Abstract
We demonstrate that trypanosomes compromised in flagellar function are rapidly cleared from infected mice. Analysis of the PFR2 bloodstream RNA interference mutant revealed that defective cell motility occurred prior to cytokinesis failure. This validation provides a paradigm for the flagellum as a target for future assays and interventions against this human pathogen.
The African trypanosome Trypanosoma brucei is a flagellated protozoan parasite and the etiological agent of n'gana in cattle and sleeping sickness in humans. Approximately 500,000 cases of sleeping sickness occur annually in sub-Saharan Africa, and the disease is invariably fatal unless treated. Unfortunately, new therapies are desperately needed to replace existing drugs, which are both toxic and associated with drug resistance.
The trypanosome flagellum contains, in addition to a “9+2” microtubule axoneme, a paraflagellar rod, which is a structure of restricted evolutionary distribution that we have shown to be important for motility (2, 3, 5, 8). Flagellum-mediated motility is used by T. brucei for its migration from the midgut to the salivary glands of its tsetse fly vector, where differentiation into the infectious metacyclic trypomastigote form occurs. However, the role(s) for flagellar motility within the circulatory system of the mammalian host is unclear: removal and internalization of antibodies bound to surface coat proteins (12, 14) could be dependent upon motility, as could migration across the blood-brain barrier or through the vasculature of various tissues. In our hands, in vitro-cultured procyclic and bloodstream form flagellar mutants produce dramatically different RNA interference (RNAi) phenotypes. Procyclic RNAi mutants exhibit defective motility or paralysis, with little, if any, detrimental effect on cell division (1, 3, 5). However, equivalent cultured bloodstream flagellar mutants exhibit a rapid and catastrophic failure of cytokinesis (5), a result subsequently echoed by other studies (4, 15). Our analysis of the bloodstream mutant phenotype suggests that cells fail in cytokinesis and twist to form grotesque cells (5). In other protozoa, such as Tetrahymena thermophila, cytokinetic mutants can be rescuedby physical manipulation (6). This raises a critical issue of whether the pressures, turbulence, and shear forces of blood flow in mammals may compensate and allow bloodstream trypanosome flagellar mutants to complete cytokinesis and develop normally within a natural host environment. In this context, it is known that turbulent fluid shear stress is powerful enough to affect endothelial cell turnover and release of mitotic cells (7, 20). Thus, an analysis of flagellar mutants in vivo is a necessary validation test in order to establish flagellar proteins as potential targets for chemotherapeutic development. We therefore sought to extend our studies and determine as a paradigm the effects of a flagellum-specific RNAi within an animal model.
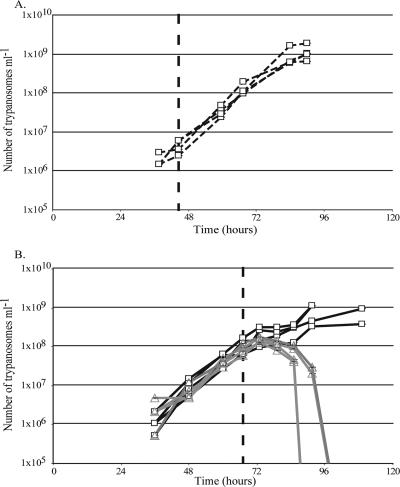
We initially examined the susceptibility of several mouse strains to the noninduced bloodstream PFR2 RNAi mutant (5) and selected the SV129/SvEv strain for subsequent experiments since with these mice no spontaneous clearance of parasitemia was observed. The original T. brucei 90-13 strain from which all our bloodstream RNAi mutants were derived was used in initial infections to ascertain the kinetics of the parasitemia (Fig. 1A). We then inoculated (intraperitoneal injection) eight 10-week-old female mice with 105 parasites of the PFR2 RNAi-inducible mutant. We followed the resulting parasitemia by determining cell density in blood obtained from tail bleeds. Seventy hours postinfection, when mice exhibited a noticeable parasitemia (∼5 × 107 cells ml−1), doxycycline was added to the drinking water (supplemented with 