Abstract
To test the hypothesis that prolonged culture would enhance the sensitivity of latent tuberculosis detection by a gamma interferon release assay, blood samples from 33 household contacts of Gambian tuberculosis patients were stimulated with Mycobacterium tuberculosis-specific antigens. After 24 h of culture, 66% were positive, compared to 93% after 6 days of culture.
The tuberculin skin test (TST), using purified protein derivative (PPD) of Mycobacterium tuberculosis, is the most commonly used test for latent tuberculosis (TB) infection. Most of the antigens present in PPD are also present in Mycobacterium bovis BCG in addition to many environmental mycobacteria, which can affect the test's specificity (1). Recently developed T-cell in vitro assays are based on M. tuberculosis-specific antigens ESAT-6, CFP-10, and TB7.7, encoded in “region of difference 1” (RD-1), which is absent from M. bovis BCG (2). Although these antigens are present in some environmental mycobacteria, these assays have shown encouraging sensitivity and specificity for the diagnosis of M. tuberculosis infection and disease, particularly in countries with low TB prevalence (4, 10-12).
A commercial whole-blood assay for M. tuberculosis infection is based on the release of gamma interferon (IFN-γ) from antigen-specific T cells previously sensitized with mycobacterial antigens (5). Such assays have incubation periods of 16 to 24 h and detect mainly recently activated lymphocytes. By diluting the whole blood 10-fold and extending the stimulation period with mycobacterial antigens to 6 days (13), a strong correlation between in vitro IFN-γ production and TST was observed in Malawi (3). We therefore hypothesized that a longer incubation period in a whole-blood assay with M. tuberculosis-specific antigens would enhance the detection of latent TB in an area of endemicity.
In this study, we modified the QuantiFERON-TB Gold in-tube assay (Cellestis Limited, Carnegie, Victoria, Australia), a commercially available whole-blood “specific antigen” assay, for the diagnosis of latent TB infection in The Gambia, which has a TB prevalence of 329/100,000 (14). We recruited 33 human immunodeficiency virus-negative household contacts of sputum smear-positive TB cases, as well as four subjects from low-TB-prevalence European countries as controls. Ethical approval for the study was granted by the London School of Hygiene & Tropical Medicine Ethics Committee, United Kingdom, and by the combined MRC/Gambia Government Ethics Committee. The contacts recruited were 15 to 70 years old (median age, 27.5 years): 21 were female, and 12 were male. All underwent a Mantoux skin test; the median diameter was 12.5 mm (0 to 40 mm). Twenty-one (64%) contacts had a positive Mantoux skin test (mean of the longitudinal and transverse diameter of ≥10 mm) and were offered a chest X ray: all had a normal chest X ray. The four control subjects were two males and two females, aged between 21 and 28 years, without history of exposure to M. tuberculosis.
Blood samples were collected into QuantiFERON-TB Gold blood collection tubes. According to the manufacturer's instructions, the samples were incubated for 24 h at 37°C in a 5% CO2 atmosphere in negative control tubes (termed nil control tubes by the manufacturer) and TB-specific antigen tubes. After collection of 200 μl plasma, the blood was diluted 10-fold with RPMI 1640 medium supplemented with glutamine and antibiotics (InVitrogen), in U-bottom 96-well tissue culture plates, and incubated for a further 5 days. Fourteen blood samples were additionally stimulated with 10 μg/ml PPD (Statens Serum Institute, Copenhagen, Denmark). After incubation, supernatants were collected and stored at −20°C.
The concentration of IFN-γ in overnight plasma samples and the 6-day culture supernatants was quantified using the QuantiFERON-TB Gold enzyme-linked immunosorbent assay kit and in-tube analysis software, and data were extrapolated above the standard curve where necessary. IFN-γ values in IU/ml in the negative control tube were subtracted from the values obtained in the TB-specific antigen tube. A positive result is defined by the manufacturer to be ≥0.35 IU of IFN-γ/ml.
