Abstract
Holstein dairy cows (four J5 vaccinates and four controls) selected for no recorded intramammary disease and low somatic cell count (SCC) during the previous lactation were challenged by intramammary infusion of Escherichia coli. Vaccination with J5 was at 8 weeks and again 4 weeks before the anticipated calving date. Cows were challenged at 8 to 16 days in milk (DIM). Shedding of E. coli in milk was significantly higher among controls than vaccinates (no shedding) from 6 h to 21 h postchallenge. From 21 h to 132 h postchallenge, SCC in challenged quarters of controls (5,429,000/ml) was significantly higher than that of vaccinates (490,000/ml). On the day after challenge, milk production in control cows was 8 kg less, while vaccinates gained 0.5 kg, a significant difference. In serum immediately prior to challenge, J5-specific immunoglobulin G1 (IgG1) was significantly higher, IgG2 was nearly significantly higher, and IgM was the same in J5 vaccinates relative to controls. Vaccinates had proportionally more IgG2 in serum postcalving and in the first 12 h following challenge and less IgG2 in milk 24 h after challenge than the controls, approaching statistical significance. The ratio of J5-specific IgG1 and IgG2 combined compared to IgM was significantly higher in vaccinates than in controls in prechallenge serum (ratios of 15.8 and 3.2, respectively) and milk (5.0 and 1.3, respectively). Cows with higher IgM titers in milk 12 h postchallenge produced significantly less milk. Vaccination with J5 was significantly associated with higher production of J5-specific IgG1 and IgG2 in early lactation, reduced SCC, faster clearance of E. coli from milk, and less milk production loss following intramammary challenge.
Bovine coliform mastitis is most commonly caused by Escherichia coli and Klebsiella spp. (16, 21, 30, 32). Coliform mastitis can cause abnormal milk, milk production loss, treatment costs, and death of cattle (20, 21, 30).
Vaccination against coliform mastitis with J5 bacterins has been used in the dairy industry for more than 15 years (10, 15). However, the effects of J5 immunization on the bovine mammary immune response have not yet been fully explained (3, 12). It is not clear whether J5 vaccination results in J5 E. coli-specific immunoglobulin M (IgM), IgG1, or IgG2 antibody production or in changes in their relative proportions produced in milk or serum or whether such changes are associated with resistance to coliform mastitis (1, 5, 17, 19, 28, 32). It is generally accepted that either IgM, IgG2, or both isotypes are particularly important in opsonization of bacteria for polymorphonuclear leukocyte (PMN) phagocytosis, and increases in these antibodies against the target mastitis pathogens are desired goals of vaccination against mastitis (1, 5).
The primary objectives were to evaluate a commercial J5 vaccine for protection against E. coli challenge and to statistically test for associations between J5 vaccination, outcome measures of clinical mastitis (CM) severity, and J5-specific IgG1, IgG2, and IgM antibodies in milk and serum before and after challenge.
MATERIALS AND METHODS
Experimental design and timeline.
Pregnant Holstein cows selected to be J5 vaccinates (n = 4) were vaccinated with J5 bacterin (J-VAC; Merial Ltd., Duluth, GA) at 8 weeks before they were due to calve (at dryoff) and again 4 weeks before the due date (mid dry period). Vaccine (2 ml) was administered subcutaneously in the supramammary lymph node region. Pregnant controls (n = 4) were not given a sham immunization. Milk samples were collected immediately prior to intramammary infusion challenge (approximately 2 weeks after calving) with E. coli O:157 and again 12 h and 24 h postchallenge. Blood samples were collected 4 weeks before the calving due date, postcalving, immediately prior to intramammary challenge, and 12 h and 24 h postchallenge.
Selection of cows for study.
Holstein dairy cows (n = 8) were purchased from a commercial dairy. All cows had completed at least one previous lactation, were in late lactation, had no recorded cases of disease in the previous lactation, and had similar milk production values for the previous lactation, and all monthly Dairy Herd Improvement Association somatic cell count (SCC) tests were <250,000/ml. Milk production of the eight cows was approximately 10,900 kg per 305 days in the previous lactation. The first four cows that calved were controls, and the last four cows that calved were J5 vaccinates.
Milking, SCC, bacteriology, and selection of challenge quarters.
As cows reached approximately 14 days before their calving due date, they were transported to a research tiestall facility at Cornell University. All eight cows calved uneventfully on or a few days before or after their due date, with live calves and no dystocia.
Cows were milked twice daily using a bucket milking system within industry mechanical performance standards. Chlorhexidine udder wash, predip, and postdip with 0.5% chlorhexidine teat dip, and forestripping were utilized. Until 3 days before challenge, cows were milked in the typical way, with milk from all four quarters harvested and weighed in the same bucket. Beginning 3 days before challenge, each quarter was milked separately and the milk from each quarter was weighed separately until the intramammary challenge. From the milking following challenge until 7 days postchallenge, the challenged quarter and the contralateral quarter were milked and weighed separately, and the other two quarters were milked together and their combined milk was weighed separately (e.g., if the right forequarter was challenged, the left forequarter was also milked separately and the right rear quarter and left rear quarter were milked and weighed together).
