Abstract
Two indirect enzyme-linked immunosorbent assays (ELISAs) were employed to measure levels of immunoglobulin G (IgG), IgM, and IgA antibodies against Salmonella in sera from 303 Danish patients diagnosed by fecal culture with either Salmonella enterica serovar Enteritidis or Salmonella enterica serovar Typhimurium infections. The ELISAs were based on serovar Enteritidis lipopolysaccharide (LPS) and serovar Typhimurium LPS. The antibody levels were assessed approximately 1, 3, 6, and 12 months after the onset of salmonellosis. Sera from 164 healthy blood donors were analyzed to establish cutoff values for each analysis. One month after the onset of symptoms, the sensitivities of the assays were 95% for patients recovering from a serovar Enteritidis infection and 89% for patients recovering from a serovar Typhimurium infection. Three months after the onset of symptoms, these values had decreased to 85% and 55%. At 6 months they were 62% and 40%, and at 12 months they were 40% and 16%, respectively. The specificities of the assays were 97% for the serovar Enteritidis LPS ELISA and 94% for the serovar Typhimurium LPS ELISA. The high values for both sensitivity and specificity make these two ELISAs useful for serodiagnoses of Salmonella infection shortly after the acute phase of the infection and of Salmonella-associated reactive arthritis, as well as for seroepidemiological studies. A mixed ELISA consisting of both antigens, i.e., serovar Enteritidis and serovar Typhimurium LPS, was developed as a diagnostic tool with very high values for both specificity and sensitivity.
In Denmark, Salmonella enterica serovar Enteritidis and Salmonella enterica serovar Typhimurium are the second and third most common causes of bacterial gastrointestinal infections, with Campylobacter spp. being the most common cause (1).
The most common vehicles of nontyphoid Salmonella infections in humans are eggs, poultry, and red meat (1, 4, 7). Gastrointestinal infections in humans with Salmonella are commonly diagnosed by culturing feces or blood and by serology. Antibodies to Salmonella are traditionally detected by tube agglutination using the Widal test; however, this test has a low sensitivity and cannot be used to discriminate between antibody classes (immunoglobulin G [IgG], IgM, and IgA) (5). Nonetheless, the detection of specific antibodies is potentially valuable both for routine diagnostic purposes, such as the diagnosis of postinfection conditions, e.g., Salmonella-triggered reactive arthritis, and for seroepidemiological studies. However, the validation and result interpretation of serological tests require extensive knowledge about antibody development and decay profiles after the onset of infection. To our knowledge, such detailed antibody studies have not been performed previously with humans.
Serology has been widely used in the veterinary sector, both for diagnosis and for seroprevalence studies; thus, a number of enzyme-linked immunosorbent assays (ELISAs) have been developed for animal testing. However, those tests are not directly applicable to human samples. Early studies regarding the use of lipopolysaccharide (LPS) in an ELISA for the detection of Salmonella antibodies have given promising results (2, 5, 8, 9); moreover, a pilot study of nine patients showed a persistence of anti-LPS IgG antibodies after gastrointestinal Salmonella infections (5). During a food outbreak involving 80 patients, antibodies were measured against serovar Enteritidis LPS, and IgA response was correlated to the development of reactive arthritis (10).
In this study, the levels of IgG, IgM, and IgA antibodies against serovar Enteritidis LPS and serovar Typhimurium LPS were measured in sera from 303 patients diagnosed by fecal culturing with either serovar Enteritidis or serovar Typhimurium. In order to assess the antibody decay over a period of approximately 2 years after Salmonella infections, two indirect ELISAs based on Salmonella LPS were employed. As a diagnostic tool, a mixed ELISA consisting of both antigens, i.e., serovar Enteritidis and serovar Typhimurium, with very high values for both specificity and sensitivity, was also developed.
MATERIALS AND METHODS
Study population and serum samples.
