. The strips were allowed to dry at ambient temperature for approximately 20 min before placement into individual lanes of a strip incubation tray (Bio-Rad). The following incubation and wash steps were performed at ambient temperature on a rocker platform unless otherwise indicated. To each lane of the tray, 5 ml of 5% (wt/vol) nonfat dried milk in PBS-T was added as a blocking agent, and strips were incubated in this solution for 5 min. The blocking solution was removed by aspiration, and each strip was washed three times with PBS-T for 5 min. Purified rabbit antibodies (3.5 ml), obtained from each of the five immunization protocols, were diluted to 25 μg/ml in PBS-T and added to a given lane of the tray. Strips were incubated for 1 h and then washed for 5 min with PBS-T that had been heated to 50°C. This wash was followed by three 5-min washes with PBS-T at ambient temperature. After aspiration of the wash solution, 5 ml of a 1:4,000 dilution of horseradish peroxidase-labeled goat anti-rabbit IgG (Bio-Rad) in PBS-T was added to each lane, and trays were incubated for 1 h. Strips were then washed four times for 5 min each with PBS-T followed by a single wash with PBS only for 5 min. Wash fluid was removed and replaced with 5 ml of a colorimetric substrate solution, made immediately before use by mixing 7.5 μl of a 30% solution of H2O2 with 75 ml of a 0.05% (wt/vol) solution of DAB (3′3′-diaminobenzidine tetrachloride dehydrate) made in PBS. Strips were incubated in the H2O2-DAB solution for 20 min before being washed three times for 5 min each with distilled H2O. Strips were allowed to dry overnight before the intensity of the colorimetric reaction was recorded.
Biotinylation of purified antibodies.
One-half of the ammonium sulfate-purified antibodies were labeled with biotin for use in a double-antibody EIA. Biotinylation of purified antibodies was conducted using the EZ-Link biotin hydrazide kit (Pierce) according to the manufacturer's instructions. Five hundred microliters of ammonium sulfate-purified antibodies (10 mg/ml) was placed in a Slide-A-Lyzer 2K Dialysis Cassette (Pierce) and dialyzed overnight at 4°C against 200 volumes of 0.1 M sodium acetate coupling buffer (86 mM sodium acetate [C2H3NaO2], 14 mM glacial acetic acid [C2H4O2], pH 5.50). The antibody was removed from the dialysis cassette and diluted with fresh coupling buffer to a final concentration of 2 mg/ml and placed at 4°C. Once the solution reached 4°C, an equal volume of sodium meta-periodate (dissolved in coupling buffer to a concentration of 20 mM and equilibrated to 4°C) was added, and the mixture was incubated in the dark for 30 min at 4°C. Glycerol was then added to a final concentration of 15 mM, and the mixture was held at 4°C for 5 min. The solution was then dialyzed overnight at 4°C against 1,000 volumes of coupling buffer. One part of 50 mM biotin hydrazide, dissolved in dimethyl sulfoxide, was added to nine parts of the antibody preparation. The mixture was agitated by rotation for 2 h at 60 rpm in the dark at ambient temperature. The sample was then dialyzed overnight at 4°C against 1,000 volumes of PBS. An equal volume of glycerol was added to the dialyzed sample to yield a final antibody concentration of 0.5 mg/ml. This solution was stored at −20°C until used.
Double-antibody sandwich EIA.
Ammonium sulfate-purified anti-H. capsulatum antibodies, produced from each of the immunization protocols 1 to 5, or anti-H. capsulatum antibodies (anti-HPA antibodies) provided by L. Joseph Wheat were diluted to 20 μg/ml in 0.01 M Tris-HCl buffer, pH 7.0. One hundred microliters of purified antibodies was then added to each well of a flat-bottom microtiter plate (Immulon 2 HB; Thermo Electron Corp., Milford, MA). The plate was covered with a plastic lid and incubated at 37°C for 1 h. Plates were then washed six times with PBS-T using an ELX50 Autostrip washer (BioTek Instruments, Inc., Winooski, VT). Residual wash buffer was removed from the microtiter plate wells by vigorous inversion of the plate onto paper towels. Two hundred microliters of 5% (wt/vol) BSA solution in 0.01 M Tris-HCl buffer, pH 7.0, was then added to each well. The plate was covered with a plastic lid, incubated at 37°C for 1 h, and then washed as described above. Urine samples from patients and healthy individuals were heated at 100°C for 5 min and allowed to cool before 100 μl of each sample was added to duplicate wells of the precoated microtiter plate. Positive and negative control wells, respectively, contained 100 μl of a 0.32 μg/ml solution of purified C-Ag and 100 μl of 0.01 M Tris-HCl buffer, pH 7.0. After addition of the test samples, the plate was covered with a plastic lid, incubated at 37°C for 1 h, and washed as before. One hundred microliters of biotinylated rabbit anti-H. capsulatum antibody in 0.1 M Tris-HCl buffer, pH 8.0, was then added to each well, and plates were incubated at 37°C for 1 h. Plates were washed as before, and 100 μl of a 1:1,000 dilution of streptavidin-horseradish peroxidase (Pierce), diluted in 0.1 M Tris-HCl buffer (pH 8.0) containing 5% (wt/vol) BSA, was added per well. The plate was again covered with a plastic lid, incubated at 37°C for 1 h, and washed as before. One hundred microliters of a colorimetric substrate solution (50:50 [vol/vol] mixture of TMB and H2O2; KPL) was then added to each well, and plates were incubated at ambient temperature for 15 min. The reaction was stopped by the addition of 100 μl of 1.0 M H2SO4 to each well, and plates were read spectrophotometrically at A450 using a SpectraMax 250 microtiter plate reader (Molecular Devices). The coefficient of variance for within-run testing ranged from 1.7 to 6.4%, and the coefficient of variance for between-run testing ranged from 3.9 to 7.7%, depending on the concentration of C-Ag tested.