Milk samples from all four quarters were tested for SCC at 7 days, 2 days, and 1 day before challenge, and duplicate milk samples collected using aseptic techniques were cultured for bacteria, including Mycoplasma spp., at 7 days and 2 days prechallenge. Milk samples for SCC enumeration were transported to the nearby Dairy Herd Improvement Association laboratory and were measured using a Fossomatic (Foss in North America, Eden Prairie, MN) cell counter; samples too thick to go through the Fossomatic were tested by direct microscopic SCC. Samples for bacteriological culture were transported cold to the Quality Milk Production Services Central Laboratory at Cornell University for microbiological culture according to protocols recommended by the National Mastitis Council (18). Contamination was defined as the isolation of more than three types of bacteria other than Staphylococcus aureus.
Challenge (and contralateral) quarters were selected based upon consistency of milk production, having no major pathogens isolated from milk cultures, and SCC of <100,000/ml.
Intramammary E. coli challenge.
Cows were challenged two at a time on the same day of the week between 7 and 14 days following their calving due dates over a period of 35 days. Immediately following the afternoon milking on the day of challenge, the chosen quarter was infused with approximately 1,000 CFU of E. coli into the mammary gland (50 CFU/ml in 20 ml of sterile saline). Each challenged quarter was aseptically sampled for milk culture immediately before the challenge infusion. A remnant of the 20-ml intramammary challenge solution was transported cold immediately back to the Quality Milk Production Services laboratory at Cornell for confirmatory counting of the CFU/ml of E. coli. The challenge strain of bacteria was an E. coli O:157 strain (available from Quality Milk Production Services, Cornell University) originally isolated from a CM case. This strain had been used for challenge experiments previously (22).
Rectal temperature and clinical signs were monitored for 24 h postchallenge. Cows’ behavior was observed several times per day for the remainder of the trial. Milk samples were collected for SCC and culture from the challenged quarter every 3 h for the first 24 h postchallenge and then every 12 h until 180 h postchallenge. The milk production for the challenged quarter, contralateral quarter, and the other two quarters combined were monitored as described above for the rest of the trial. Cows were sold 8 days postchallenge.
J5-specific antibody.
Blood samples for measurement of serum J5-specific antibodies (IgM, IgG1, and IgG2) were collected 4 weeks before the calving due date and within 4 h following calving, and both milk and blood samples were collected immediately prior to challenge and 12 h and 24 h postchallenge. No blood samples were collected 8 weeks before calving because based on preliminary data from one of the authors (J. L. Burton), we did not expect much seroconversion after only the first vaccination. Blood samples were centrifuged (750 × g, 15 min, 5°C), and the serum was stored at −80°C in five 1-ml aliquots in microtubes. Milk was not centrifuged, and milk samples were stored at −80°C in five 1-ml aliquots in microtubes. Antibody was determined by an enzyme-linked immunosorbent assay as described previously (6).
Briefly, flat-bottom 96-well enzyme-linked immunosorbent assay plates (BD Falcon, Bedford, MA) were coated with 100 μl of J5 E. coli (1 × 109 CFU/ml in sterile saline) as the target antigen (killed with 1% phenol), covered with an adhesive plate sealer, and left on a flat surface at room temperature for 12 h to enable the bacteria to adhere to the wells. There were four negative-control wells with no E. coli added on each plate to assess nonspecific binding of antibodies to plastic. To perform the assay, plates were washed three times with a wash solution (0.1% Tween 20 in aqueous normal 0.9% saline) that functioned as a protein blocker, and various control and test samples were added to the appropriate wells. Four wells of each plate received a 1:400 dilution of low immunoglobulin fetal bovine serum as the assay negative control. An additional four wells received a 1:400 dilution of serum from a dairy cow immunized with J5 six times as the assay positive control. Any plate for which the standard deviation of the mean optical density (OD) of this positive control was more than 1.0 was repeated on a different assay day. Finally, additional control wells included two blanks (no E. coli or other test reagents) against which the plate reader was blanked, two wells with no E. coli or test serum but with all other test reagents added, two wells with E. coli but no other test reagents except serum, and two wells with E. coli plus all other test reagents except serum. The remaining 80 wells per plate received test milk or sera as described below after receiving 125 μl of diluent (10% 10× phosphate-buffered saline [pH 7.3], 0.05% Tween 20 in sterile water) as the solution for serial dilutions of these test samples.