Sera from 303 Danish patients with gastroenteritis diagnosed by fecal culturing with infection by either serovar Enteritidis (153 patients) or serovar Typhimurium (150 patients) were collected. According to the laboratory-based national surveillance data for gastrointestinal infections, none of these patients were known to have suffered from bacterial gastroenteritis of a known etiology within the previous year. All patients were asked to deliver a blood sample shortly after the time of diagnosis as well as 3, 6, and 12 months later. At the 12-month collection, 186 patients remained in the study. The times of the onset of symptoms were recorded for all patients, and the median time spans from the onset of symptoms to the collection of the three serum samples were 21, 106, 210, and 435 days for the serovar Enteritidis patients and 27, 108, 202, and 422 days for the serovar Typhimurium patients.
The distribution by sex of all patients was 56% women and 44% men, and they ranged in age from 11 to 97 years, with a median age of 48 years. The Danish Central Scientific Ethical Committee (reference no. 11-097/02) and the Danish College of General Practitioners (reference no. MPU-02/2001) have given their approval for contacting doctors to ask the patients for first, second, and third blood samples. All involved patients and doctors were provided with a letter of information.
Moreover, sera from 164 healthy Danish blood donors were analyzed in order to determine the baseline levels of Salmonella LPS antibodies in the general population. For each of the three immunoglobulin classes, the cutoff values were calculated using the “mean plus 2 standard deviations” method. The optical density (OD) values had a lognormal distribution, and the cutoff values were calculated as 10(mean + 2 standard deviations), where the mean was that of the log-transformed OD values.
Sera from patients with high levels of antibodies against other bacteria causing gastrointestinal illnesses were also analyzed in order to determine the extent of cross-reactions. Thus, 33 sera from patients with one of the following bacterial were tested in our assays: Escherichia coli, Yersinia enterocolitica, Campylobacter jejuni, Campylobacter coli, and Helicobacter pylori.
All antisera were supplemented with 0.01% sodium azide and stored at −20°C. In the analyses of sera from patients diagnosed with either serovar Enteritidis or serovar Typhimurium infections, the detection rate was defined as the percentage of sera producing detectable antibodies against Salmonella LPS, i.e., generating an OD value in the ELISA above the cutoff value.
Tube agglutination assay.
A standard tube agglutination assay (the Widal test) was performed for the determination of antibody titers against Salmonella. Whole-cell antigens were prepared from overnight cultures (SSI standard strains) either by formalin or alcohol fixation. O (LPS) and H (flagella) whole-cell-antigen preparations were controlled by including standards and positive controls each time a new preparation was made as well as each time the assay was performed.
All patient sera were analyzed for agglutination to S. enterica serovar Typhi O antigen, S. enterica serotype Paratyphi A O antigen, and serotype Paratyphi B O antigen. The O antigens from serovar Typhi (O:9,12) are very similar to the O antigens from serovar Enteritidis (O:1,9,12), and the O antigens from serotype Paratyphi B (O:1,4,5,12) are identical to the O antigens from serovar Typhimurium (O:1,4,5,12). Furthermore, sera from patients diagnosed with serovar Enteritidis infections were analyzed for agglutination to serovar Enteritidis H antigen (g,m), and sera from patients diagnosed with serovar Typhimurium infections were analyzed for agglutination to serovar Typhimurium H antigens (i:1,2).
Specific LPS ELISA development for serovar Enteritidis and serovar Typhimurium.
An indirect ELISA was developed for the determination of the contents of antibodies against Salmonella LPS in human sera. For the determination of antibodies against serovar Enteritidis LPS, commercially available LPS (Sigma-Aldrich, Denmark) from serovar Enteritidis was used. Likewise, for the determination of antibodies against serovar Typhimurium LPS, commercially available LPS (Sigma-Aldrich, Denmark) from S. enterica serovar Typhimurium was used. A number of different batches and lot numbers were tested and gave the same results. LPS preparations were checked for protein impurities by silver stain polyacrylamide gel electrophoresis.