The enzyme immunoassay index (EI) values were calculated by dividing the mean A450 value obtained for wells containing patient urine by the mean A450 value obtained for wells containing urine from healthy controls. EI values for the positive antigen control wells were calculated by dividing the mean A450 value obtained for wells containing purified C-Ag by the mean A450 value obtained for wells that received 0.01 M Tris-HCl buffer, pH 7.0. A positive EIA result using patient urine was defined as an EI value of ≥1.
Limit of sensitivity for the detection of serially diluted antigen.
The lower limit of assay sensitivity was determined using fivefold serial dilutions of chromatographically purified C-Ag (range, 0.1 to 8,000 ng/ml) in a double-antibody sandwich EIA using antibodies produced by the yeast-i.v. immunization regimen.
Statistical analyses.
Differences between antibody titers obtained for each of the immunization regimens were determined using the exact Wilcoxon-2 sample test. Differences between immunization groups in the mean EI values obtained using the indirect antibody EIA and the double-antibody sandwich EIA were determined using the paired Student t test. Pearson's r correlation coefficient was calculated to determine correlations between test groups. Differences between comparison groups as determined using any of the statistical tests employed were considered to be significant when the P value was ≤0.05.
RESULTS
Screening rabbit antisera in an indirect EIA.
To evaluate and compare sera produced by each of five different immunization regimens for their potential use in the detection of H. capsulatum urinary antigen, 180 sera from 19 rabbits (representing three to four rabbits from each of the five regimens examined) were collected at regular intervals throughout the immunization process. Sera were then tested in an indirect EIA against chromatographically purified H. capsulatum C-Ag (44). C-Ag was used to coat microtiter plates for the initial screening assay because the HPA urinary antigen detected by commercial anti-HPA antibodies has never been purified to homogeneity or fully characterized. Therefore, HPA was not available in purified form for use as a screening antigen. For this reason and because C-Ag shares many of the properties attributed to HPA (e.g., HPA is primarily [>95%] carbohydrate by weight, is stable to boiling, is not destroyed by pronase or nuclease treatment, is destroyed by mixed glycosidases or periodate treatment, and is removed by binding to concanavalin A) (42), C-Ag served as a logical antigen for screening rabbit antisera for use in the detection of H. capsulatum antigenuria. Further, the commercial HPA-EIA has also characteristically demonstrated cross-reactivity among specimens obtained from patients with diseases caused by fungi known to share common cell wall carbohydrate antigens such as the C-Ag (2, 11, 26, 33, 35, 44).
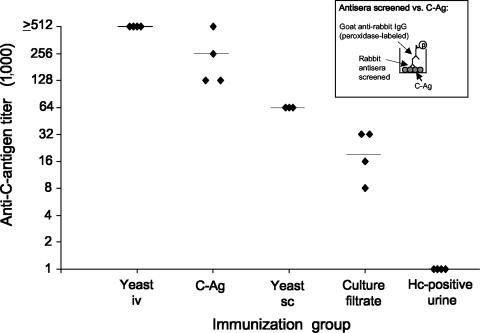
Figure 1 depicts the peak anti-C-Ag antibody titers obtained for each of the various immunization regimens employed when tested in the indirect EIA. Rabbits immunized using the yeast-i.v. protocol most consistently demonstrated high peak antibody titers. All four rabbits in this group demonstrated antibody titers of ≥1:512,000 (Fig. 1). Rabbits immunized using the C-Ag protocol demonstrated peak titers that varied from 1:128,000 to ≥1:512,000 among the four rabbits immunized. Peak antibody titers ranged from 1:8,000 to 1:32,000 among the four rabbits immunized with H. capsulatum yeast-phase culture filtrate antigens (culture filtrate) (Fig. 1). Three rabbits immunized with whole, killed H. capsulatum yeast-phase cells, administered subcutaneously and intramuscularly (yeast-s.c.), produced peak antibody titers of 1:64,000. In contrast, rabbits immunized with concentrated urine from a patient with disseminated histoplasmosis (H. capsulatum antigen-positive urine) displayed the lowest antibody response (i.e., all four rabbits produced peak antibody titers of only 1:1,000).
FIG. 1.
Evaluation of antisera to detect C-Ag in an indirect EIA. Rabbits were either immunized intravenously using whole, killed H. capsulatum yeast-phase cells (yeast-i.v.) or immunized subcutaneously and intramuscularly using one of the following immunogens: chromatographically purified C-antigen (C-Ag); whole, killed H. capsulatum yeast-phase cells (yeast-s.c.); filtrates from H. capsulatum yeast-phase cell cultures (culture filtrate); or concentrated urine from a patient with disseminated histoplasmosis (H. capsulatum antigen-positive urine). Sera were screened against chromatographically purified C-Ag in an indirect EIA (see inset). Maximum EIA titers obtained for three to four rabbits in each immunization group are shown. Each symbol (⧫) represents one rabbit. The geometric mean antibody titer obtained for each immunization group is represented by a horizontal bar; maximum titers varied among immunization groups from as high as ≥1:512,000 (yeast-i.v. and C-Ag) to as low as 1:1,000 (H. capsulatum antigen-positive urine). Hc, H. capsulatum.
Pairwise comparisons between immunization groups indicated that sera from rabbits immunized intravenously with whole, killed H. capsulatum yeast-phase cells (yeast-i.v.), as well as rabbits immunized with purified C-Ag, demonstrated peak antibody titers that were significantly greater (P < 0.03) than those obtained using any of the remaining three immunization regimens (Fig. 1). It was further shown that use of the yeast-s.c. protocol resulted in peak antibody titers that were significantly greater (P < 0.03) than those for both the culture filtrate and the H. capsulatum antigen-positive urine protocols. Peak antibody titers for rabbits immunized by the culture filtrate protocol were also found to be significantly greater (P < 0.03) than those for rabbits immunized by the H. capsulatum antigen-positive urine protocol (Fig. 1). Therefore, rabbits immunized using the yeast-i.v. protocol demonstrated the highest peak antibody titers and also the most consistently high peak antibody titers (i.e., all four rabbits gave antibody titers of ≥1:512,000).