Serum test samples for detection of J5-specific IgG1 were plated in 30 serial (doubling) dilutions from 1:2 to 1:1,073,741,824. Serum test samples for detection of J5-specific IgG2 and J5-specific IgM were plated in 20 doubling dilutions from 1:2 to 1:1,048,576. Whole milk was used for detection of J5-specific IgG1, IgG2, and IgM; all samples were plated in 15 doubling dilutions from 1:2 to 1:32,768. These ranges of dilutions were sufficient so that all milk or serum samples for each class of antibody were eventually diluted to an OD reading of <0.100, which was the OD of the fetal bovine serum negative control and thus used as an indication of endpoint titer. Therefore, the highest dilutions of test samples that resulted in ODs of <0.100 were recorded as the titers.
Statistical analyses.
Shedding of E. coli in milk (CFU/ml) was tested for differences between controls and J5 vaccinates with mixed linear models and least-squares means (Statistical Analysis System [SAS]; SAS Institute, Cary, NC) (PROC MIXED). Differences between controls and vaccinates in SCC/ml in milk, mean daily milk production change from the 8 days before challenge versus the 7 days after challenge, and J5-specific antibody titers preceding and following challenge were tested with analysis of variance (ANOVA) (PROC ANOVA). Ratios of IgG1:IgG2 and of IgG1 and IgG2 to IgM were evaluated using linear regression with general linear models (PROC GLM). Linear regression was also used to evaluate other possible explanatory variables associated with shedding of E. coli in milk 3 h postchallenge and with differences in milk production between controls and vaccinates following challenge. Quarter milk production change was similar to total cow milk loss; therefore, only the latter was analyzed because of its practical importance. A mixed linear model with least-squares means (PROC MIXED) was developed to explain variation in total cow milk production on a given day of lactation, whether before or after the challenge. Repeated measures of the same cow were taken into account using a random cow effect. The daily milk production models included the effects of days in milk (DIM) on total cow milk production. The ratios of IgG1:IgG2 and IgG1 and IgG2 (combined) to IgM were also tested as potential explanatory variables (covariates) for each of the models examining variation among cows in outcomes following mastitis. All statistical analyses were performed using SAS 8.2.
For example, evaluation of J5-specific antibody response at a given time, specific isotype, and in milk or serum used ANOVA (PROC ANOVA): Y = J5 Vacc + e. Expanded ANOVA was as follows: Y = J5 Vacc + LACT + e, where Y is the serum titer of IgG2 24 h postchallenge, J5 Vacc is the J5 vaccinate (value of 1) or control (value of 0), LACT is the lactation number 2, 3, or 4, and e is unexplained variation.
RESULTS
Bacteriology and SCC before intramammary challenge.
Challenged quarters had no major pathogens isolated before the challenge. Up until immediately prior to challenge, all 16 challenged and contralateral quarters had consistent milk production. Mean prechallenge SCC was 45,000/ml in challenged quarters, ranging from 16,000 to 87,000/ml.
Clinical response and follow-up culture of challenge solution after intramammary challenge.
Cows were challenged at a median of 13 DIM (range, 8 to 16 DIM). There were no clinical signs of mastitis after challenge, and except for one control cow with a rectal temperature of 104.5°C at 12 h postchallenge, all rectal temperatures at 12 h and 24 h postchallenge were normal. All cows had normal appetite following challenge. Cultures of the remnants of all eight challenge infusion solutions revealed E. coli between 600 and 1,700 CFU/20 ml, all within 1 log unit of the target of 1,000 CFU E. coli/20 ml.
Shedding of E. coli in milk after intramammary challenge.
Shedding of E. coli in milk was significantly higher among controls than vaccinates between 6 h and 21 h postchallenge (all P values were ≤0.05, mixed linear model, PROC MIXED). During that time, CFU/ml of E. coli ranged between 195 and 5,500 for three of the four control cows. Of the four vaccinates, one cow shed 10 CFU/ml, and another cow shed 20 CFU/ml of E. coli at 3 h postchallenge; otherwise, the four vaccinates shed no bacteria from 3 to 21 h postchallenge. From 24 h postchallenge on, no statistically significant differences were observed between vaccinates and controls.
Because most of the shedding of E. coli in milk among J5 vaccinates took place at 3 h postchallenge, that time point was investigated using a general linear model. Vaccination with J5 was associated with less shedding of E. coli in milk (P < 0.0001, linear regression, PROC GLM). Within only the control cow population, serum J5-specific IgM antibody just prior to challenge was associated with reduced E. coli shedding at 3 h postchallenge (significant interaction term, P < 0.0001). This model for shedding of E. coli in milk at 3 h postchallenge was highly explanatory (R2 = 0.99, P < 0.0001, PROC GLM).
SCC in milk after intramammary challenge.
