Abstract
Results from two dengue rapid tests, the PanBio Duo cassette and the SD Bioline strip test, were compared to those of enzyme-linked immunosorbent assays (Focus Diagnostics) from sera of 200 Vietnamese febrile patients. The PanBio assay was superior, with sensitivity and specificity values for acute-phase serum samples of 54% and 70% (immunoglobulin M) and 70% and 88% (immunoglobulin G), respectively.
Vietnam is a region of endemicity for dengue virus, with an annual incidence of first infections exceeding 10% (9). Dengue is often misdiagnosed or ignored, and routine notification of dengue virus infections grossly underestimates the burden of disease (6, 7). Routine serological diagnosis is usually based on the detection of dengue-specific serum immunoglobulin M (IgM) and IgG in paired sera using enzyme-linked immunosorbent assay (ELISA) (8, 11). ELISA is also the basis for an abundance of rapid tests (1). The latest models make use of the lateral flow principle, with the advantage that the test sample can be applied directly on the test pad together with a buffer solution (2). In this study we compared a newly developed lateral flow test for dengue to another commercially available product with a population of febrile patients from Vietnam, an area where dengue is highly endemic.
The study site has been described elsewhere (5, 6). Patients with acute fever without signs of severe systemic or organ-specific disease were included. Two blood samples were collected by venous puncture on presentation (at time 0 [t0]) and after 3 weeks (t3 weeks); sera were stored in a freezer at −70°C until analysis. Paired serum samples were tested with a commercially available IgM capture and an IgG ELISA (Focus Diagnostics Inc., Cypress, CA) (4). Details regarding both the IgM capture ELISA and the IgG ELISA and the interpretation of the results have been described previously (4, 10). Two rapid tests were evaluated: the PanBio Dengue Duo Cassette (PanBio Ltd., Queensland, Australia) and the SD Bioline Dengue IgG/IgM strip test (Standard Diagnostics Inc., Kyonggi-do, Korea). The rapid tests were read according to the manufacturers' instructions by two independent investigators. Results were read as positive and negative. Indeterminate results were recorded as such. Acute primary dengue infection was definedas an IgM-positive and IgG-negative result; acute secondary dengue infection was defined as an IgM-positive and IgG-positive or IgM-negative and IgG-positive result.
The overall agreement, sensitivities, specificities, and predictive values and their 95% confidence intervals (95% CI) were determined using SPSS for Windows (version 12.2; SPSS, Inc., Chicago, IL). Agreement was assessed by calculating the kappa value.
Serum samples from 200 febrile patients were tested, of whom 162 had dengue antibodies. Based on the Focus ELISA results, 51 patients were classified as having acute primary dengue infection, 44 as having acute secondary dengue infection, and 67 as having had past dengue infections. In 38 patients, no dengue antibodies were demonstrated.
The results of the rapid tests, applied to the t3-week sera, were read as positive or negative and compared to the results of the ELISA. The IgG test was always very clear. Five PanBio IgM and three SD IgM test samples showed a very faint band, even after a second reading 15 minutes later. In the following analysis, these were classified as negative.
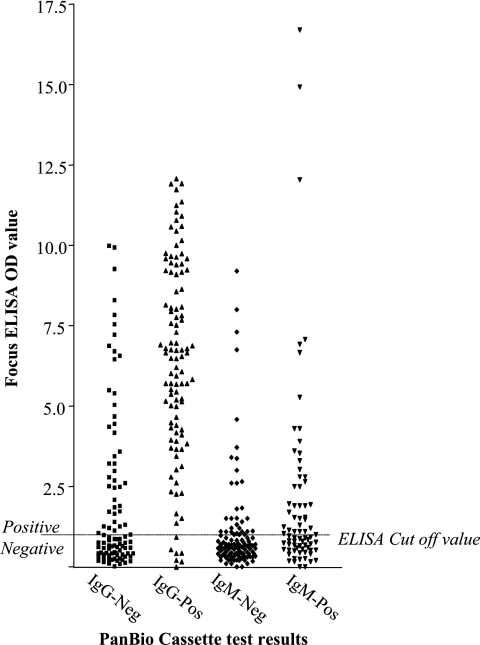
With the PanBio test, 78 samples were positive for IgM and 100 for IgG. With the SD tests, 12 samples were positive for IgM and 138 for IgG. Sensitivity and specificities, positive predictive values, and negative predictive values using the Focus ELISA as the gold standard are presented in Table 1. The results of the PanBio test were regarded as the best, and therefore, this assay was also applied to the t0 sample. Figure 1 shows the relationship between the PanBio cassette test and the optical density (OD) values of the Focus ELISA. Especially for IgM, there is no clear distinction between positive and negative results.