The ELISA was performed with microtiter plates (Nunc-Immuno PolySorp F96 plates) using a BioMek 2000 robot (Beckman Coulter Inc.). Wells were coated overnight at 4°C with 0.1 μg antigen in 100 μl coating buffer (carbonate buffer at pH 9.60 containing 0.2% phenol red). Wells were washed four times with wash buffer (i.e., phosphate-buffered saline at pH 7.40 with 0.1% Tween 20) and thereafter blocked with 250 μl wash buffer per well for 30 min at room temperature. The wells were subsequently washed (four times), 100 μl of serum diluted 1:400 in dilution buffer (phosphate-buffered saline at pH 7.40 with 0.1% Tween 20 and 0.2% phenol red) was added per well in duplicate, and then the plates were incubated at room temperature for 30 min. After the wells were washed (four times), 100 μl of horseradish peroxidase-conjugated rabbit anti-human IgG, IgM, or IgA (catalog no. P02014, P0215, and P0216; DakoCytomation) was added per well (anti-IgG, 1:2,500; anti-IgM, 1:1,000; and anti-IgA, 1:500) and incubated for 30 min at room temperature. After the wells were washed, 100 μl of a 3,3′,5,5′-tetramethyl-benzidine solution (catalog no. 4380A; Kem-En-Tec Diagnostics) was added per well and incubated for 15 min at room temperature. The reaction was stopped with 100 μl 1 M H2SO4, and the OD at 490 nm (650 nm was used as a reference) was finally determined. Each serum sample was analyzed for the presence of IgG, IgM, and IgA anti-LPS antibodies. Day-to-day variations were minimized by including the dilution series of a standard for each assay.
Reproducibility was tested using duplicates of sera both within the plate and between different days, and a coefficient of variation under 10% was observed.
Mixed-LPS ELISA for diagnosis of Salmonella antibodies.
To investigate the potential benefits of an ELISA combining the two assays described above, a study of a “mixed ELISA,” incorporating equal amounts of serovar Enteritidis and serovar Typhimurium LPS as the antigen, was conducted. The final amount of antigen per microtiter well was 0.1 μg LPS. All other steps in the mixed ELISA were exactly the same as for the single-LPS ELISA described in detail above.
RESULTS
Tube agglutination assays.
The tube agglutination assays showed significantly low sensitivities. In an analysis of 154 sera from patients with culture-confirmed serovar Enteritidis infection, 67 (44%) of these sera were found positive by agglutination; 34 sera agglutinated with serovar Enteritidis-similar O antigen, and 54 sera agglutinated with serovar Enteritidis H antigen. Only 9 (8%) of 113 sera from patients with culture-confirmed serovar Typhimurium infection were found to agglutinate; 6 sera agglutinated with serovar Typhimurium-similar O antigen, and 3 sera agglutinated with serovar Typhimurium H antigen.
Cutoff determination of the Salmonella LPS ELISA.
By analyzing blood donor sera using the serovar Enteritidis LPS ELISA, we determined the following cutoff values for, respectively, IgG, IgM, and IgA: 0.67, 0.68, and 0.29. For the detection of serovar Typhimurium LPS antibodies, the corresponding values were 0.83, 0.69, and 0.54. With these cutoff values, very high specificities of up to 99% were achieved (Table 1).
TABLE 1.
Specificities of the developed LPS ELISAs determined by analyses of sera from healthy Danish blood donorsa
| LPS ELISA | % Specificity (no. of sera negative for indicated antibody)
|
|||
|---|---|---|---|---|
| IgG | IgM | IgA | IgG, IgM, or IgA | |
| Serotype Enteritidis | 99 (162) | 99 (162) | 98 (160) | 97 (159) |
| Serotype Typhimurium | 98 (160) | 98 (160) | 96 (157) | 94 (154) |
The specificities of the two ELISAs (based on serotype Enteritidis LPS and serotype Typhimurium LPS) are the percentages of sera with ODs below cutoff values. The cutoff values are means plus 2 standard deviations.
Antibody decay profiles of the Salmonella LPS ELISA.
