Abstract
Targeting of membrane proteins into the lysosomal/vacuolar lumen for degradation requires their prior sorting into multivesicular bodies (MVB). The MVB sorting pathway depends on ESCRT-0, -I, -II, and -III protein complexes functioning on the endosomal membrane and on additional factors, such as Bro1/Alix and the ubiquitin ligase Rsp5/Nedd4. We used the split-ubiquitin two-hybrid assay to analyze the interaction partners of yeast Bro1 at its natural cellular location. We show that Bro1 interacts with ESCRT-I and -III components, including Vps23, the Saccharomyces cerevisiae homologue of human Tsg101. These interactions do not require the C-terminal proline-rich domain (PRD) of Bro1. Rather, this PRD interacts with the Doa4 deubiquitinating enzyme to recruit it to the endosome. This interaction is disrupted by a single amino acid substitution in the conserved ELC box motif in Doa4. The PRD of Bro1 also mediates an association with Rsp5, and this interaction appears to be conserved, as Alix, the human homologue of Bro1, coimmunoprecipitates with Nedd4 in yeast lysates. We further show that the Bro1 PRD domain is essential to MVB sorting of only cargo proteins whose sorting to the vacuolar lumen is dependent on their own ubiquitination and Doa4. The Bro1 region preceding the PRD, however, is required for MVB sorting of proteins irrespective of whether their targeting to the vacuole is dependent on their ubiquitination and Doa4. Our data indicate that Bro1 interacts with several ESCRT components and contributes via its PRD to associating ubiquitinating and deubiquitinating enzymes with the MVB sorting machinery.
The multivesicular body (MVB) pathway occurs at the limiting membranes of endosomes of all eukaryotic cells and consists of the formation of vesicles pinching outward from the cytoplasm into the lumen of these organelles (4, 21, 26, 61). Membrane proteins are specifically incorporated into these endosomal vesicles, and this sorting targets most of them for degradation upon fusion of MVBs with the vacuole/lysosome. Besides degradation of many membrane proteins, the MVB pathway also plays an important role in antigen presentation (45), and many retroviruses usurp the MVB machinery for budding at the limiting membrane of infected cells (11, 42). MVB sorting is dependent on the sequential functions of the conserved Vps27/Hse1 (Hrs/STAM, or ESCRT-0) and ESCRT-I, -II, and -III protein complexes, as well as on additional factors (4, 21, 26, 61). Human Alix (also called AIP1) was recently proposed to play a central role in the MVB pathway. Alix was suggested to participate in membrane deformation and/or vesicle formation, as it binds endophilin and the conical lysobisphosphatidic acid, which is abundant in endosomal vesicles (9, 37). It also interacts with components of the ESCRT-I (Tsg101) and -III (Chmp4) complexes and is therefore proposed to mediate an interaction between these two core protein complexes of the MVB sorting pathway (36, 67, 71). Furthermore, by binding to viral structural proteins, e.g., the human immunodeficiency virus Gag polyprotein, Alix enables recruitment of the MVB sorting machinery for virus release (36, 67, 71). Bro1, the Saccharomyces cerevisiae homologue of Alix (46, 65), is required for normal MVB sorting of both biosynthetic and endocytic cargo proteins (48, 51).
Ubiquitin is the main signal for sorting of most cargo proteins into the MVB pathway and is recognized by ubiquitin-binding domains exhibited by components of the ESCRT-0, -I, and -II complexes (19, 21, 61). In yeast, cargoes undergo Doa4-mediated deubiquitination just before their incorporation into endosomal vesicles (2, 14, 25). Ubiquitin has likewise been proposed to regulate some factors of the MVB sorting machinery. Ubiquitination of mammalian Hrs (the Hrs/STAM complex) and Tsg101 (the ESCRT-I complex) has been shown to impair their capacities to bind ubiquitin and hence to recognize ubiquitinated cargoes (3, 24, 35, 40). The exact mechanism is unclear, but intramolecular ubiquitin is proposed to mask the ubiquitin-binding sites of these factors (12, 20). In mammalian cells, Nedd4 and Itch/AIP4, both members of the Rsp5/Nedd4 family of HECT ubiquitin ligases, mediate ubiquitination of Hrs (24, 35). Furthermore, Rsp5, the sole yeast member of the family, is recruited to the endosome and is associated with the Vps27/Hse1 complex (8, 13, 27, 56, 72). Ubiquitin is also required for budding of many enveloped viruses (42). Furthermore, like Alix, the Rsp5/Nedd4 ubiquitin ligases bind viral structural proteins and are required for virus release (7, 30, 60, 75).