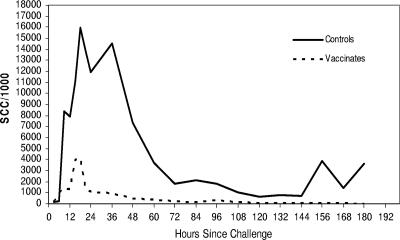
At 21 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, and 132 h following challenge, SCC in challenged quarter milk was significantly higher for control cows than for vaccinates. Most of the controls’ challenged quarter SCC/ml values were between 1,805,000/ml and 14,559,000/ml, with a mean of 5,429,000/ml, while the vaccinates’ challenged quarter SCC/ml were mainly between 81,000/ml and 373,000/ml, with a mean of 490,000/ml (all P values were <0.05, ANOVA; Fig. 1).
FIG. 1.
SCC/ml in milk following intramammary infusion challenge in controls and J5 vaccinates. There was significantly higher SCC in controls at 21 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, and 132 h postchallenge (all P values were <0.05, ANOVA).
Milk production change after intramammary challenge.
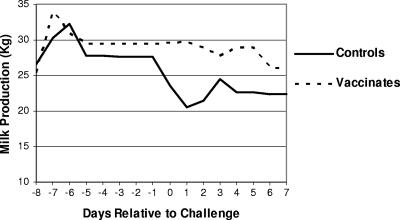
Control cows showed a significant reduction in milk yield on the day after challenge (−7.7 kg) compared to essentially no change by vaccinated cows (+0.5 kg) (P < 0.02, mixed linear model, PROC MIXED). Thereafter, milk production remained lower in control cows compared to vaccinated cows but was not significantly different due to the relatively small study size and the large between-cow variation (Fig. 2).
FIG. 2.
Total cow milk production (kg) before and after intramammary infusion challenge of controls and J5 vaccinates. There was significantly higher milk production among vaccinates for 1 day following challenge (P < 0.02, mixed linear model, PROC MIXED). From 2 through 7 days postchallenge, milk production was higher among vaccinates but not statistically different.
J5-specific antibodies in milk and serum.
Negative-control and blank wells all had ODs of <0.100. Titers for the three classes of J5-specific antibody were not significantly different in serum 4 weeks before calving (just preceding the second and last immunization), in serum immediately following calving (approximately 4 weeks after the last J5 immunization), or in milk immediately prior to intramammary challenge (at 8 to 16 DIM), between vaccinates and controls (all P values were ≥0.11, ANOVA).
However, immediately prior to challenge, serum J5-specific IgG1 antibody was significantly higher for J5 vaccinates (mean titer, 1:3,584) than for the controls (mean titer, 1:1,024) (P = 0.003, ANOVA; Fig. 3). Serum J5-specific IgG2 antibody was also higher for J5 vaccinates (mean titer,1:1,088) than for the controls (mean titer, 1:240) prior to challenge; the difference approached statistical significance (P = 0.07, ANOVA; Fig. 3). Serum J5-specific IgM antibody was not significantly different between J5 vaccinates (mean titer, 1:320) and controls (mean titer, 1:448) prior to challenge (P = 0.21, ANOVA; not shown).
FIG. 3.
Change in (total cow) daily milk production (PRODDIFF in kg) following intramammary infusion challenge at 8 to 16 DIM among four controls (C) and four J5 vaccinates (V). Blood serum titers of J5-specific IgG1 and IgG2 antibodies (Ab) (divided by 100) immediately prior to challenge are shown. Serum IgG1 titers were significantly higher prechallenge among vaccinates (P = 0.003, ANOVA). Mean serum IgG2 titers were higher prechallenge among vaccinates, and the differences approached statistical significance (P = 0.07, ANOVA). Other differences were not significant.
By 12 h postchallenge, J5-specific serum IgM response in controls (mean titer, 1:576) was higher than that for vaccinates (mean titer, 1:200), approaching statistical significance (P = 0. 07, ANOVA). J5-specific IgG1 and IgG2 in serum and all three antibody classes in milk were not significantly different among the treatment groups at 12 h postchallenge (all P values were ≥0.22, ANOVA). At 24 h postchallenge, all three classes of J5-specific antibody were not different in milk or serum between vaccinates and controls (all P values were ≥0.11, ANOVA).
Ratios of J5-specific antibody isotypes.
There were differences in the IgG1:IgG2 ratios approaching statistical significance between J5 vaccinates and controls as follows: postcalving serum ratios, 0.6 in vaccinates and 1.9 in controls (P = 0.15, type 3 SS F test, linear regression); 12 h postchallenge serum ratios, 3.3 in vaccinates and 17.0 in controls (P = 0.16); and 24 h postchallenge milk ratios, 404.0 in vaccinates and 44.0 in controls (P = 0.18). There was a trend that vaccinates had proportionally more IgG2 in serum and less IgG2 in milk than controls.