TABLE 1.
Two rapid test results compared to ELISA of dengue in sera from Vietnamese febrile patientsa
| Sample type (collection point) and test and/or antibody | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Convalescent-phase serum (t3 weeks) | ||||
| PanBio Dengue Duo Cassette | ||||
| IgM | 67.3 (57.8-75.6) | 91.7 (84.4-95.7) | 89.7 (81.1-94.7) | 72.1 (63.6-79.3) |
| IgG | 66.4 (58.4-73.6) | 94.4 (84.9-98.1) | 97.0 (91.6-99.0) | 51.0 (41.4-60.6) |
| SD Bioline Dengue IgG/IgM strip | ||||
| IgM | 10.6 (6.0-18.0) | 99.0 (94.3-99.8) | 91.7 (64.6-98.5) | 50.5 (43.5-57.6) |
| IgG | 90.4 (84.6-94.2) | 88.9 (77.8-94.8) | 95.7 (90.8-98.0) | 77.4 (65.6-86.0) |
| Acute-phase serum (t0) | ||||
| IgM | 54.3 (42.7-65.4) | 69.7 (61.0-77.1) | 50.1 (39.6-61.7) | 72.7 (64.0-79.9) |
| IgG | 70.0 (62.0-77) | 88.3 (77.8-94.2) | 93.3 (86.9-96.7) | 55.8 (45.8-65.4) |
Serum samples from 200 patients were tested. Values are percentages. Values in parentheses are 95% CI. PPV, positive predictive value; NPV, negative predictive value.
FIG. 1.
The Focus Dengue ELISA OD values for acute-phase serum samples tested with the PanBio Dengue Duo Cassette test.
The interpretation of results for the PanBio cassette test for four diagnostic groups in comparison to that for the Focus ELISA is shown in Table 2. The overall agreement was poor (kappa, 0.426; χ2, 160; P < 0.001). The rapid test, applied to the t0 sample, had difficulties in discriminating acute from past disease and acute primary from acute secondary dengue infection.
TABLE 2.
Diagnostic classification of dengue with the PanBio Dengue Duo Cassette test on acute-phase serum of Vietnamese febrile patients, compared to ELISA on paired serum samplesa
| Diagnostic group | No. of samples found positive by ELISA for paired sera
|
||||
|---|---|---|---|---|---|
| Acute primary dengue | Acute secondary dengue | No dengue | Recent or past dengue | Total | |
| Acute primary dengue | 10 | 12 | 0 | 2 | 24 |
| Acute secondary dengue | 15 | 25 | 0 | 16 | 56 |
| No dengue | 21 | 3 | 38 | 9 | 71 |
| Recent or past dengue | 5 | 4 | 0 | 40 | 49 |
| Total | 51 | 44 | 38 | 67 | 200 |
The diagnostic groups were assigned based on the results of the PanBio cassette test conducted at t0. The paired sera were collected at t0 and t3 weeks and tested by the Focus Diagnostics ELISA.
When the outcomes were combined to determine acute or not acute dengue infection, the Focus ELISA identified 95 patients with acute dengue infection and 105 patients with no acute dengue infection. Of these patients, the PanBio rapid test, applied to the acute sample, identified 80 patients with acute dengue and 120 without acute dengue (positive predictive value, 77.5%; negative predictive value, 72.5%; kappa, 0.485; P < 0.001).
The manufacturers have succeeded in making tests that are easy to handle, but the sensitivity of both assays is poor. The high cutoff levels that both manufacturers apply are necessary to increase the specificity in areas where dengue is endemic, but this is done at the expense of the sensitivities of both tests. The poor sensitivities of both rapid tests, especially that of the SD IgM test (∼11%), compares to results of a recent study of a series of rapid tests that included the previous generation PanBio test, a strip test, and the SD test (1).