In this study, we further assessed the role of Bro1, the yeast homologue of mammalian Alix/AIP1, in MVB sorting. Previous work showed that Bro1 interacts with the Doa4 deubiquitinating enzyme (34) and is required for deubiquitination of the yeast Gap1 permease after its internalization (48). In systematic studies of protein interactions, Bro1 was found to interact with Snf7 (8, 16), and recruitment of Bro1 to the endosome was indeed shown to be dependent on Snf7 (31, 51). Bro1 differs from the classical Vps factors forming the core machinery of MVB sorting in that a deficiency of Bro1 leads to recycling of internalized Gap1 permease to the cell surface instead of its accumulation in the class E compartment (48), an enlarged late endosome typical of class E vps mutants. Alix and Bro1 have in common a conserved N-terminal domain (the “Bro1 domain”) that is also conserved in other proteins known to associate with endosomes, including the fungal Rim20/PalA proteins involved in activation of the transcription factors Rim101/PacC in the pH response pathway (53, 69, 73, 74). This domain, whose crystal structure has recently been solved (31), interacts with Snf7, thus mediating endosomal localization (31, 69, 74). Yeast Bro1 and human Alix differ from Rim20/PalA in that their C-terminal domains contain a proline-rich domain (PRD) whose function is still unclear.
We used the split-ubiquitin two-hybrid assay, as well as coimmunoprecipitation experiments, to further explore the interactions between Bro1 and protein components of the MVB machinery. We report for the first time that yeast Bro1 interacts with Vps23, a component of the ESCRT-I complex, an observation consistent with the previously reported interaction between the human orthologues of these proteins, namely, Alix and Tsg101 (36, 67, 71). We also confirm that Bro1 interacts with Snf7 (ESCRT-III). Whereas the C-terminal PRD of Bro1 is dispensable for these interactions, it is essential and sufficient for interaction with the Doa4 deubiquitinating enzyme. A Bro1 protein with its PRD truncated fails to recruit Doa4 to the endosome. The interaction between Bro1 and Doa4 and the localization of Doa4 to the endosome are also impaired by a single amino acid substitution in a Doa4 sequence (the ELC box) highly conserved in other deubiquitinating enzymes. Interestingly, the PRD of Bro1 also mediates association with the Rsp5 ubiquitin ligase. Finally, we show that the PRD domain of Bro1 is specifically required for MVB sorting of ubiquitinated cargoes that are dependent on Doa4 for correct delivery to the vacuolar lumen. Our results are consistent with a pivotal role of Bro1 in the association of ubiquitinating and deubiquitinating enzymes with the MVB sorting machinery.
MATERIALS AND METHODS
Strains, plasmids, and media.
The S. cerevisiae strains used in this study are presented in Table 1. In the npi3/bro1ΔPRD strain (27086c), Bro1 largely lacks the conserved C-terminal PRD due to excision of a guanosine base, which results in a frameshift at position 732 of the polypeptide and a preliminary stop. This Bro1ΔPRD variant still contains the PAPPLPPL sequence comprising residues 716 to 723. Gene deletions and excision of the KanMX4 cassette and transformation of the cells were performed as previously described (62). Cells were grown in minimal buffered medium (pH 6.1) (22) unless otherwise indicated. Glucose (3%) was used as the carbon source and proline (0.1%) as the nitrogen source if not otherwise indicated.
TABLE 1.
Yeast strains used in this study
| Straina | Genotype | Source or reference |
|---|---|---|
| 23344c | ura3 | Laboratory collection |
| 27092a | bro1Δ ura3 | 65 |
| 27086c | npi3/bro1ΔPRDura3 | 65 |
| ME029 | snf7Δ ura3 | 47 |
| ME027 | vps4Δ ura3 | 47 |
| EN079 | vps4Δ bro1Δ ura3 | This study |
| EN082 | snf7Δ bro1Δ gap1Δ ura3 | This study |
| EN035 | GAL-GFP-BRO1 ura3 | This study |
| EN040 | GAL1-GFP-BRO1ΔPRD ura3 | This study |
| THY.AP4 | lexA-lacZ-TRP1 lexA-HIS3 lexA-ADE2 ura3 leu2 | 49 |
| THY.AP5 | lexA-ADE2 leu2 trp1 his3 | 49 |
| EN155 | DOA4-13Myc | This study |
| EN156 | VPS23-13Myc ura3 | This study |
| OS20-3 | doa4Δ ura3 | This study |
| 36329a | doa4Δ vps4Δ gap1Δ ura3 | This study |
| 27040d | npi2/doa4W782Rura3 | 64 |
| 27061b | ura3 trp1 | Laboratory collection |
Except for those in boldface, strains are congenic with Σ1278b.
mbSUS.