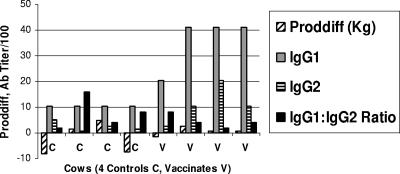
The ratio of J5-specific IgG1 and IgG2 combined to IgM (IgGs:IgM) was investigated. The milk and serum of J5 vaccinates were significantly higher in J5-specific IgG1 plus IgG2 relative to J5-specific IgM than the milk and serum of control cows immediately prechallenge (Fig. 4a). The ratios of IgGs:IgM for J5 vaccinates and controls, respectively, were as follows: 2.4 and 2.4 in serum 4 weeks before calving (P = 0.99, ANOVA); 6.1 and 0.5 in postcalving serum (P = 0.10); 15.8 and 3.2 in prechallenge serum (P = 0.02); 5.0 and 1.3 in prechallenge milk (P = 0.03) (Fig. 4a); 51.5 and 7.6 in serum 12 h postchallenge (P = 0.27); 76.8 and 66.0 in milk 12 h postchallenge (P = 0.91) (Fig. 4b); 15.3 and 7.6 in serum 24 h postchallenge (P = 0.18); and 306.7 and 35.4 in milk 24 h postchallenge (P = 0.31, all tests by ANOVA) (Fig. 4c). This indicated that J5 vaccinated cows’ immune response was more toward J5-specific IgG1 and IgG2 antibodies while that of controls had proportionally more J5-specific IgM antibody, especially just before challenge. Both the IgG1-to-IgM ratio and IgG2-to-IgM ratio were similar (data not shown).
FIG. 4.
(a) Mean titers of J5-specific antibodies in serum and milk samples from J5 vaccinates and controls just prior to intramammary challenge infusion with E. coli. Significant differences were higher serum IgG1 prechallenge (P = 0.003, ANOVA), higher serum IgG2 prechallenge (borderline significant at P = 0.07), and higher IgGs:IgM ratio in serum and milk (P = 0.02 and 0.03, respectively) in J5 vaccinates than in control cows. (b) Mean titers of J5-specific antibodies in serum and milk samples from J5 vaccinates and controls 12 h after intramammary challenge infusion with E. coli. Serum IgM was higher in controls than in J5 vaccinates (borderline significant at P = 0. 07, ANOVA). All other antibody titers and ratios were not significantly different in controls and vaccinates. (c) J5-specific antibodies in serum and milk 24 h after intramammary challenge infusion with E. coli. No significant differences between controls and vaccinates were observed.
Milk production change and J5-specific IgM.
Diagnostic plots (including residual analysis) for the milk production linear model revealed that one cow was driving the model as an outlier. Therefore, that cow was excluded (she was excluded only from this analysis of milk production change), which resulted in a better fitting model according to diagnostic plots. The resultant model (for the difference in mean daily milk production for the 8 days prechallenge compared with the 7 days postchallenge) was highly explanatory (R2 = 0.78, P = 0.009, linear regression, PROC GLM). Cows having relatively high milk IgM antibodies against J5 by 12 h postchallenge were significantly more likely to have greater milk production loss following the E. coli challenge.
A linear model for milk production change (with all eight cows included) following challenge showed strong trends but was not significant (R2 = 0.50, P = 0.18, linear regression, PROC GLM; Table 1). Cows that were J5 vaccinates and that had higher J5-specific IgG1:IgG2 ratio in serum after calving had less milk production loss following CM (Table 1). The two effects were only marginally statistically significant (P = 0.10 and 0.11, respectively).
TABLE 1.
Linear regression model evaluating factors associated with change in daily milk productiona
| Parameter | Change in daily production after challenge (kg of milk)b
|
t | Probable associationc | |
|---|---|---|---|---|
| Estimate | SE | |||
| Intercept | −0.9 | 2.11 | −0.41 | 0.702 |
| Variables | ||||
| J5 vaccination | 6.7 | 3.33 | 2.00 | 0.102 |
| J5-specific antibody titerd | 2.8 | 1.43 | 1.93 | 0.111 |
For the overall linear regression model, R2 = 0.50 and P = 0.18.
The change in daily milk production was calculated by taking the difference between the mean of the 8 days before intramammary E. coli challenge and the mean of the 7 days following challenge.
Values indicate the probability that the parameter is significantly related to the change in milk production; lower values indicate greater probability. Values were calculated by comparison of the calculated t value to the critical t value. The SAS program indicates this comparison by the shorthand notation “Pr > t,” where Pr is probability.
Ratio of J5-specific IgG1 antibody to IgG2 antibody. Serum antibody titers were measured just after calving.
DISCUSSION
J5 efficacy.