The mean time required for rabbits that had been immunized by the yeast-i.v. regimen to reach peak antibody titers was 32 days (range, 28 to 42 days). This compares to 49 days (H. capsulatum antigen-positive urine group), 53 days (culture filtrate group), 71 days (C-Ag group), and 79 days (yeast-s.c. group) to reach peak antibody titers when rabbits were immunized by other methods. Peak antibody titers remained stable for 32 to 58 days (32 days for the yeast-i.v. group and 58 days for the C-Ag group; rabbits in all other immunization groups sustained peak antibody levels for intervals between 32 and 58 days). These data suggest that the yeast-i.v. immunization regimen resulted in the most consistently high peak antibody titers and that peak titers for this group were attained in a little over 1 month following initial immunization. Interestingly, the highest peak EIA titers obtained for antibodies from the yeast-i.v. immunization group were obtained before subcutaneous booster immunizations were initiated. Therefore, these data indicate that the intravenous immunization of rabbits with whole, killed H. capsulatum yeast-phase cells alone was responsible for the majority of the immune response observed and that subsequent subcutaneous booster immunizations are not necessary.
Reactivity of purified antibodies, including anti-HPA antibodies, with C-Ag in a dot blot enzyme immunoassay.
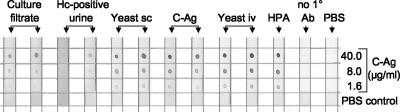
Sera from two rabbits from each immunization group that demonstrated the highest titers in the indirect EIA were selected, purified by ammonium sulfate precipitation, and adjusted to a 25 μg/ml concentration. These antibodies, along with anti-HPA antibodies obtained from L. Joseph Wheat, were evaluated in an indirect dot immunoblot assay to detect C-Ag. Replicate nitrocellulose membrane strips were dotted with three concentrations of serially diluted, chromatographically purified C-Ag (40, 8, and 1.6 μg/ml, respectively) and reacted with antibodies from each of the immunization groups or with anti-HPA antibodies (Fig. 2). Antibodies from rabbits that had been immunized using the yeast-i.v. protocol demonstrated the strongest reactivity to C-Ag, and this reactivity was dose dependent. Reactivity of a similar, dose-dependent intensity was observed using rabbit anti-HPA antibodies (Fig. 2, HPA). These data indicate that the antibodies used in the standard HPA-EIA urinary antigen test (13) detect the C-Ag of H. capsulatum in a dose-dependent manner and suggest that the antigen detected in the HPA-EIA may be, at least in part, the C-Ag of H. capsulatum.
FIG. 2.
Reactivity of purified antibodies, including anti-HPA antibodies, with C-Ag in a dot blot enzyme immunoassay. C-Ag was dotted onto nitrocellulose membrane strips at concentrations of 40, 8, or 1.6 μg/ml and reacted with purified antibodies from each of the immunization groups described in the legend of Fig. 1. In addition, antibodies produced for use in the commercial HPA-EIA (HPA) were tested in parallel. Membrane strips that received PBS instead of primary antibody (no 1° Ab) or instead of all reagents (PBS) served as negative controls and did not react with any antibody tested. Pooled normal human urine also did not react with any antibody tested (not shown). In contrast, all antibodies reacted with C-Ag in a dose-dependent manner, although to various degrees. Hc, H. capsulatum.
Antibodies produced by rabbits in the remaining immunization groups also reacted with purified C-Ag in a dose-dependent manner; however, the intensity of this reactivity varied considerably among the different immunization groups. For example, antibodies produced using H. capsulatum antigen-positive urine as the immunogen demonstrated weak reactivity with C-Ag (Fig. 2). Antibodies from rabbits immunized by the yeast-s.c. and C-Ag regimens gave reactions that were robust, although less intense than those for the yeast-i.v. immunization group. Lastly, antibodies from rabbits immunized using the culture filtrate immunization regimen reacted even less strongly than antibodies from the yeast-s.c. and C-Ag groups. No C-Ag reactivity was observed using normal human urine (not shown), PBS as a negative control (Fig. 2, PBS), or in the absence of primary antibody (Fig. 2, no 1° Ab).
Comparison of the anti-C-Ag reactivities of antibodies from each immunization group using a double-antibody sandwich EIA.
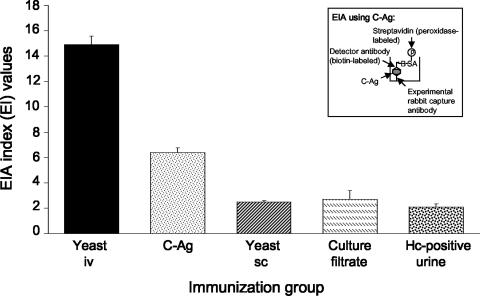
Antibodies from the most C-Ag-reactive rabbit from each immunization group were then tested in a colorimetric double-antibody sandwich EIA to measure the capacity of each to capture purified C-Ag onto microtiter plates (Fig. 3). The same biotinylated anti-HPA detector antibody was used throughout so that the only variable among test groups was the capture antibody employed. Five (replicate) tests were performed using antibodies from each of the five immunization groups. Antibodies obtained from the yeast-i.v. immunization regimen demonstrated the greatest capacity to capture C-Ag (mean EI value ± standard deviation [SD], 14.9 ± 0.6) (Fig. 3) among antibodies from all immunization groups. Anti-HPA antibodies demonstrated lower reactivity when employed as the capture antibodies (mean EI value ± SD, 11.3 ± 0.6; data not shown in Fig. 3) than those from the yeast-i.v. immunization group. Antibodies obtained from the C-Ag immunization group demonstrated substantially lower reactivity than antibodies from the yeast-i.v. immunization group (mean EI value ± SD, 6.4 ± 0.4) but displayed higher reactivity than antibodies from all remaining immunization groups (mean EI value ± SD for all remaining immunization groups combined, 2.4 ± 0.3).