The mating-based split-ubiquitin system (mbSUS) plasmids used in this study are presented in Table 2. The plasmids were prepared and the assay was performed as described previously (49). All primer sequences are available upon request. Cells were grown on YNB (Difco) plates containing 3% glucose as the carbon source and 0.1% urea as the nitrogen source and also on plates supplemented with 0.01, 0.05, 0.1, 0.25, or 0.5 mM methionine to attenuate expression of the Cub constructions from the MET25 promoter. On nonselective plates, adenine and histidine were added, each to a final concentration of 0.0025%.
TABLE 2.
Plasmids used in the mbSUS assay
| Plasmid | Gene insertion | Reference |
|---|---|---|
| pNubWT-2 | NubWt-3HA | 49 |
| pNXgate | NubG-3HA | 49 |
| pMetYCgate | Met25-Cub-PLV | 49 |
| pEN114 | NubG-3HA-BRO1 | This study |
| pEN133 | NubG-3HA-BRO1ΔPRD | This study |
| pCJ167 | NubG-3HA-PRD-BRO1a | This study |
| pEN120 | NubG-3HA-DOA4 | This study |
| pEN129 | NubG-3HA-DOA4W782R | This study |
| pEN118 | NubG-3HA-SNF7 | This study |
| pEN113 | NubG-3HA-RSP5 | This study |
| pEN141 | NubG-3HA-VPS27 | This study |
| pEN117 | NubG-3HA-VPS23 | This study |
| pEN144 | NubG-3HA-VPS36 | This study |
| pEN106 | Met25-BRO1-Cub-PLV | This study |
| pEN165 | Met25-BRO1ΔPRD-Cub-PLV | This study |
| pEN110 | Met25-DOA4-Cub-PLV | This study |
| pEN164 | Met25-DOA4W782R-Cub-PLV | This study |
| pEN108 | Met25-SNF7-Cub-PLV | This study |
| pEN105 | Met25-RSP5-Cub-PLV | This study |
| pEN157 | Met25-VPS27-Cub-PLV | This study |
| pEN107 | Met25-VPS23-Cub-PLV | This study |
| pEN152 | Met25-VPS36-Cub-PLV | This study |
Amino acids 702 to 844.
Immunoprecipitations and immunoblotting.
To prepare the hemagglutinin (HA)-Bro1 (pEN001) plasmid, BRO1 was cloned into the NotI site in the pYeF1 vector (2μ GAL1-HA URA3) (10). The HA-Bro1ΔPRD (pEN079) plasmid was created from the pEN001 plasmid by Quick site-directed mutagenesis (Stratagene). Both constructs were verified by sequencing them. To prepare the Doa4-13Myc (EN155)- or Vps23-13Myc (EN156)-expressing strains, a 13Myc-kanMX6 fragment, amplified by using the pFA6a-13Myc-kanMX6 plasmid as a template (32), was appended downstream from the DOA4 or VPS23 gene in wild-type (23344c) cells. Mouse Alix cDNA was a kind gift of R. Sadoul. Alix was first cloned into the NotI site of the pYeF1 vector to obtain HA-tagged Alix. An HA-Alix fragment with flanking ClaI and XhoI restriction sites was amplified, restricted, and ligated into the p424 vector to create the pEN187 plasmid (CEN/ARS Met25-HA-Alix TRP1) (44). The pKT10-Nedd4 plasmid was used to express mouse Nedd4 in yeast (a kind gift of S. Kumar). For anti-HA immunoprecipitations, cells (except those expressing HA-Alix) were cultured on medium containing 3% raffinose and 0.3% glucose and harvested on 3% galactose after 4 h of growth. Cells expressing HA-Alix, like the cells used in anti-c-myc immunoprecipitations, were cultured on medium containing 3% galactose and 0.3% glucose. The cells were