The maximum sensitivity was obtained by including measurements of all three immunoglobulin classes and assigning positivity when at least one measurement was above the cutoff level. Approximately 1 month after the onset of symptoms, the sensitivity was 95% for serovar Enteritidis patients and 89% for serovar Typhimurium patients. Three months after the onset of symptoms, these percentages had decreased to 85% and 55%; 6 months after the onset of symptoms, the detection rates were 62% and 40%; and 12 months after onset of symptoms, the detection rates were 40% and 16% (Tables 2 and 3).
TABLE 2.
Analysis of sera from a cohort of patients diagnosed as having serotype Enteritidis by fecal culturing
| Mo. of test (median no. of days) | No. (%) of sera positive for indicated antibody/total no. of seraa
|
|||
|---|---|---|---|---|
| IgG | IgM | IgA | IgG, IgM, or IgA | |
| 1 (21) | 122/153 (80) | 121/153 (79) | 138/153 (90) | 145/153 (95) |
| 3 (106) | 94/132 (71) | 26/132 (20) | 66/132 (50) | 112/132 (85) |
| 6 (210) | 67/131 (51) | 18/131 (14) | 35/131 (27) | 81/131 (62) |
| 12 (435) | 35/100 (35) | 2/100 (2) | 21/100 (21) | 40/100 (40) |
Ratios and percentages of sera producing OD values above the cutoff of an ELISA based on LPS antigen from serotype Enteritidis. All sera were from patients initially diagnosed with serotype Enteritidis by fecal culture and were analyzed for the presence of IgG, IgM, and IgA LPS antibodies. Sera were obtained and analyzed approximately 1, 3, 6, and 12 months after the onset of symptoms.
TABLE 3.
Analysis of sera from a cohort of patients diagnosed as having serotype Typhimurium by fecal culturing
| Mo. of test (median no. of days) | No. (%) of sera positive for indicated antibody/total no. of seraa
|
|||
|---|---|---|---|---|
| IgG | IgM | IgA | IgG, IgM, or IgA | |
| 1 (27) | 85/149 (57) | 110/149 (74) | 86/149 (58) | 132/149 (89) |
| 3 (108) | 55/124 (44) | 25/124 (20) | 21/124 (17) | 68/124 (55) |
| 6 (202) | 32/103 (31) | 9/103 (9) | 11/103 (11) | 41/103 (40) |
| 12 (422) | 9/86 (11) | 5/86 (6) | 6/86 (7) | 14/86 (16) |
Ratios and percentages of sera producing OD values above the cutoff of an ELISA based on LPS antigen from serotype Typhimurium. All sera were from patients initially diagnosed with serotype Typhimurium by fecal culture and were analyzed for the presence of IgG, IgM, and IgA LPS antibodies. Sera were obtained and analyzed approximately 1, 3, 6, and 12 months after the onset of symptoms.
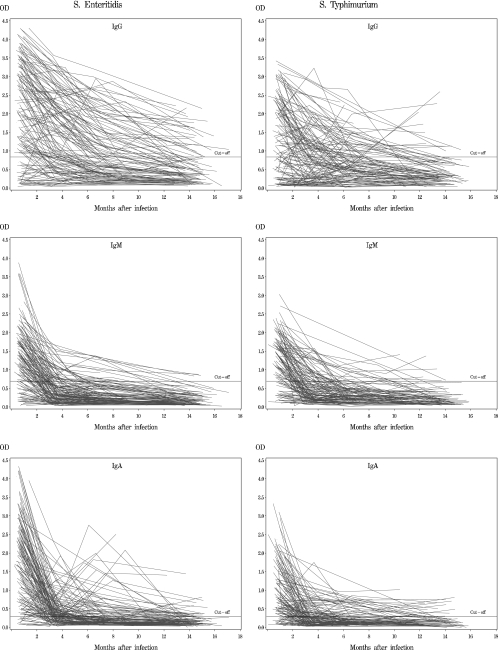
Figure 1 shows the individual antibody decay profiles of the patients. For a considerable proportion of patients, anti-LPS IgG antibodies persisted for several months after infection. In fact, 35% of the patients with a serovar Enteritidis infection had detectable IgG antibodies 12 months after the onset of the infection, as did 11% of the serovar Typhimurium patients. The levels of both anti-LPS IgM and anti-LPS IgA antibodies declined 3 to 4 months after the onset of the infection (Fig. 1).