Vaccination with this J5 bacterin conferred protection following intramammary infusion challenge with E. coli. Vaccination was associated with almost immediate clearance of bacteria from milk, while controls shed E. coli in their milk for approximately 24 h. The SCC in milk of vaccinates was approximately 10% of SCC in controls following challenge, less than the mean values of 500,000/ml for vaccinates and more than 5,000,000/ml for control cows. Reduced SCC is an important indicator of reduced mammary inflammation and less milk loss following CM and is an important test of milk quality in the dairy industry (11, 25). After 60 h postchallenge, the vaccinates had SCCs of <300,000/ml, and the SCCs of the controls were between 1,000,000 and 2,000,000/ml. Vaccinates had less milk production loss following challenge than controls by more than 3.0 kg per day, although this was statistically significant for only one day postchallenge.
J5 vaccination and production of J5-specific antibodies.
Both controls and vaccinates had some J5-specific antibody of all three classes in serum and milk at all stages of the experiment. This suggests that all cows had some previous exposure to coliform mastitis pathogens sufficient to mount some immune response, including memory type responses. Considering that coliforms are widespread in dairy cow environments, this is reasonable (21, 32).
Immediately prior to challenge at approximately 2 weeks into lactation and 6 weeks after the second of two vaccinations, serum E. coli J5-specific IgG1 antibody was significantly higher in J5 vaccinates than controls, and serum IgG2 was also higher in vaccinates at borderline statistical significance. The proportions of IgG1 and IgG2 (compared separately or combined) to IgM were significantly higher in the J5 vaccinates immediately before challenge, 5 times higher in serum and 4 times higher in milk.
Thus, the controls had relatively more IgM antibody to E. coli J5 just prior to intramammary infusion challenge, while J5 vaccinates had more IgG1 and IgG2 antibody. Exposure of B cells to antigen within a given host and a particular cytokine milieu stimulates a class switch from production of IgM to IgG1, IgG2, or other isotypes. A key component of effective host defense and immunological memory is this irreversible B-cell genetic change from IgM to production of other antibody isotypes, including IgG1 and IgG2 (5, 14). Originally described in mice, an antibody response in other species, including cows, featuring proportionally more production of IgG2 antibody has been called part of a type 1 response, while a response with more IgG1 is part of a type 2 response (4, 13, 27).
It has been suggested that an IgG2 type 1 response should be beneficial against bovine mastitis because IgG2 is an important opsonizing antibody aiding in neutrophil phagocytosis of bacteria, and IgG2 has the ability to readily fix complement (2, 5, 12, 24). In milk, the most important opsonizing antibody against coliform bacteria is IgG2, especially early in the infection (5, 7). Phagocytosis by PMN and the associated clearance of coliform bacteria from the mammary gland has been reported as optimal when IgG2 increases within 4 h following intramammary infection, 6 to 12 h before the greatest influx of PMN from blood to milk (5, 23). The availability of more IgG2 against J5 immediately after bacteria enter the mammary gland appears to be an important benefit of J5 vaccination; because of previous natural exposure, control cows produced a similar response but only 12 h after infusion of E. coli. The immune response of dairy cows in early lactation has a type 2 bias, while later in lactation, the bias is type 1, which may be associated with greater protection against mastitis (26). These present results suggest that increased production of both J5-specific IgG1 and IgG2 are important mechanisms of J5 vaccine protection, including production of a higher proportion of IgG2 (lower IgG1:IgG2 ratio) than in control cows. In fact, the postcalving serum ratio of IgG1:IgG2 was less than 1 (0.6) in vaccinates, the only time in all of the data points where the immune response showed higher J5-specific IgG2 than IgG1, a type 1 response.
It has been previously speculated that increased production of J5-specific IgG2 could be a key mechanism of protection by J5 immunization in cattle (1, 5, 12, 28). Calves were reported more resistant to pneumonia caused by Haemophilus somnus in association with higher IgG2 antibody specific for H. somnus, while other antibody isotypes were not protective (8, 9). However, it has also been reported that while J5 vaccinates had higher IgG1 and IgG2 following vaccination as well as following CM, these antibodies were not considered protective based on CM signs (17). The present study demonstrated reduced milk loss, bacterial shedding, and SCC following mastitis, together with higher IgG1 and IgG2 response among J5 vaccinates, especially in the first 12 h after bacteria entered the gland.
In a previous study, the antibody responses of 136 Holsteins in three dairy herds (48 cows in first lactation and 88 older cows) were studied following vaccination with ovalbumin and J5. It was concluded that there are many sources of variation in CM among herds and that herds should probably be evaluated separately for J5 effects on antibody and associations with CM (29). The current study used cows that originated from one commercial dairy herd, so the herd effect could not be studied. Conversely, there is some benefit from this in that there was no herd effect to possibly affect results between cows.
From the present study as well as some previous reports, it seems that J5 vaccination is associated with a memory antibody response of IgG1 and IgG2 isotypes and that this is also associated with protection against mastitis. This leads to some important questions to be investigated further, such as what are the relevant antigen(s) in J5 bacterins? This has never been conclusively shown (3, 6, 12). Indeed, there is recent evidence that the most immunogenic antigen(s) in J5 bacterin is not lipopolysaccharide or lipid A (6).