washed once with 1 mM NaN3 and suspended in lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Nonidet P-40) supplemented with Complete EDTA-free protease inhibitor cocktail (Roche), 1 mM phenylmethylsulfonyl fluoride, and 10 mM N-ethylmaleimide. The cells were lysed by vortexing them with glass beads and incubated for 1 h on ice. This was followed by a 15-min centrifugation at 12,000 × g. Samples were cleared with Protein G-Sepharose 4 Fast Flow (Amersham Pharmacia) under gentle rotation for 1 h at 4°C. For anti-HA immunoprecipitations, samples were incubated overnight at 4°C with rat anti-HA affinity matrix (clone 3F10; Roche) under gentle rotation. For anti-c-myc immunoprecipitations, samples were incubated for 3 h with anti-c-myc antibodies (clone 9E10; Roche) under gentle rotation at 4°C, which was continued overnight after the addition of Protein G-Sepharose 4 Fast Flow. The matrix was washed four times with cold lysis buffer. Bound material was eluted with 50 mM glycine-HCl, pH 2.5, and neutralized with 1 M Tris, pH 9.0, followed by trichloroacetic acid precipitation and immunoblotting. Crude extracts were obtained as previously described (18). Rsp5/Nedd4 and ubiquitin immunoblotting were performed as described previously (47, 62-64). For Western blotting, mouse anti-HA (clone 12CA5; Roche) and anti-c-myc antibodies were used at 1:10,000 and 1:5,000 dilutions, respectively.
Fluorescence microscopy.
The pGO45 (green fluorescent protein [GFP]-carboxypeptidase S [CPS]) plasmid was a kind gift of S. D. Emr (50), and the Sna3-GFP and Ub-GFP-Phm5 plasmids were kind gifts of H. R. Pelham (55). For Bro1 localization, the GAL1-GFP fragment, amplified by using the pFA6a-kanMX6-PGAL1-GFP plasmid as a template (32), was placed upstream from the genomic copy of BRO1 or BRO1ΔPRD/NPI3 to create the EN035 and EN040 strains, respectively. The GFP-Bro1 generated in this way was also cloned into the XhoI-BamHI site of the p416 vector (44) to create the pEN009 plasmid (CEN/ARS GAL1-GFP-BRO1 URA3). The Doa4-GFP plasmid (pEN124) (47) was used to prepare the Doa4W782R-GFP plasmid (pEN127) by Quick site-directed mutagenesis (Stratagene). Both the pEN009 and pEN127 plasmids were verified by sequencing them. The localization of GFP-Bro1 or GFP-Bro1ΔPRD was determined after 2 h of synthesis and Doa4-GFP or Doa4W782R-GFP after 3 h of synthesis induced by adding galactose up to 3% in cells cultured in medium containing 3% raffinose and 0.3% glucose. To visualize vacuoles, cells were incubated at 30°C for 15 min with shaking in medium containing FM4-64 (40 μM; Molecular Probes, Eugene, OR), transferred to fresh medium, and chased for approximately 1 h. The cells were viewed with a Nikon Eclipse E600 microscope equipped with appropriate fluorescent-light filter sets. Images were captured with a Nikon DXM1200 digital color camera and processed using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA).
RESULTS
Bro1 interacts with itself and with components of the ESCRT-I and -III complexes.