FIG. 1.
Decay profiles for antibodies against Salmonella LPS. Each line represents a single patient. (Left panels) Patients with a serovar Enteritidis fecal culture and whose sera were analyzed by the ELISA based on the serovar Enteritidis LPS antigen. (Right panels) Patients with a serovar Typhimurium fecal culture and whose sera were analyzed by the ELISA based on the serovar Typhimurium LPS antigen.
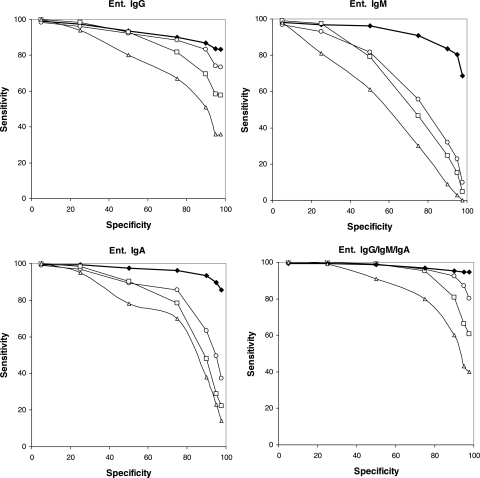
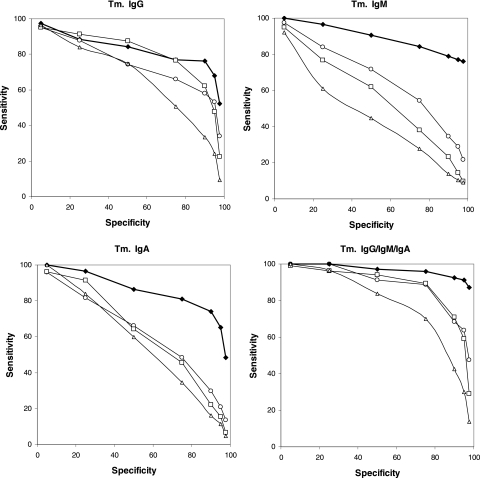
When we plotted receiver operating characteristic (ROC) curves for the obtained sensitivities, it appeared that the antibody response against serovar Enteritidis was stronger than the response against serovar Typhimurium. Also, a long-lasting persistence of anti-LPS IgG antibodies with a faster decay of IgM and IgA antibodies was observed (Fig. 2 and 3).
FIG. 2.
ROC curves depicting the sensitivity and specificity of an ELISA based on serovar Enteritidis LPS. The analyzed sera are from patients diagnosed with serovar Enteritidis (Ent.) infection. The analyzed sera were obtained approximately 1 month (filled diamonds), 3 months (open circles), 6 months (open squares), and 12 months (open triangles) after the onset of symptoms. The ROC curves for the detection of just IgG (top left), just IgM (top right), just IgA (bottom left), and either IgG, IgM, or IgA (bottom right) are shown.
FIG. 3.
ROC curves depicting the sensitivity and specificity of an ELISA based on serovar Typhimurium LPS. The analyzed sera are from patients diagnosed with serovar Typhimurium (Tm.) infection. The analyzed sera were obtained approximately 1 month (filled diamonds), 3 months (open circles), 6 months (open squares), and 12 months (open triangles) after the onset of symptoms. The ROC curves for the detection of just IgG (top left), just IgM (top right), just IgA (bottom left), and either IgG, IgM, or IgA (bottom right) are shown.
The effects of age and/or sex were calculated according to the first blood sample taken. No significant effects of either age or sex for immunoglobulin classes G and A were found. The IgM response was also found to be independent of sex but was dependent on age (P = 0.0002) for younger patients with serovar Enteritidis infection. No age effect on the IgM response was found with the serovar Typhimurium patients (Table 4).