5%, wt/vol, sucrose) of four mice (200 μg ml−1). In mice not provided with doxycycline a humane end point (109 parasites ml−1) was reached within 96 h. In contrast, addition of doxycycline even at high parasitemia resulted in a rapid clearance of circulating parasites (Fig. 1B) with kinetics (within ∼16 h) that were comparable to those for the appearance of the previously reported in vitro phenotype (5). Cells counted displayed normal morphology, suggesting that once they began to display abnormalities they were removed rapidly from the circulation. No recrudescence was observed in the subsequent 7 days. Thus, effective flagellar function is essential for viability in vivo, just as it is in cultured PFR2 bloodstream RNAi parasites, and is absolutely necessary for cytokinesis. The early in vitro RNAi phenotype that we described previously (5) involved incomplete ingression of a cleavage furrow and failure of cytokinesis. The in vivo experiments described here indicate that blood flow and blood vessel turbulence and shear forces provide no assistance for cytokinesis completion in flagellar RNAi mutants.
FIG. 1.
The induced PFR2 mutant is not viable in mice. These growth curves chart parasitemia following intraperitoneal inoculation of 105 bloodstream forms of the parental T. brucei cell line 90-13 (A) or the PFR2 RNAi mutant (B). Dashed vertical lines indicate the times at which doxycycline was added to the drinking water of selected animals. (A) Application of doxycycline does not result in the clearance of parental bloodstream form trypanosomes (open squares). (B) Application of doxycycline to the PFR2 RNAi cell line (triangles) results in the rapid clearance of bloodstream form cells from the mouse, which is not seen in mice not given doxycycline (squares). The data indicate that clearance can be observed at 16 h after doxycycline application. Counts were made in duplicate using a Neubauer hemocytometer; standard errors were too small to be observable on the charts shown. The graph in panel B is representative of two experiments.
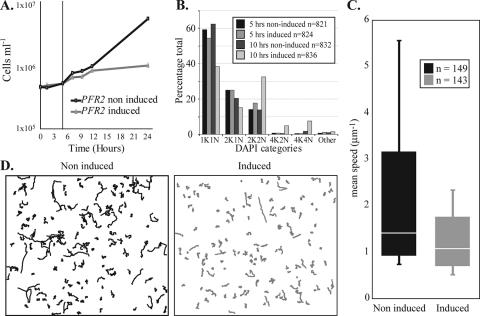
New flagellum construction precedes cytokinesis. Thus, we performed further experiments using cultured bloodstream parasites with the aim of formally demonstrating that a motility defect preceded a failure in cytokinesis. Several parameters complicate the interpretation of data from any experiment of this nature. (i) RNAi can be induced only within asynchronous populations, which means that mRNA degradation will initiate in parasites that are at different points in the cell division cycle. (ii) We have already shown that the presentation kinetics of cytokinesis defects differs between RNAi mutants (e.g., PFR2 verses TAX-1 [5]), which may reflect the relative severity of the motility defect and hence the ability of cells to complete cytokinesis early in the induction when only the new flagellum lacks the RNAi target. (iii) With current technology it is very difficult to ascertain whether subtle changes in the effective motility of bloodstream T. brucei occur during the cell division cycle of wild-type parasites. The key question to ask, while recognizing these caveats, is if cell population motility was compromised before cytokinesis failure. An extensive series of motility analyses were made using established methods (5, 8) on the PFR2 bloodstream RNAi mutant, which presents its cytokinesis defect within 10 h of RNAi induction. We determined that at 5 h postinduction (i.e., before the appearance of significant numbers of multinucleate or even postmitotic cells possessing two flagella) motility was significantly compromised in induced populations compared with the noninduced controls (Fig. 2). These data are persuasive that the cytokinesis failure of bloodstream T. brucei flagellar RNAi mutants occurs as a consequence of defective motility.