FIG. 3.
Comparison of antibodies from each immunization group using a double-antibody sandwich EIA. The most reactive antibodies within each immunization group were used to capture C-Ag onto microtiter plates in a double-antibody sandwich EIA (see inset). Immunization groups were as noted in the legend of Fig. 1. Biotinylated anti-HPA antibodies were used throughout as detector antibodies. The bars and corresponding standard deviations represent the mean and standard deviations of five replicate EIA index measurements. Antibodies produced by the yeast-i.v. immunization regimen demonstrated the highest reactivity to C-Ag compared to all other immunization groups. Reactivity of anti-HPA antibodies (not shown) was intermediate to that of the yeast-i.v. and C-Ag group antibodies. Hc, H. capsulatum.
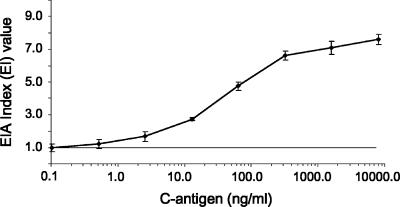
Limit of sensitivity to detect antigen.
Fivefold serial dilutions of purified C-Ag (range, 0.1 to 8,000 ng/ml) were tested in the double-antibody sandwich EIA format using antibodies produced by the yeast-i.v. immunization regimen. The results are shown in Fig. 4. Using one EI unit as the cutoff value for a positive reaction, the assay could detect between 0.1 and 0.5 ng/ml of C-Ag.
FIG. 4.
Limit of sensitivity for the detection of antigen. Antibodies produced by the yeast-i.v. immunization regimen were used to determine the lower limit of assay sensitivity. Fivefold serial dilutions of purified C-Ag (range, 0.1 to 8,000 ng/ml) were tested in the double-antibody sandwich EIA format. The results represent the mean and standard deviation of results from three independent experiments. The horizontal line represents a positive cutoff value defined as one EI unit.
Evaluating the double-antibody sandwich EIA using urine from patients with histoplasmosis.
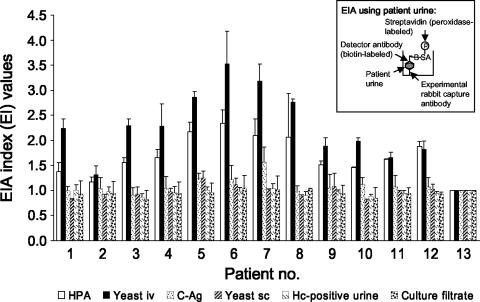
To ensure that the C-Ag reactivity of the antibodies produced by each of the various immunization regimens correlated with their capacity to detect antigenuria in patients, antibodies from each of the immunization groups were used in a double-antibody sandwich EIA to detect antigenuria in patients with confirmed histoplasmosis (Fig. 5). Antibodies produced using the yeast-i.v. protocol detected antigen in the urine of 12 of 12 patients (100%) with histoplasmosis as did anti-HPA antibodies (Fig. 5, HPA). EIA reactivity was higher using antibodies derived from the yeast-i.v. immunization regimen (mean EI value ± SD, 2.32 ± 0.65; n = 12) than using anti-HPA antibodies (mean EI value ± SD, 1.74 ± 0.36; n = 12; P < 0.02). Nonetheless, antibodies from both sources detected all cases of histoplasmosis (12 of 12 patients). Antibodies produced using any of the other immunization regimens also detected antigenuria in histoplasmosis patients to various degrees: 10 of 12 (83%, C-Ag regimen), 6 of 12 (50%, yeast-s.c. regimen), 3 of 12 (25%, culture filtrate regimen), and 3 of 12 (25%, H. capsulatum antigen-positive urine regimen).
FIG. 5.
Comparison of anti-HPA antibodies and antibodies from five different immunization regimens to detect antigen in the urine of patients with histoplasmosis by double-antibody sandwich EIA. Immunization groups are as noted in the legend of Fig. 1. Antibodies produced using the yeast-i.v. immunization regimen detected 12 of 12 histoplasmosis patients (100%) as did anti-HPA antibodies (HPA) in the double-antibody sandwich EIA format (inset). Antibodies produced using any of the other immunization regimens also reacted with patient urines to various degrees, although all were significantly less sensitive (P < 0.02) than antibodies produced by the yeast-i.v. and C-Ag immunization regimens. A direct correlation (r = 0.97, P < 0.001) was observed between antibody reactivity to C-Ag and the detection of antigenuria by double-antibody sandwich EIA. The bars and corresponding standard deviations shown represent the mean and standard deviations of two replicate EIA index measurements for urines from each of 12 histoplasmosis patients (patient numbers 1 to 12) and a healthy control (specimen number 13). Hc, H. capsulatum.
A direct correlation (r = 0.97, P < 0.001) was observed between antibody reactivity to C-Ag and the detection of antigenuria by enzyme immunoassay. For example, antibodies produced using the yeast-i.v. immunization regimen demonstrated the greatest mean reactivity to C-Ag (mean EI value ± SD, 14.9 ± 0.6) and also demonstrated the greatest mean reactivity to patient urine (mean EI value ± SD, 2.32 ± 0.6). Mean reactivity to C-Ag was lowest for antibodies produced by the culture filtrate, H. capsulatum antigen-positive urine, and yeast-s.c. immunization regimens, and these antibodies gave the lowest mean reactivity to patient urine.
Correlation of results between the HPA-EIA and the double-antibody EIA using antibodies from each of five immunization regimens for the detection of histoplasmosis antigenuria.