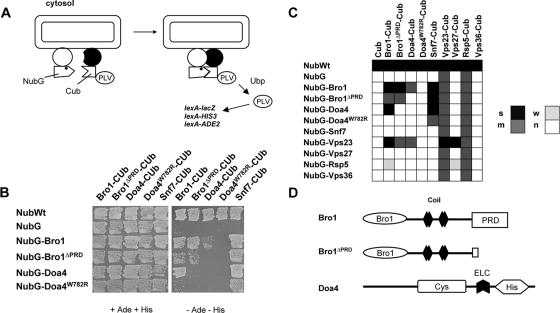
The results of previous genetic-epistasis studies indicate that Bro1 intervenes late in the MVB sorting pathway, downstream from the ESCRT-III complex component Snf7 and upstream from or in conjunction with the Doa4 deubiquitinating enzyme (48, 51). To assess further the role of Bro1 in MVB sorting, we sought to identify new Bro1-interacting proteins. For this, we opted for the mbSUS (49, 66). In this two-hybrid method, the proteins of interest are appended to either the N- or C-terminal half of ubiquitin (NubG or Cub) (Fig. 1A). If the two proteins interact, the two halves of ubiquitin are brought together to reconstitute a functional ubiquitin, which is recognized by ubiquitin hydrolases. As a consequence, the artificial transcription factor ProtA-LexA-Vp16 (PLV) appended to the Cub is released and enters the nucleus to initiate transcription of the ADE2, HIS3, and lacZ reporter genes (Fig. 1A). A major advantage of the mbSUS method compared to the classical two-hybrid assay is that protein-protein interactions can be analyzed at the sites of their natural locations in the cell, i.e., the proteins of interest do not need to enter the nucleus for activation of the reporter gene (66). This two-hybrid assay has recently been applied to 705 yeast integral membrane proteins (39). Here, we applied the mbSUS assay to Bro1 and other proteins of the MVB sorting machinery, all associated with the peripheral membrane of the late endosome. A strain coexpressing NubG-Bro1 and Bro1-Cub fusion proteins grew well on the selective medium, indicating that Bro1 interacts with itself (Fig. 1B). The Bro1-Bro1 interaction detected with mbSUS was in fact expected, since Alix, the mammalian homologue of Bro1, also interacts with itself (9, 36, 71). Bro1 was also found to interact in the mbSUS with the ESCRT-III complex component Snf7 (Fig. 1B), a result also obtained using various other experimental approaches (8, 16, 31).
FIG. 1.
Identification of Bro1-interacting proteins by means of the mbSUS. (A) Schematic presentation of the principle of the split-ubiquitin two-hybrid assay (mbSUS) for detecting interaction between two membrane-associated proteins. The N-terminal half of ubiquitin has Ile13 mutated to Gly (NubG), thus preventing nonspecific binding to the C-terminal half of ubiquitin (Cub). When the two proteins interact, the reconstituted split-ubiquitin heterodimer is recognized by a deubiquitinating enzyme (Ubp) that cleaves and liberates the in-frame-fused PLV transcription factor. PLV can then enter the nucleus and activate reporter genes containing upstream LexA binding sites. (B) Growth tests of diploid yeast clones coexpressing various NubG- and Cub-PLV fused proteins. Expression of soluble Nub with (NubG) or without (Nub-wt) the Ile13Gly substitution were used as controls. Cells were grown for 3 days on media containing urea as a sole nitrogen source and lacking adenine and histidine (− Ade − His) in order to detect interacting pairs (right) or on the same medium with adenine and histidine (+ Ade + His) used as controls. (C) Recapitulative matrix showing the data of the experiment shown in panel A plus additional data. The gray scale represents the extent of growth after 3 days on the adenine- and histidine-free medium. s, strong; m, medium; w, weak; n, no growth. (D) Domain structures of Bro1 and Doa4. Bro1 contains a conserved N-terminal “Bro1 domain”, two central coiled-coil domains, and a conserved C-terminal PRD, mostly absent from the Bro1ΔPRD/Npi3 variant. The ubiquitin hydrolase Doa4 has two catalytically important domains, histidine and cysteine boxes, and a widely conserved ELC box.
Human Alix was recently proposed to associate with the ESCRT-I and -III complexes through mediating interactions with the Tsg101 and Chmp4 proteins, homologous to yeast Vps23 and Snf7, respectively (36, 67, 71). Although the interaction between yeast Bro1 and Snf7 has been illustrated in several studies (8, 16, 31), Bro1 has thus far not been reported to interact with Vps23. As the MVB sorting core machinery appears to be conserved in yeast and mammalian cells (21, 26, 61), we speculated that Bro1 might interact with Vps23. Accordingly, this interaction was clearly detected in the mbSUS (Fig. 1C). To confirm this, we immunoprecipitated the HA-Bro1-tagged protein from cells that also expressed Vps23 fused with the 13Myc epitope. The Vps23-13Myc protein was clearly associated with anti-HA immunoprecipitates of HA-Bro1-expressing cells but not of control cells (Fig. 2). On immunoblots of the HA-Bro1 protein, we observed an additional minor band that comigrated with HA-Bro1ΔPRD, a mutant form of Bro1 in which most of the C-terminal PRD is deleted (see below). This faster-migrating form is likely a degradation product of HA-Bro1. In the mbSUS assay, Vps23 also interacted with the Vps27/Hrs complex component Vps27 (Fig. 1C), and this is consistent with data obtained by other methods (6, 8, 28). However, even though Vps23 interacts with both Bro1 and Vps27, no interaction was observed between the last two proteins (Fig. 1C). Similarly, no interaction between Vps27/Hrs and Bro1/Alix was detected in previous analyses of protein-protein interactions that have included these factors (8, 36, 71). The lack of mbSUS interaction between Bro1 and Vps27 also suggests that the Bro1-Bro1, Bro1-Snf7, Bro1-Vps23, and Vps27-Vps23 interactions detected in the mbSUS are specific.