These results also raise the question of the optimum J5 immunization schedule for producing the most efficacious immune response. In a previous study of steers vaccinated with a J5 bacterin, five immunizations with J5 over 72 days were necessary for J5-specific IgG2 antibody to be significantly increased over the preimmunization levels in serum. The authors suggested that multiple doses of J5 bacterin may be needed to result in high concentrations of IgG2 reactive against J5 (6). Further investigations into the antigen(s) in J5 bacterin and the most cost-effective immunization schedule as measured by protection against naturally occurring mastitis cases in lactating dairy cattle are needed.
Conclusions.
Vaccination with this J5 bacterin according to the described protocol was associated with faster clearance of E. coli from milk, reduced SCC, and less milk production loss following intramammary challenge. The vaccine was also associated with higher J5-specific IgG1 and IgG2 antibody in blood before challenge. While the controls relied more on IgM antibody response to E. coli postchallenge, the vaccinates produced an IgG1 and IgG2 immune response to E. coli, including a higher proportion of IgG2 (type 1 response). Vaccination with J5 appears to promote more IgG2 early in the course of intramammary infection, which is beneficial to phagocytosis by PMN and clearance of coliform bacteria from the mammary gland. Further investigations into the antigen(s) in J5 bacterin and the most cost-effective immunization schedule for dairy cattle are needed.
Acknowledgments
This work was funded by grants from USDA award 98-5204-6489 and the Cornell University Center for Biotechnology, a New York State Center for Advanced Technology supported by New York State and industrial partners.
We appreciate the cooperation of the personnel in the laboratories of Jeanne Burton and Phil Sears at Michigan State University as well as the invaluable technical help of Suzanne Klaessig and Al Keyes at Cornell University. We also appreciate the help of Ruben González and Carlos Santisteban at the Quality Milk Production Services and Gerald Willis at the dairy barns of the College of Veterinary Medicine at Cornell University.
This paper is Journal Series no. 7886 of the Utah Agricultural Experiment Station.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Barrio, M. B., P. Rainard, F. B. Gilbert, and B. Poutrel. 2003. Assessment of the opsonic activity of purified bovine sIgA following intramammary immunization of cows with Staphylococcus aureus. J. Dairy Sci. 86:2884-2894. [DOI] [PubMed] [Google Scholar]
- 2.Bastida-Corcuera, F. D., J. E. Butler, S. Yahiro, and L. B. Corbeil. 1999. Differential complement activation by bovine IgG2 allotypes. Vet. Immunol. Immunopathol. 71:115-123. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner, J., D. Heumann, T. Calandra, and M. P. Glauser. 1991. Antibodies to lipopolysaccharides after immunization of humans with the rough mutant Escherichia coli J5. J. Infect. Dis. 163:769-772. [DOI] [PubMed] [Google Scholar]
- 4.Brown, W. C., A. C. Rice-Ficht, and D. M. Estes. 1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:45-55. [DOI] [PubMed] [Google Scholar]
- 5.Burton, J. L., and R. J. Erskine. 2003. Immunity and mastitis. Some new ideas for an old disease. Vet. Clin. N. Am. Food Anim. Pract. 19:1-45. [DOI] [PubMed] [Google Scholar]
- 6.Chaiyotwittayakun, A., J. L. Burton, P. S. Weber, K. Kizilkaya, F. F. Cardoso, and R. J. Erskine. 2004. Hyperimmunization of steers with J5 Escherichia coli bacterin: effects on isotype-specific serum antibody responses and cross reactivity with heterogeneous gram-negative bacteria. J. Dairy Sci. 87:3375-3385. [DOI] [PubMed] [Google Scholar]
- 7.Colditz, I., and D. Watson. 1985. The immunophysiological basis for vaccinating ruminants against mastitis. Aust. Vet. J. 62:145-153. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil, L. B. 2002. Antibodies as effectors. Vet. Immunol. Immunopathol. 87:169-175. [DOI] [PubMed] [Google Scholar]
- 9.Corbeil, L. B., R. P. Gogolewski, I. Kacskovics, K. H. Nielsen, R. R. Corbeil, J. L. Morrill, R. Greenwood, and J. E. Butler. 1997. Bovine IgG2 antibodies to Haemophilus somnus and allotype expression. Can. J. Vet. Res. 61:207-213. [PMC free article] [PubMed] [Google Scholar]
- 10.Cullor, J. S. 1991. The Escherichia coli J5 vaccine: investigating a new tool to combat mastitis. Vet. Med. 86:836-844. [Google Scholar]
- 11.Dohoo, I. R., A. H. Meek, and S. W. Martin. 1984. Somatic cell counts in bovine milk: relationships to production and clinical episodes of mastitis. Can. J. Comp. Med. 48:130-135. [PMC free article] [PubMed] [Google Scholar]
- 12.Dosogne, H., F. Vangroenweghe, and C. Burvenich. 2002. Potential mechanism of action of J5 vaccine in protection against severe bovine coliform mastitis. Vet. Res. 33:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Estes, D. M., and W. C. Brown. 2002. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet. Immunol. Immunopathol. 90:1-10. [DOI] [PubMed] [Google Scholar]
- 14.Estes, D. M., W. C. Brown, and A. Hirano. 1998. CD40 ligand-dependent signaling of bovine B lymphocyte development and differentiation. Vet. Immunol. Immunopathol. 63:15-20. [DOI] [PubMed] [Google Scholar]
- 15.González, R., J. Cullor, D. Jasper, T. Farver, R. Bushnell, and M. Oliver. 1989. Prevention of clinical coliform mastitis in dairy cows by a mutant Escherichia coli vaccine. Can. J. Vet. Res. 53:301-305. [PMC free article] [PubMed] [Google Scholar]
- 16.González, R. N., D. J. Wilson, H. O. Mohammed, P. M. Sears, A. L. Rivas, and S. G. Campbell. 1996. A placebo-controlled trial of an Escherichia coli J5 bacterin and the ribotyping-based assessment of coliform bacteria diversity on a dairy farm, p. 277-280. In Proceedings of the 19th World Buiatrics Congress, Edinburgh, Scotland.