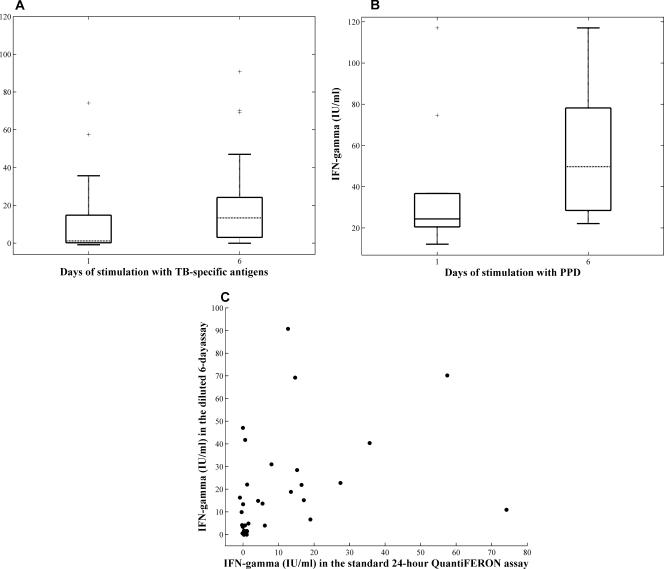
The median IFN-γ production in TB contact samples after 24 h of stimulation with TB-specific antigens was 1.15 ± 5.8 IU/ml (median ± 95% confidence interval), and this was significantly higher following 6 days of stimulation of diluted whole blood, with median IFN-γ production of 13.35 ± 7.7 IU/ml (P = 0.0010 by Wilcoxon's signed-rank test; Fig. 1A). The PPD-induced IFN-γ response was higher than that to the M. tuberculosis-specific antigens at both time points (Fig. 1B). In all controls, both the overnight and 6-day IFN-γ responses to TB-specific antigens were negative, with median levels of IFN-γ production of 0.045 ± 0.13 IU/ml and 0.21 ± 0.11 IU/ml, respectively.
FIG. 1.
Comparison of IFN-γ production after 24 h and 6 days of incubation with the antigens. (A) Whole blood from 33 TB contacts was tested with the TB-specific antigens in 24-h and extended 6-day IFN-γ assays. The box plots represent the median, lower, and upper quartiles of IFN-γ values after 24 h and 6 days of stimulation after subtraction of the negative control values. Lines extending from each end of the box show the extent of the rest of the data (1.5× interquartile range), and crosses represent outliers. (B) Whole blood from 14 subjects was stimulated with PPD for 1 or 6 days. (C) Scatter plot representing the correlation between 24-h undiluted and extended 6-day diluted IFN-γ assays (Spearman's rho = 0.5, P < 0.001).
As shown in Table 1, using the standard 24-h QuantiFERON-TB Gold in-tube method, 22 TB contacts tested positive and 11 were negative. The agreement between the QuantiFERON-TB Gold test and the skin test was 73%, with 17 (52%) of the skin test-positive individuals being positive by the QuantiFERON test and 7 (21%) of the QuantiFERON test-negative individuals being negative by the skin test. After 6 days of incubation of diluted blood with TB-specific antigens, 31 individuals were positive and only 2 individuals remained negative: both of these had been skin test positive. The agreement between the original, 24-h QuantiFERON Gold in-tube assay and the 6-day diluted assay was 67%, with 10 TB contacts testing positive in the extended assay but not with the standard overnight assay (Table 1). One individual who was positive with the 24-h assay had values of IFN-γ below 0.35 IU/ml in the extended 6-day assay, possibly due to activation-induced cell death if circulating TB-specific T cells were already activated in vivo. Overall, the correlation coefficient between the two assays was 0.5 (Fig. 1C).
TABLE 1.
Comparison between TB-specific 24-h and extended 6-day IFN-γ assaysa
| Overnight undiluted IFN-γ assay result | No. (%) with result by 6-day diluted IFN-γ assay
|
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 1 (3) | 10 (30) | 11 (33) |
| Positive | 1 (3) | 21 (64) | 22 (67) |
| Total | 2 (6) | 31 (94) | 33 |
TB contacts were tested for IFN-γ responses to TB-specific antigens in either a standard QuantiFERON-TB Gold overnight undiluted assay or a 6-day diluted assay. The cutoff for positivity was an IFN-γ concentration of 0.35 IU/ml, as determined by the manufacturer: negative, <0.35 IU/ml; positive, ≥0.35 IU/ml. The agreement between the assays is 63.4%.
We found that 67% of the 41 TB contacts tested were positive for latent TB infection by the 24-h QuantiFERON-TB Gold in-tube assay; 94% tested positive by the modified, extended IFN-γ assay; and none of the four controls tested positive with either assay. This was in keeping with our hypothesis that since overnight assays mainly detect IFN-γ released from recently activated lymphocytes, but not central memory lymphocytes, people with latent infection from past exposure may test negative by overnight assay and positive after extended culture.
QuantiFERON TB-Gold is a useful tool for TB contact investigation studies after recent exposure to M. tuberculosis. However, it might not detect a reservoir of people with latent TB who may have been infected with M. tuberculosis previously. Our data support such a premise, although long-term follow-up of patients tested with different test methods will be needed to answer this question definitively. Of note, TST appears to be slightly more sensitive at detecting latent TB infection from recent exposure than the standard overnight IFN-γ release assay in The Gambia (7). It has been suggested that regular ingestion of environmental mycobacteria might down-regulate skin test reactivity, especially that due to transient infection by nontuberculous mycobacteria (9). However, in Malawi, the IFN-γ response to PPD in a 6-day diluted-whole-blood assay strongly correlated with TST induration in healthy individuals not known to be contacts of TB patients (3).