TABLE 4.
Age dependency of the anti-LPS IgM response after the onset of salmonellosisa
| LPS ELISA | Value for age range
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <20 yr
|
21-40 yr
|
41-60 yr
|
>60 yr
|
|||||
| No. of positive sera/total no. of sera | Odds ratio | No. of positive sera/total no. of sera | Odds ratio | No. of positive sera/total no. of sera | Odds ratio | No. of positive sera/total no. of sera | Odds ratio | |
| Serotype Enteritidis | 12/13 | 3.3 | 27/30 | 2.2 | 65/79 | 1.6 | 17/31 | 1.0 (ref) |
| Serotype Typhimurium | 9/13 | 1.1 | 30/37 | 1.1 | 42/63 | 0.88 | 28/34 | 1.0 (ref) |
The presented data are all from sera collected approximately 1 month after the onset of symptoms (medians, 21 days for the serotype Enteritidis patients and 27 days for the serotype Typhimurium patients). Sera from patients diagnosed with serotype Enteritidis infections were analyzed by the serotype Enteritidis LPS-based ELISA, and sera from patients diagnosed with serotype Typhimurium infections were analyzed by the serotype Typhimurium LPS-based ELISA.
Salmonella LPS ELISA cross-reactions.
In an analysis of cross-reactions, a proportion of sera from patients diagnosed with other gastrointestinal pathogens were found to generate OD values above the cutoffs in both ELISAs. For example, 24% and 30% of sera from patients with high levels of antibodies against Helicobacter pylori showed cross-reactions in the serovar Enteritidis and serovar Typhimurium ELISAs, respectively. Sera from patients diagnosed with either diarrheagenic E. coli, Y. enterocolitica, or Campylobacter sp. infection showed cross-reactions ranging from 3% to 21%.
Swap tests were performed to evaluate the abilities of the two ELISAs to discriminate between sera from patients with confirmed serovar Enteritidis and serovar Typhimurium infections. When we tested sera from the 1-month collections, 94% (30 of the 32 tested) of sera from patients diagnosed with serovar Enteritidis gave OD values above the cutoff in the serovar Typhimurium-based ELISA. This was a significantly higher sensitivity than obtained when serovar Typhimurium-infected patients were analyzed with the serovar Enteritidis-based ELISA; 63% (20 of the 32 tested) were found to be positive (P = 0.005, Fisher's exact test) (Table 5).
TABLE 5.
Analyses of sera from patients diagnosed by fecal culturing with either diarrheagenic E. coli, Y. enterocolitica, C. jejuni, C. coli, H. pylori, S. enterica serotype Typhimurium, or S. enterica serotype Enteritidis infectiona
| Organism(s) | No. (%) of sera positive for antigen/total no. of sera
|
|
|---|---|---|
| IgG, IgM, or IgA for serotype Enteritidis | IgG, IgM, or IgA for serotype Typhimurium | |
| E. coli | 1/33 (3) | 4/33 (12) |
| Y. enterocolitica | 5/33 (15) | 7/33 (21) |
| C. jejuni and C. coli | 4/33 (12) | 4/33 (12) |
| H. pylori | 10/33 (30) | 8/33 (24) |
| S. enterica serotype Typhimurium | 20/32 (63) | |
| S. enterica serotype Enteritidis | 30/32 (94) | |
Sera were analyzed for the presence of IgG, IgM, and IgA LPS antibodies in separate runs. The ratios and percentages of sera producing OD values above the cutoff for any of the three immunoglobulin classes are presented.
Diagnostic mixed ELISA for determination of Salmonella antibodies.