FIG. 2.
Defective motility in the induced PFR2 mutant. (A) Representative growth curve for the PFR2 bloodstream RNAi mutant in the presence and absence of doxycycline. Cell motility was determined at the 5-h time point (represented by the vertical line). Cell densities were determined using a Casy counter (Schärfe Systems, GmbH). (B) Five hours after the addition of doxycycline the accumulation of postmitotic (2K2N) cells or cells that had failed in cytokinesis but subsequently entered a new cell division cycle (4K2N, 4K4N, other) is not evident. Data from two experiments were pooled; mitochondrial (kinetoplast [K]) and nuclear (N) DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). (C) Five hours postinduction the motility of RNAi-induced cells is significantly reduced (t test from two experiments; P = 1.4 × 10−6 and 2.8 × 10−7) compared to that of cells grown in the absence of doxycycline. Cells were maintained at 37°C throughout the analysis, and the results shown represent the pooled data from both experiments. Boxes encompass mean speeds between the 5th and 95th percentiles; the white bars indicate the median mean speeds; the range of mean speeds is indicated by the horizontal bars. (D) Individual trajectories for cells used in the analysis shown in panel C also illustrate the defective motility of the PFR2 RNAi-induced mutant. Addition of doxycycline exerted no significant effect on the motility of the parental 90-13 cell line, from which the PFR2 bloodstream RNAi mutant was derived (data not shown).
Cytokinesis defects have been observed in several bloodstream mutants induced for RNAi against different cell cycle regulatory proteins (9-11, 13, 16, 18, 19). In the example of the T. brucei RACK1 homologue, the induced mutant is not viable in mice (16). Similarly, RNAi directed against the protective variant surface glycoprotein coat results in rapid arrest at a precytokinesis point, and induced cells are also not viable in mice (17). All the available data therefore suggest that in postmitotic cells a variety of delays in, or aberrations during, cytokinesis leave trypanosomes particularly vulnerable to clearance by the host immune system. This is likely to have consequences for how the timing of cell cycle events and checkpoints has evolved in this morphological form. Our results provide a contrast to those obtained with the ciliate Tetrahymena thermophila, where mechanical force can rescue a cytokinesis defect present in mutants that are unable to assemble cilia (6). Moreover, the efficient clearance reported here of the induced PFR2 bloodstream RNAi mutant from a relevant animal model completes a reverse genetic validation revealing that effective flagellar function is essential for cell morphogenesis and proliferation and that inhibition of flagellar function is likely to provide a novel credible avenue for future drug development against African sleeping sickness.
Acknowledgments
We are grateful to Gloria Rudenko for her advice regarding the animal experiments and to the staff of our animal facility for the care of the animals used in this study.
The use of animals was in accordance with institutional and United Kingdom Home Office guidelines.
This work was supported by grants from the Wellcome Trust, BBSRC, Royal Society, and the E. P. Abraham Trust. K.G. is a Wellcome Trust Principal Research Fellow. P.R.T. is a Wellcome Trust Research Career Development Fellow (grant 070579). M.L.G. is a Royal Society University Research Fellow.