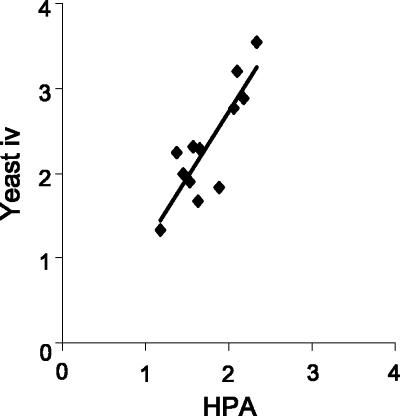
The correlation between results obtained for the HPA-EIA and those for the double-antibody EIA to detect antigenuria in histoplasmosis patients was strongest when antibodies from intravenously immunized rabbits (yeast-i.v.) were employed (Pearson's r = 0.85, P < 0.001) (Fig. 6). These data indicate that the double-antibody sandwich EIA using antibodies from the yeast-i.v. immunization regimen correlates favorably with the HPA-EIA for the detection of histoplasmosis antigenuria. The correlation between the results obtained for the HPA-EIA and the double-antibody sandwich EIA using antibodies from the other immunization groups was less strong (Pearson's r ranged from 0.24 to 0.67; P > 0.05 to P < 0.02).
FIG. 6.
Direct correlation between results from the HPA-EIA and the double-antibody EIA to detect antigenuria in histoplasmosis patients. EI values obtained using the HPA-EIA test to detect antigenuria directly correlated with those from the double-antibody EIA test when antibodies from the yeast-i.v. immunization regimen were employed (Pearson's r = 0.85, P < 0.001).
DISCUSSION
Of the major endemic mycoses, histoplasmosis is responsible for the greatest number of hospitalizations in the United States and has a mortality rate of 7.5% in this setting (9). In human immunodeficiency virus (HIV)-infected persons, institution of highly active antiretroviral therapy has been associated with a reduced risk of developing the most severe, life-threatening form of the disease (15). However, access to highly active antiretroviral therapy is limited in many developing countries in which histoplasmosis is endemic, and incidence rates have been reported to be as high as 21% (6) and mortality rates to be as high as 32% (10). Early detection and implementation of appropriate therapy have been shown to reduce morbidity and mortality (32). Therefore, a rapid, effective, and low-cost diagnostic test is needed in countries where both HIV and histoplasmosis are endemic. In order to produce a cost-effective test, an improved method must be developed to efficiently produce antibodies for the detection of H. capsulatum antigen.
The primary objective of the research reported here was to compare and contrast the use of a variety of immunization routes, schedules, and H. capsulatum-derived antigens to determine the optimum method for the production of antibodies useful for the detection of H. capsulatum antigenuria. Antibodies were then compared to those produced for use in a standard commercial HPA-EIA antigenuria test. We found that rabbits immunized by the intravenous route with whole, killed H. capsulatum yeast-phase cells produced the most consistently reactive antibodies for the detection of chromatographically purified C-Ag as well as for the detection of antigenuria in patients with histoplasmosis. Reactivity of these antibodies with C-Ag and with patient urine gave results comparable to those obtained using antibodies produced for the HPA-EIA.
Intravenous immunization of rabbits with whole, killed yeast-phase cells produced antisera with consistently high titers in 100% of rabbits immunized (4 of 4 rabbits immunized gave titers of ≥1:512,000). This contrasts to methods used to produce antibodies for the HPA-EIA, where immunization of as many as 40 rabbits was required to produce a single, high-responding rabbit. Production of antibodies for the HPA-EIA used antigen in incomplete Freund's adjuvant as part of the immunization regimen as well as intravenous injection of live H. capsulatum yeast-phase cells (42). This immunization regimen was later modified so that intravenous immunizations were no longer given; intramuscular and subcutaneous injections of antigen in Freund's complete adjuvant, followed by booster immunizations in Freund's incomplete adjuvant, were used. In contrast, rabbits producing antisera with consistently high titers in our study were immunized by the intravenous route, and no adjuvant was necessary to obtain peak antibody titers. Indeed, although an adjuvant (TiterMax) was employed for subcutaneous booster injections of the yeast-i.v. immunization group, antibody titers peaked in these rabbits before booster injections were initiated. This lack of dependence on adjuvant for the production of highly reactive antibodies is an especially important feature of this method not only because it saves time, labor, and expense but also because of animal welfare considerations. Although there is some controversy regarding whether judicious use of newer formulations of Freund's adjuvant may be acceptable for the augmentation of polyclonal antibody production in rabbits (18, 28), historical evidence indicates that deleterious side effects such as focal necrosis and ulceration of the skin can occur in a dose-dependent manner (5). Finally, the amount of time required to obtain peak antibody titers using the intravenous immunization method was only 32 days, and titers remained at peak levels for at least an additional 32 days. In contrast, rabbits were not reported to have reached peak antibody titers until 104 or more days after initial immunization in the HPA-EIA antibody production regimen (although no data were presented regarding test bleeds or antibody titers at earlier time points).
A second objective of the research reported here was to better understand the nature of the antigen being detected by anti-HPA antibodies and by antibodies raised by our own immunization regimens. The intravenous immunization method used whole, formalin-killed H. capsulatum yeast-phase cells as the immunogen. This was the same immunogen employed to produce antibodies for use in the HPA-EIA test (29, 42). The rationale for using C-Ag to screen rabbit antisera for use in our test was based on the following considerations. One of the limitations of the HPA-EIA test has been its detection of a largely unknown and poorly characterized antigen that has not been purified to homogeneity. Studies to date have indicated that this antigen is primarily carbohydrate in nature, based on its stability during boiling, resistance to degradation by various proteases and nucleases, destruction by mixed glycosidases or periodate treatment, and removal by binding to concanavalin A (42). The HPA-EIA has also characteristically demonstrated cross-reactivity when specimens from patients with diseases caused by fungi known to share common cell wall carbohydrate antigens (e.g., blastomycosis and paracoccidioidomycosis) (2, 14, 35) are tested. It was therefore postulated that the antigen detected in the HPA-EIA test may be, at least in part, the C-Ag of H. capsulatum. The true identity of the H. capsulatum polysaccharide antigen may never be determined, primarily because it is found in such low concentrations in patient urine (i.e., in nanogram-per-milliliter concentrations in urine) (references 14 and 42a and the present study) that purification to homogeneity would be difficult. Concentration of patient urine before purification may be helpful, as a 20-fold concentration of HPA-positive patient urine resulted in a total carbohydrate content of 19.9 mg/ml (this study). However, it is uncertain how much of the carbohydrate content measured in the concentrated HPA-positive urine was contributed by H. capsulatum antigen and how much came from dietary or other sources. It would be interesting to determine if anti-HPA antibodies might be used to affinity purify the antigen of interest from patient urine. Nonetheless, obtaining sufficient amounts of pure antigen for specific structural analyses would be challenging.