Cutoff values were determined on the basis of sera from 33 healthy Danish blood donors and calculated by the “mean plus 2 standard deviations” method. These cutoff values for IgG, IgM, and IgA, respectively, were 0.65, 0.84, and 0.74. Using these cutoffs, the specificity of the assay was 100%, since all of the analyzed blood donor sera produced OD values below the cutoffs. When 28 sera from the 1-month collection of patients with proven serovar Enteritidis infection were analyzed, a maximum detection rate of 93% (n = 26) was reached. Likewise, when 26 sera from the 1-month collection of patients with proven serovar Typhimurium infection were analyzed, a maximum detection rate also of 93% (n = 24) was reached. The sensitivities for the three individual antibody classes were 86%, 82%, and 86% (24, 23, and 24 sera) for serovar Enteritidis IgG, IgM, and IgA antibodies, respectively, and 73%, 69%, and 73% (19, 18, and 19 sera) for serovar Typhimurium antibodies, respectively.
DISCUSSION
Patients with fecal-culture-confirmed infections with either serovar Enteritidis or serovar Typhimurium were followed for a 2-year time period and tested for the presence of specific Salmonella antibodies. Patient sera were analyzed by the in-house-developed ELISA, and results were compared to those from the traditional tube agglutination assay (the Widal assay). The described ELISA was found to be highly sensitive and specific and suitable for the diagnosis of gastrointestinal salmonellosis. Furthermore, the Salmonella anti-LPS antibody decay profile was determined for both serovar Enteritidis and serovar Typhimurium.
The specificity of the assay was based on samples from healthy Danish blood donors, and from these the cutoff values for the assays were calculated. The specificities ranged from 97 to 99% for both assays and for all immunoglobulin classes (Table 1).
The sensitivities of the assays were calculated during the whole time period, and they were shown to be superior to that of the conventionally used Widal assay. Approximately 1 month after the onset of salmonellosis symptoms, 95% of the serovar Enteritidis patients were found positive for the presence of anti-LPS (O:1,9,12) antibodies, while 89% of the serovar Typhimurium patients were found positive for the presence of anti-LPS (O:4,5,12) antibodies. In comparison, the sensitivities obtained by standard Widal agglutinations were 44% and 8% for the serovar Enteritidis and serovar Typhimurium patients, respectively. Three months after the onset of symptoms, the ELISA detection rates had decreased to 85% and 55%; after 6 months, the detection rates were 62% and 40%; and after 12 months, the detection rates were 40% and 16%, respectively. In particular, anti-LPS IgG antibodies were long-lasting in sera from a large subset of patients, whereas the levels of anti-LPS IgM and IgA antibodies generally diminished after 3 to 4 months (Tables 2 and 3).
As expected, a correlation of young age with a high IgM response in patients infected with serovar Enteritidis was observed (Table 4). Similarly, it has been previously shown that the presence of anti-Campylobacter IgM antibodies after Campylobacter gastroenteritis was age related (8). Surprisingly, this was not the situation for serovar Typhimurium (Table 4). A possible explanation could be that in Denmark the incidence of this serotype is lower than that of serotype Enteritidis, and therefore a larger proportion of infections in elderly patients might be primary infections. Alternatively, this may reflect the possibility that serovar Enteritidis is more immunogenic than serovar Typhimurium. Generally, the OD values as well as the agglutination levels (Widal titers) for sera indicating the presence of serovar Enteritidis antibodies were higher than the values obtained for sera indicating the presence of serovar Typhimurium antibodies. The notion of serovar Enteritidis LPS being more immunogenic is also supported by the observation that more serovar Enteritidis samples tested positive in the serovar Typhimurium assay than vice versa.
Cross-reactions of sera from patients with other gastrointestinal infections were observed; in particular, 24 to 30% of sera from patients with high anti-H. pylori antibodies gave rise to OD values above the cutoffs (Table 5). The risk of incorrect diagnosis due to this cross-reactivity is low since the symptoms of nontyphoid salmonellosis are very distinct from the symptoms of H. pylori infection. Minor cross-reactions of sera from patients diagnosed with infections by Y. enterocolitica, E. coli, or Campylobacter were observed. The basis for these cross-reactions is not clear; there may be common antigenic structures at the LPS level recognized by different antibodies, or the human subpopulation may be more susceptible to a number of gastrointestinal infections.