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Bastin, P., K. Ellis, L. Kohl, and K. Gull. 2000. Flagellum ontogeny in trypanosomes studied via an inherited and regulated RNA interference system. J. Cell Sci. 113:3321-3328. [DOI] [PubMed] [Google Scholar]
- 2.Bastin, P., and K. Gull. 1999. Assembly and function of complex flagellar structures illustrated by the paraflagellar rod of trypanosomes. Protist 150:113-123. [DOI] [PubMed] [Google Scholar]
- 3.Bastin, P., T. Sherwin, and K. Gull. 1998. Paraflagellar rod is vital for trypanosome motility. Nature 391:548. [DOI] [PubMed] [Google Scholar]
- 4.Branche, C., L. Kohl, G. Toutirais, J. Buisson, J. Cosson, and P. Bastin. 2006. Conserved and specific functions of axoneme components in trypanosome motility. J. Cell Sci. 119:3443-3455. [DOI] [PubMed] [Google Scholar]
- 5.Broadhead, R., H. R. Dawe, H. Farr, S. Griffiths, S. R. Hart, N. Portman, M. K. Shaw, M. L. Ginger, S. J. Gaskell, P. G. McKean, and K. Gull. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440:224-227. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. M., N. A. Fine, G. Pandiyan, R. Thazhath, and J. Gaertig. 2003. Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene. Mol. Biol. Cell 14:3192-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, P. F., A. Remuzzi, E. J. Gordon, C. F. Dewey, Jr., and M. A. Gimbrone, Jr. 1986. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. USA 83:2114-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadelha, C., B. Wickstead, W. de Souza, K. Gull, and N. Cunha-e-Silva. 2005. Cryptic paraflagellar rod in endosymbiont-containing kinetoplastid protozoa. Eukaryot. Cell 4:516-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammarton, T. C., J. Clark, F. Douglas, M. Boshart, and J. C. Mottram. 2003. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J. Biol. Chem. 278:22877-22886. [DOI] [PubMed] [Google Scholar]
- 10.Hammarton, T. C., S. G. Lillico, S. C. Welburn, and J. C. Mottram. 2005. Trypanosoma brucei MOB1 is required for accurate and efficient cytokinesis but not for exit from mitosis. Mol. Microbiol. 56:104-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, Z., and C. C. Wang. 2006. Changing roles of aurora-B kinase in two life cycle stages of Trypanosoma brucei. Eukaryot. Cell 5:1026-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Beirne, C., C. M. Lowry, and H. P. Voorheis. 1998. Both IgM and IgG anti-VSG antibodies initiate a cycle of aggregation-disaggregation of bloodstream forms of Trypanosoma brucei without damage to the parasite. Mol. Biochem. Parasitol. 91:165-193. [DOI] [PubMed] [Google Scholar]
- 13.Oberholzer, M., G. Marti, M. Baresic, S. Kunz, A. Hemphill, and T. Seebeck. 2007. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J. 21:720-731. [DOI] [PubMed] [Google Scholar]
- 14.Pal, A., B. S. Hall, T. R. Jeffries, and M. C. Field. 2003. Rab5 and Rab11 mediate transferrin and anti-variant surface glycoprotein antibody recycling in Trypanosoma brucei. Biochem. J. 374:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralston, K. S., and K. L. Hill. 2006. Trypanin, a component of the flagellar Dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog. 2:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothberg, K. G., D. L. Burdette, J. Pfannstiel, N. Jetton, R. Singh, and L. Ruben. 2006. The RACK1 homologue from Trypanosoma brucei is required for the onset and progression of cytokinesis. J. Biol. Chem. 281:9781-9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheader, K., S. Vaughan, J. Minchin, K. Hughes, K. Gull, and G. Rudenko. 2005. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc. Natl. Acad. Sci. USA 102:8716-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu, X., and C. C. Wang. 2004. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. J. Biol. Chem. 279:20519-20528. [DOI] [PubMed] [Google Scholar]
- 19.Tu, X., and C. C. Wang. 2005. Pairwise knockdowns of cdc2-related kinases (CRKs) in Trypanosoma brucei identified the CRKs for G1/S and G2/M transitions and demonstrated distinctive cytokinetic regulations between two developmental stages of the organism. Eukaryot. Cell 4:755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechezak, A. R., R. F. Viggers, D. E. Coan, and L. R. Sauvage. 1994. Mitosis and cytokinesis in subconfluent endothelial cells exposed to increasing levels of shear stress. J. Cell Physiol. 159:83-91. [DOI] [PubMed] [Google Scholar]