Results from the present study and from studies using monoclonal antibodies directed against chromatographically purified C-Ag (25; S. Das, L. Benjamin, M. D. Lindsley, B. Samayoa, E. Arathoon, J. Morgan, and C. J. Morrison, unpublished data) indicate that C-Ag may, at least in part, be responsible for the observed reactivity of patient urine in the HPA-EIA. In addition, the C-Ag may be partially responsible for test cross-reactivity with specimens from patients living in areas where fungal diseases other than histoplasmosis are endemic. Indeed, it has been suggested that the carbohydrate antigen common to H. capsulatum, Paracoccidioides brasiliensis, and Blastomyces dermatitidis is a galactomannan and that this carbohydrate is responsible for test cross-reactivity (2, 11, 26, 33). In any case, when tested in an indirect dot blot immunoassay, anti-HPA antibodies gave positive reactions with purified C-Ag in a dose-dependent manner.
Another advantage to the use of chromatographically purified C-Ag as a control antigen is the capacity to generate a standard curve against which the amount of urinary antigen could be measured and quantified. The standard HPA-EIA urinary antigen test has long relied on the use of HPA-positive patient urine as a positive control and normal urine as a negative control against which to measure test results (13, 39). Obtaining sufficiently large quantities of uniformly positive and negative control urine to use as a test standard over time can be difficult. Storage of such samples so that they do not gain or lose reactivity can be problematic. In contrast, chromatographically purified C-Ag can be produced in large quantities (44) and can be standardized for carbohydrate content. Although a preliminary report (42a) indicated that a standard curve has now been developed for use in the HPA-EIA, the nature of the antigen being detected has not been revealed for proprietary reasons (patent pending). Now that we have established that the antibodies produced by our yeast-i.v. immunization regimen are equivalent to those developed for the HPA-EIA for detecting H. capsulatum antigenuria, future studies can employ a C-Ag standard curve to better quantify the actual antigen concentrations found in patient urine.
A second-generation HPA-EIA test has recently been described (37). In this format, the HPA-EIA has been modified to reduce cross-linking of capture and detector antibodies by human anti-rabbit antibodies (HARA) which have been reported to occur in 16% of patients receiving rabbit anti-thymocyte globulin for the reduction of allograft rejection (38). Sera containing HARA have been demonstrated to produce false-positive HPA-EIA results (38). The nature of the HPA-EIA modification to produce the second-generation test is unknown (proprietary; patent pending) but may involve an alteration of the detector antibody or incorporation of proteolytic enzymes to reduce the activity of the HARA cross-linking antibodies in serum. However, our test was designed to detect H. capsulatum antigenuria rather than antigenemia, given reports that HPA detection is more sensitive in urine than in serum (41); therefore, interference in our antigenuria test by HARA would not be expected.
Concurrent with the present work, we have developed animal infection models of histoplasmosis, blastomycosis, penicilliosis marneffei, aspergillosis, cryptococcosis, and candidiasis (9a) in order to obtain well-characterized urine specimens to test in our EIA. We are also prospectively collecting urine from HIV-seropositive patients with histoplasmosis, coccidioidomycosis, cryptococcosis, and other respiratory infections to fully evaluate our H. capsulatum antigenuria EIA. In addition, test parameters are being optimized (27a) and monoclonal as well as polyclonal detector antibodies are being evaluated for use in the detection of H. capsulatum antigenuria by EIA (25; Das et al., unpublished).
Although it is expected that the antibodies produced in this study will cross-react with urinary antigens produced during other fungal infections, particularly those caused by B. dermatitidis and P. brasiliensis, in a manner much like that reported for the HPA-EIA test, our test should nonetheless be useful as a screening tool to rule out respiratory infections from other causes. The United States is not an area of endemicity for paracoccidioidomycosis, and although blastomycosis has the same areas of endemicity as histoplasmosis, it is a much rarer disease (3, 9). In addition, both histoplasmosis and blastomycosis can be successfully treated with similar antifungal drug regimens (8, 34).
To our knowledge, we are the first to conduct a systematic assessment of immunization schedules, routes, and antigens to optimize the production of antibodies for use in the detection of H. capsulatum polysaccharide antigenuria. As a result, we developed an immunization regimen that is faster, easier, more humane, and more consistently reliable than regimens employed previously to produce antibodies to detect HPA antigenuria. Antibodies produced by the yeast-i.v. immunization regimen were equivalent to those produced for the standard HPA-EIA test in detecting chromatographically purified C-Ag as well as in detecting H. capsulatum antigenuria in histoplasmosis patients. Further studies are currently under way to improve and optimize test parameters.
Finally, there is an urgent public health need for the availability of reagents that can be used for the detection of significant fungal diseases outside the United States, particularly in resource-poor countries lacking the means to identify patients with these infections. Outside the United States, histoplasmosis cannot be readily discriminated from a variety of bacterial and parasitic diseases, especially when observed in the disseminated form in HIV-infected individuals in the regions of Central and South America where it is endemic. A rapid urine antigen test will be of great value in these countries so that patients who require specific antifungal therapy can be readily identified and treated. These studies will hopefully contribute to the development and distribution of such materials in resource-poor countries throughout the world.