The Focus ELISA is a rather sensitive assay, and the algorithm that was used for diagnostic classification in this study was also sensitive, with small increases of antibody concentrations leading to a diagnosis of acute dengue infection. However, the lack of sensitivity of the rapid assays for detecting antibodies in this study was at the basis of the diagnostic misclassification.
Although the PanBio cassette test performs somewhat better than its paper strip predecessor and much better than the SD strip test, the results of this study again confirm that rapid tests that are based on antibody detection still have a limited value for diagnosing dengue infection in its acute stage. Rapid tests for the detection of dengue NS1 antigen are being developed and are a promising alternative for tests that are based on antibody detection (3, 12).
In conclusion, the PanBio cassette and SD strip tests for dengue infection are easy to use with clear results. They show a high specificity with poor sensitivity, especially with respect to the detection of IgM. The PanBio cassette test performed better than the SD test but, applied to the acute-phase serum sample, also did not discriminate clearly between infection at the acute primary or secondary dengue or past dengue phase.
Acknowledgments
This study was carried out with the support of The Netherlands Foundation for the Advancement of Tropical Research (WOTRO).
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Blacksell, S. D., P. N. Newton, D. Bell, J. Kelley, M. P. Mammen, Jr., D. W. Vaughn, V. Wuthiekanun, A. Sungkakum, A. Nisalak, and N. P. Day. 2006. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clin. Infect. Dis. 42:1127-1134. [DOI] [PubMed] [Google Scholar]
- 2.Charrel, R. N., and X. de Lamballerie. 2002. Low specificity of an immunochromatographic serological assay for diagnosis of dengue fever in travelers returning with malaria. Clin. Diagn. Lab. Immunol. 9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dussart, P., B. Labeau, G. Lagathu, P. Louis, M. R. Nunes, S. G. Rodrigues, C. Storck-Herrmann, R. Cesaire, J. Morvan, M. Flamand, and L. Baril. 2006. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin. Vaccine Immunol. 13:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groen, J., P. Koraka, J. Velzing, C. Copra, and A. D. Osterhaus. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phuong, H. L., P. J. de Vries, N. Nagelkerke, P. T. Giao, L. Q. Hung, T. Q. Binh, T. T. Nga, N. V. Nam, and P. A. Kager. 2006. Acute undifferentiated fever in Binh Thuan province, Vietnam: imprecise clinical diagnosis and irrational pharmacotherapy. Trop. Med. Int. Health 11:869-879. [DOI] [PubMed] [Google Scholar]
- 6.Phuong, H. L., P. J. de Vries, T. T. Nga, P. T. Giao, L. Q. Hung, T. Q. Binh, N. V. Nam, N. Nagelkerke, and P. A. Kager. 2006. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect. Dis. 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phuong, H. L., P. J. de Vries, K. T. Thai, T. T. Nga, L. Q. Hung, P. T. Giao, T. Q. Binh, N. V. Nam, and P. A. Kager. Dengue virus infections in Vietnam: the tip of the iceberg. Dengue Bull., in press.
- 8.Teles, F. R., D. M. Prazeres, and J. L. Lima-Filho. 2005. Trends in dengue diagnosis. Rev. Med. Virol. 15:287-302. [DOI] [PubMed] [Google Scholar]
- 9.Thai, K. T., T. Q. Binh, P. T. Giao, H. L. Phuong, L. Q. Hung, N. Van Nam, T. T. Nga, J. Groen, N. Nagelkerke, and P. J. de Vries. 2005. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop. Med. Int. Health 10:379-386. [DOI] [PubMed] [Google Scholar]
- 10.Tran, T., P. de Vries, L. Hoang, G. Phan, H. Le, B. Tran, C. Vo, N. Nguyen, P. Kager, N. Nagelkerke, and J. Groen. 2006. Enzyme-linked immunoassay for dengue virus IgM and IgG antibodies in serum and filter paper blood. BMC Infect. Dis. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention, and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 12.Xu, H., B. Di, Y. X. Pan, L. W. Qiu, Y. D. Wang, W. Hao, L. J. He, K. Y. Yuen, and X. Y. Che. 2006. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 44:2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]