Further studies with the modified, 6-day diluted-whole-blood assay might include an assessment against a TB exposure gradient (8) and more controls without known exposure to TB to confirm specificity (1).
Our results suggest that a negative overnight assay in individuals from a setting of TB endemicity should be interpreted with caution. In an area of M. tuberculosis endemicity, it may be more important to identify individuals with recent infection with a 24-h assay, but a 6-day incubation may provide useful information on the extent of latent infection. In settings of nonendemicity, a correct diagnosis of latent infection is of particular importance in certain patients, such as those with rheumatoid arthritis undergoing anti-tumor necrosis factor therapy (6). A 6-day diluted-whole-blood assay with TB-specific antigens may enhance detection of latent M. tuberculosis infection and should be considered in situations in which it is crucial to identify as many individuals as possible who are harboring the organism.
Acknowledgments
We thank the technical staff of the TB Immunology Group, MRC, The Gambia, for their help and support with the study.
Footnotes
Published ahead of print on 25 April 2007.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 3.Black, G. F., P. E. M. Fine, D. K. Warndorff, S. Floyd, R. E. Weir, J. M. Blackwell, L. Bliss, L. Sichali, L. Mwaungulu, S. Chaguluka, E. Jarman, B. Ngwira, and H. M. Dockrell. 2001. Relationship between IFN-gamma and skin test responsiveness to Mycobacterium tuberculosis PPD in healthy, non-BCG-vaccinated young adults in northern Malawi. Int. J. Tuberc. Lung Dis. 5:664-672. [PubMed] [Google Scholar]
- 4.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 5.Desem, N., and S. L. Jones. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardam, M. A., E. C. Keystone, R. Menzies, S. Manners, E. Skamene, R. Long, and D. C. Vinh. 2003. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect. Dis. 3:148-155. [DOI] [PubMed] [Google Scholar]
- 7.Hill, P. C., R. H. Brookes, A. Fox, D. Jackson-Sillah, M. D. Lugos, D. J. Jeffries, S. A. Donkor, R. A. Adegbola, and K. P. McAdam. 2006. Surprisingly high specificity of the PPD skin test for M. tuberculosis infection from recent exposure in The Gambia. PLoS ONE 1:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill, P. C., R. H. Brookes, A. Fox, K. Fielding, D. J. Jeffries, D. Jackson-Sillah, M. D. Lugos, P. K. Owiafe, S. A. Donkor, A. S. Hammond, J. K. Otu, T. Corrah, R. A. Adegbola, and K. P. McAdam. 2004. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin. Infect. Dis. 38:966-973. [DOI] [PubMed] [Google Scholar]
- 9.Hoft, D. F., R. M. Brown, and R. B. Belshe. 2000. Mucosal bacille calmette-Guerin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin. Infect. Dis. 30(Suppl. 3):S217-S222. [DOI] [PubMed] [Google Scholar]
- 10.Leyten, E. M. S., B. Mulder, C. Prins, K. Weldingh, P. Andersen, T. H. M. Ottenhoff, J. T. van Dissel, and S. M. Arend. 2006. Use of enzyme-linked immunospot assay with Mycobacterium tuberculosis-specific peptides for diagnosis of recent infection with M. tuberculosis after accidental laboratory exposure. J. Clin. Microbiol. 44:1197-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori, T., M. Sakatani, F. Yamagishi, T. Takashima, Y. Kawabe, K. Nagao, E. Shigeto, N. Harada, S. Mitarai, M. Okada, K. Suzuki, Y. Inoue, K. Tsuyuguchi, Y. Sasaki, G. H. Mazurek, and I. Tsuyuguchi. 2004. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59-64. [DOI] [PubMed] [Google Scholar]
- 12.Ravn, P., M. E. Munk, Å. B. Andersen, B. Lundgren, J. D. Lundgren, L. N. Nielsen, A. Kok-Jensen, P. Andersen, and K. Weldingh. 2005. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 12:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weir, R. E., P. J. Brennan, C. R. Butlin, and H. M. Dockrell. 1999. Use of a whole blood assay to evaluate in vitro T cell responses to new leprosy skin test antigens in leprosy patients and healthy subjects. Clin. Exp. Immunol. 116:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. 2005. TB country profile, Gambia. World Health Organization, Geneva, Switzerland. http://www.who.int/GlobalAtlas/predefinedReports/TB/PDF_Files/GM_2004_Brief.pdf. Accessed 28 January 2007.