Acknowledgments
We thank L. Joseph Wheat for providing the antisera and urine that were used in the evaluation of our reagents.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Ajello, L. 1964. Relationship of Histoplasma capsulatum to avian habitats. Public Health Rep. 79:266-270. [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma, I., F. Kanetsuna, Y. Tanaka, Y. Yamamura, and L. M. Carbonell. 1974. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blastomyces dermatitidis. Mycopathol. Mycol. Appl. 54:111-125. [DOI] [PubMed] [Google Scholar]
- 3.Baumgardner, D. J., E. M. Knavel, D. Steber, and G. R. Swain. 2006. Geographic distribution of human blastomycosis cases in Milwaukee, Wisconsin, USA: association with urban watersheds. Mycopathologia 161:275-282. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Broderson, J. R. 1989. A retrospective review of lesions associated with the use of Freund's adjuvant. Lab. Anim. Sci. 39:400-405. [PubMed] [Google Scholar]
- 6.Cahn, P., W. H. Belloso, J. Murillo, and G. Prada-Trujillo. 2000. AIDS in Latin America. Infect. Dis. Clin. N. Am. 14:185-209. [DOI] [PubMed] [Google Scholar]
- 7.Chamany, S., S. A. Mirza, J. W. Fleming, J. F. Howell, S. W. Lenhart, V. D. Mortimer, M. A. Phelan, M. D. Lindsley, N. J. Iqbal, L. J. Wheat, M. E. Brandt, D. W. Warnock, and R. A. Hajjeh. 2004. A large histoplasmosis outbreak among high school students in Indiana, 2001. Pediatr. Infect. Dis. J. 23:909-914. [DOI] [PubMed] [Google Scholar]
- 8.Chapman, S. W., R. W. Bradsher, Jr., G. D. Campbell, Jr., P. G. Pappas, and C. A. Kauffman. 2000. Practice guidelines for the management of patients with blastomycosis. Clin. Infect. Dis. 30:679-683. [DOI] [PubMed] [Google Scholar]
- 9.Chu, J. H., C. Feudtner, K. Heydon, T. J. Walsh, and T. E. Zaoutis. 2006. Hospitalizations for endemic mycoses: a population-based national study. Clin. Infect. Dis. 42:822-825. [DOI] [PubMed] [Google Scholar]
- 9a.Das, S., M. D. Lindsley, and C. J. Morrison. 2005. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. F-016.
- 10.de Francesco Daher, E., F. A. de Sousa Barros, G. B. da Silva, Jr., C. F. Takeda, R. M. Mota, M. T. Ferreira, J. C. Martins, S. A. Oliveira, and O. A. Gutierrez-Adrianzen. 2006. Risk factors for death in acquired immunodeficiency syndrome-associated disseminated histoplasmosis. Am. J. Trop. Med. Hyg. 74:600-603. [PubMed] [Google Scholar]
- 11.Domer, J. E. 1971. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J. Bacteriol. 107:870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois, M., H. Gillis, J. Hamilton, A. Rebers, and R. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Biochem. 28:250-256. [Google Scholar]
- 13.Durkin, M. M., P. A. Connolly, and L. J. Wheat. 1997. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 35:2252-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garringer, T. O., L. J. Wheat, and E. J. Brizendine. 2000. Comparison of an established antibody sandwich method with an inhibition method of Histoplasma capsulatum antigen detection. J. Clin. Microbiol. 38:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman, M., R. Zackin, C. J. Fichtenbaum, D. J. Skiest, S. L. Koletar, R. Hafner, L. J. Wheat, P. M. Nyangweso, C. T. Yiannoutsos, C. T. Schnizlein-Bick, S. Owens, J. A. Aberg, and AIDS Clinical Trials Group A5038 Study Group. 2004. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin. Infect. Dis. 38:1485-1489. [DOI] [PubMed] [Google Scholar]
- 16.Hage, C. A., T. E. Davis, L. Egan, M. Parker, D. Fuller, A. M. Lemonte, M. Durkin, P. Connelly, L. J. Wheat, D. Blue-Hnidy, and K. S. Knox. 2007. Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay. Respir. Med. 101:43-47. [DOI] [PubMed] [Google Scholar]
- 17.Hajjeh, R. A., P. G. Pappas, H. Henderson, D. Lancaster, D. M. Bamberger, K. J. Skahan, M. A. Phelan, G. Cloud, M. Holloway, C. A. Kauffman, L. J. Wheat, and National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2001. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin. Infect. Dis. 32:1215-1220. [DOI] [PubMed] [Google Scholar]
- 18.Halliday, L. C., J. E. Artwohl, R. M. Bunte, V. Ramakrishnan, and B. T. Bennett. 2004. Effects of Freund's complete adjuvant on the physiology, histology, and activity of New Zealand white rabbits. Contemp. Top. Lab. Anim. Sci. 43:8-13. [PubMed] [Google Scholar]
- 19.Hebert, G. A. 1976. Improved salt fractionation of animal serums for immunofluorescence studies. J. Dent. Res. 55:A33-37. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman, C. A. 2001. Pulmonary histoplasmosis. Curr. Infect. Dis. Rep. 3:279-285. [DOI] [PubMed] [Google Scholar]
- 21.Lindsley, M. D., D. W. Warnock, and C. J. Morrison. 2006. Serological and molecular diagnosis of fungal infections, p. 569-605. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of molecular and clinical laboratory immunology, 7th ed. ASM Press, Washington, DC.
- 22.Lyon, G. M., A. V. Bravo, A. Espino, M. D. Lindsley, R. E. Gutierrez, I. Rodriguez, A. Corella, F. Carrillo, M. M. McNeil, D. W. Warnock, and R. A. Hajjeh. 2004. Histoplasmosis associated with exploring a bat-inhabited cave in Costa Rica, 1998-1999. Am. J. Trop. Med. Hyg. 70:438-442. [PubMed] [Google Scholar]
- 23.Pine, L. 1957. Studies of the growth of Histoplasma capsulatum. III. Effects of thiamin and other vitamins on the growth of the yeast and mycelial phases of Histoplasma capsulatum. J. Bacteriol. 74:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiss, E., L. Kaufman, J. A. Kovacs, and M. D. Lindsley. 2002. Clinical immunomycology, p. 559-583. In N. R. Rose, R. G. Hamilton, and B. Detrick (ed.), Manual of clinical laboratory immunology, 6th ed. ASM Press, Washington, DC.