- 17.Hill, A. W. 1991. Vaccination of cows with rough Escherichia coli mutants fails to protect against experimental intramammary bacterial challenge. Vet. Res. Commun. 15:7-16. [DOI] [PubMed] [Google Scholar]
- 18.Hogan, J. S., R. N. González, R. J. Harmon, S. C. Nickerson, S. P. Oliver, J. W. Pankey, and K. L. Smith. 1999. Laboratory handbook on bovine mastitis, rev. ed., p. 85-111. NMC Inc., Madison, WI.
- 19.Hogan, J. S., K. L. Smith, D. A. Todhunter, and P. S. Schoenberger. 1992. Field trial to determine efficacy of an Escherichia coli J5 mastitis vaccine. J. Dairy Sci. 75:78-84. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, E., and J. Bramley. 1983. Coliform mastitis. In Pract. 5:135-146. [DOI] [PubMed] [Google Scholar]
- 21.Jones, G., and G. Ward. 1989. Cause, occurrence, and clinical signs of mastitis and anorexia in cows in a Wisconsin study. J. Am. Vet. Med. Assoc. 195:1108-1113. [PubMed] [Google Scholar]
- 22.Lohuis, J. A., Y. H. Schukken, J. H. Verheijden, A. Brand, and A. S. Van Miert. 1990. Effect of severity of systemic signs during the acute phase of experimentally induced Escherichia coli mastitis on milk production losses. J. Dairy Sci. 73:333-341. [DOI] [PubMed] [Google Scholar]
- 23.Rainard, P. 1983. Experimental mastitis with Escherichia coli: sequential response of leukocytes and opsonic activity in milk of immunized and unimmunized cows. Ann. Rech. Vet. 14:281-286. [PubMed] [Google Scholar]
- 24.Rainard, P., Y. Lautrou, and B. Poutrel. 1988. Ingestion and killing of Streptococcus agalactiae by bovine granulocytes in the presence of natural opsonins. Vet. Microbiol. 18:41-50. [DOI] [PubMed] [Google Scholar]
- 25.Reneau, J. K. 1986. Effective use of dairy herd improvement somatic cell counts in mastitis control. J. Dairy Sci. 69:1708-1720. [DOI] [PubMed] [Google Scholar]
- 26.Shafer-Weaver, K. A., C. M. Corl, and L. M. Sordillo. 1999. Shifts in bovine CD4+ subpopulations increase T-helper-2 compared with T-helper-1 effector cells during the postpartum period. J. Dairy Sci. 82:1696-1706. [DOI] [PubMed] [Google Scholar]
- 27.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 28.Tomita, G. M., C. H. Ray, S. C. Nickerson, W. E. Owens, and G. F. Gallo. 2000. A comparison of two commercially available Escherichia coli J5 vaccines against E. coli intramammary challenge. J. Dairy Sci. 83:2276-2281. [DOI] [PubMed] [Google Scholar]
- 29.Wagter, L. C., B. A. Mallard, B. N. Wilkie, K. E. Leslie, P. J. Boettcher, and J. C. Dekkers. 2000. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J. Dairy Sci. 83:488-498. [DOI] [PubMed] [Google Scholar]
- 30.Wenz, J. R., G. M. Barrington, F. B. Garry, K. D. McSweeney, R. P. Dinsmore, G. Goodell, and R. J. Callan. 2001. Bacteremia associated with naturally occurring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. 219:976-981. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Wilson, D. J., R. N. González, and H. H. Das. 1997. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 80:2592-2598. [DOI] [PubMed] [Google Scholar]