The two ELISAs showed remarkably high specificity and sensitivity. This fact, combined with the fast, easy, and reliable characteristics of an automated ELISA, makes the two assays very useful for routine analyses of human sera when gastrointestinal Salmonella infections are suspected and the bacteria are no longer shed. The use of Salmonella LPS as the antigen in a diagnostic ELISA is superior to the use of flagella as the antigen. We have previously shown that the specific antibody levels against H antigens decrease very rapidly and are not suitable for the diagnosis of salmonellosis (3).
In viewing the antibody decay profiles of the human antibody response against Salmonella LPS, it is clear that IgG antibodies can be detected for several months after the infection in a subset of patients (Fig. 1). Thus, an LPS-based ELISA may be useful for both acute-phase and postinfection diagnoses of gastrointestinal salmonellosis. The specificity and sensitivity are based on results for a Danish population, which also makes the assays useful for seroepidemiological studies. Although no clinical data regarding reactive arthritis were available for these 303 patients, it was previously shown that the diagnosis of Salmonella-induced arthritis is feasible by LPS ELISA (6).
For the mixed ELISA, very high values for both specificity and sensitivity were obtained. Such an ELISA for the detection of the two most common agents of nontyphoid salmonellosis, therefore, seems a promising method for a fast and easy serodiagnosis of both gastrointestinal salmonellosis and Salmonella-associated reactive arthritis and may also be useful for seroepidemiological studies.
Acknowledgments
This study was partially supported by the Ministry of Food, Agriculture and Fisheries, Directorate for Food Fisheries and Agro Business (FØS100-SSI-7).
We thank Jacob Brundsted Simonsen for his valuable help and advice regarding the statistical analysis of the data.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Anonymous. 2005. Annual report on zoonoses in Denmark. Ministry of Food, Agriculture and Fisheries, Copenhagen, Denmark.
- 2.Carlsson, H. E., A. A. Lindberg, S. Hammarstrom, and A. Ljunggren. 1975. Quantitation of Salmonella O-antibodies in human sera by enzyme-linked immunosorbent assay (ELISA). Int. Arch. Allergy Appl. Immunol. 48:485-494. [DOI] [PubMed] [Google Scholar]
- 3.Dalby, T., M. A. Strid, N. H. Beyer, J. Blom, K. Mølbak, and K. A. Krogfelt. 2005. Rapid decay of Salmonella flagella antibodies during human gastroenteritis: a follow up study. J. Microbiol. Methods 62:233-243. [DOI] [PubMed] [Google Scholar]
- 4.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 5.Isomaki, O., R. Vuento, and K. Granfors. 1989. Serological diagnosis of salmonella infections by enzyme immunoassay. Lancet i:1411-1414. [DOI] [PubMed] [Google Scholar]
- 6.Locht, H., K. Mølbak, and K. A. Krogfelt. 2002. High frequency of reactive joint symptoms after an outbreak of Salmonella enteritidis. J. Rheumatol. 29:767-771. [PubMed] [Google Scholar]
- 7.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Baumler. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3:1335-1344. [DOI] [PubMed] [Google Scholar]
- 8.Strid, M. A., J. Engberg, L. B. Larsen, K. Begtrup, K. Mølbak, and K. A. Krogfelt. 2001. Antibody response to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin. Diagn. Lab. Immunol. 8:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenungsson, B., H. Jorbeck, and A. A. Lindberg. 1979. Diagnosis of Salmonella infections: specificity of indirect immunofluorescence for rapid identification of Salmonella enteritidis and usefulness of enzyme-linked immunosorbent assay. J. Infect. Dis. 140:927-936. [DOI] [PubMed] [Google Scholar]
- 10.Thomson, G. T., M. Alfa, K. Orr, B. R. Thomson, and N. Olson. 1994. Secretory immune response and clinical sequelae of Salmonella infection in a point source cohort. J. Rheumatol. 21:132-137. [PubMed] [Google Scholar]