- 25.Reiss, E., J. B. Knowles, S. L. Bragg, and L. Kaufman. 1986. Monoclonal antibodies against the M-protein and carbohydrate antigens of histoplasmin characterized by the enzyme-linked immunoelectrotransfer blot method. Infect. Immun. 53:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiss, E., W. O. Mitchell, S. H. Stone, and H. F. Hasenclever. 1974. Cellular immune activity of a galactomannan-protein complex from mycelia of Histoplasma capsulatum. Infect. Immun. 10:802-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathapatayavongs, B., B. E. Batteiger, J. Wheat, T. G. Slama, and J. L. Wass. 1983. Clinical and laboratory features of disseminated histoplasmosis during two large urban outbreaks. Medicine (Baltimore) 62:263-270. [DOI] [PubMed] [Google Scholar]
- 27a.Scheel, C., L. Benjamin, J. Morgan, S. Hurst, B. Samayoa, M. Lindsley, B. Arthington-Skaggs, and E. Arathoon. 2006. Abstr. 16th Congr. Int. Soc. Hum. Anim. Mycol., abstr. P-0422.
- 28.Stills, H. F., Jr. 2005. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 46:280-293. [DOI] [PubMed] [Google Scholar]
- 29.Tewari, R. P., D. K. Sharma, and A. Mathur. 1978. Significance of thymus-derived lymphocytes in immunity elicited by immunization with ribosomes or live yeast cells of Histoplasma capsulatum. J. Infect. Dis. 138:605-613. [DOI] [PubMed] [Google Scholar]
- 30.Tobon, A. M., C. A. Agudelo, D. S. Rosero, J. E. Ochoa, C. De Bedout, A. Zuluaga, M. Arango, L. E. Cano, J. Sampedro, and A. Restrepo. 2005. Disseminated histoplasmosis: a comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. Am. J. Trop. Med. Hyg. 73:576-582. [PubMed] [Google Scholar]
- 31.Wheat, J. 1996. Histoplasmosis in the acquired immunodeficiency syndrome. Curr. Top. Med. Mycol. 7:7-18. [PubMed] [Google Scholar]
- 32.Wheat, J. 1997. Histoplasmosis. Experience during outbreaks in Indianapolis and review of the literature. Medicine (Baltimore) 76:339-354. [DOI] [PubMed] [Google Scholar]
- 33.Wheat, J., M. L. French, S. Kamel, and R. P. Tewari. 1986. Evaluation of cross-reactions in Histoplasma capsulatum serologic tests. J. Clin. Microbiol. 23:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheat, J., G. Sarosi, D. McKinsey, R. Hamill, R. Bradsher, P. Johnson, J. Loyd, and C. Kauffman. 2000. Practice guidelines for the management of patients with histoplasmosis. Clin. Infect. Dis. 30:688-695. [DOI] [PubMed] [Google Scholar]
- 35.Wheat, J., H. Wheat, P. Connolly, M. Kleiman, K. Supparatpinyo, K. Nelson, R. Bradsher, and A. Restrepo. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 24:1169-1171. [DOI] [PubMed] [Google Scholar]
- 36.Wheat, L. J. 2003. Current diagnosis of histoplasmosis. Trends Microbiol. 11:488-494. [DOI] [PubMed] [Google Scholar]
- 37.Wheat, L. J., P. Connolly, M. Durkin, B. K. Book, and M. D. Pescovitz. 2006. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transpl. Infect. Dis. 8:219-221. [DOI] [PubMed] [Google Scholar]
- 38.Wheat, L. J., P. Connolly, M. Durkin, B. K. Book, A. J. Tector, J. Fridell, and M. D. Pescovitz. 2004. False-positive Histoplasma antigenemia caused by antithymocyte globulin antibodies. Transpl. Infect. Dis. 6:23-27. [DOI] [PubMed] [Google Scholar]
- 39.Wheat, L. J., P. Connolly-Stringfield, R. B. Kohler, P. T. Frame, and M. R. Gupta. 1989. Histoplasma capsulatum polysaccharide antigen detection in diagnosis and management of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am. J. Med. 87:396-400. [DOI] [PubMed] [Google Scholar]
- 40.Wheat, L. J., P. A. Connolly-Stringfield, R. L. Baker, M. F. Curfman, M. E. Eads, K. S. Israel, S. A. Norris, D. H. Webb, and M. L. Zeckel. 1990. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 69:361-374. [DOI] [PubMed] [Google Scholar]
- 41.Wheat, L. J., T. Garringer, E. Brizendine, and P. Connolly. 2002. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn. Microbiol. Infect. Dis. 43:29-37. [DOI] [PubMed] [Google Scholar]
- 42.Wheat, L. J., R. B. Kohler, and R. P. Tewari. 1986. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N. Engl. J. Med. 314:83-88. [DOI] [PubMed] [Google Scholar]
- 42a.Wheat, L. J., E. J. Hackett, and P. A. Connolly. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1611.
- 43.Williams, B., M. Fojtasek, P. Connolly-Stringfield, and J. Wheat. 1994. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis, Ind. Arch. Pathol. Lab. Med. 118:1205-1208. [PubMed] [Google Scholar]
- 44.Zancope-Oliveira, R. M., S. L. Bragg, S. F. Hurst, J. M. Peralta, and E. Reiss. 1993. Evaluation of cation exchange chromatography for the isolation of M glycoprotein from histoplasmin. J. Med. Vet. Mycol. 31:29-41. [PubMed] [Google Scholar]
- 45.Zancope-Oliveira, R. M., S. L. Bragg, E. Reiss, and J. M. Peralta. 1994. Immunochemical analysis of the H and M glycoproteins from Histoplasma capsulatum. Clin. Diagn. Lab. Immunol. 1:563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]