FIG. 2.
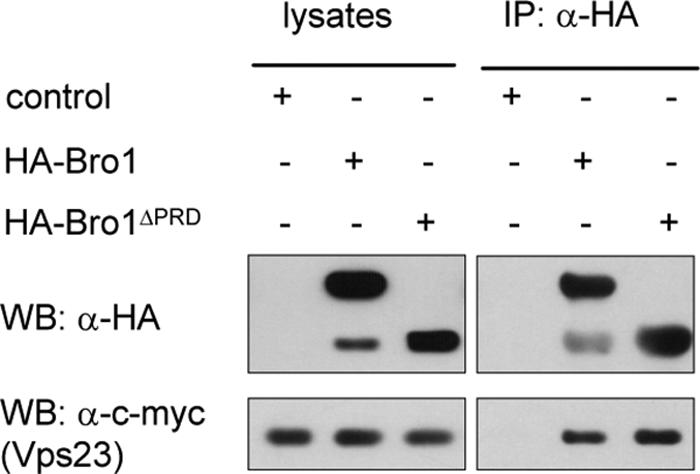
Bro1 immunoprecipitates with the ESCRT-I complex component Vps23. Anti-HA immunoprecipitates (IP: α-HA) from lysates of cells expressing Vps23-13Myc (EN156) transformed with the empty control (pYeF1), HA-Bro1 (pEN001), or HA-Bro1ΔPRD (pEN079) plasmid were immunoblotted with anti-HA (WB: α-HA) to detect Bro1 and Bro1ΔPRD or with anti-c-myc antibodies (WB: α-c-myc) to detect Vps23. Lysate samples were collected before immunoprecipitation.
Bro1 with its C-terminal PRD largely truncated remains competent for self-oligomerization, interaction with ESCRT components, and recruitment to the endosome.
We further studied Bro1 multimerization and interactions with the Snf7 and Vps23 proteins using a truncated form of Bro1, encoded by the npi3/bro1 mutant allele. This mutant was initially isolated in a screen for mutations stabilizing the Gap1 permease at the plasma membrane (17, 65). It codes for Bro1 lacking most of the conserved C-terminal PRD (Fig. 1D). This truncation does not affect self-oligomerization of the protein, as Bro1ΔPRD was found to interact, in the mbSUS assay, both with full-length Bro1 and with the Bro1ΔPRD mutant (Fig. 1B). Moreover, the Bro1ΔPRD form also proved competent for interaction with the ESCRT-III complex component Snf7 (Fig. 1B). This was somewhat expected, since two Bro1 homologues, yeast Rim20 and human Alix, have been shown to interact with Snf7 and Chmp4, respectively, via their conserved N-terminal Bro1 domains (31, 36, 74). The PRD of Bro1 is likewise not required for interaction with Vps23 (ESCRT-I), since the Bro1ΔPRD-Vps23 interaction was also observed in the mbSUS assay (Fig. 1C) and since Vps23-13Myc coimmunoprecipitated with HA-Bro1ΔPRD as efficiently as with the full-length HA-Bro1 (Fig. 2). Recruitment of Bro1 to the endosomal membrane was shown to depend on Snf7 (ESCRT-III) (31, 51). As Bro1ΔPRD interacts normally with Snf7 in the mbSUS assay (Fig. 1B), we expected the truncated protein to localize correctly to the endosome. In keeping with this expectation, cells expressing GFP-tagged versions of Bro1 or Bro1ΔPRD displayed similar hazy fluorescence throughout the cytoplasm, together with localization to structures next to the vacuole that correspond to endosomes (Fig. 3A). This pattern is similar to that recently reported for native Bro1 fused to GFP (31, 51). In conclusion, Bro1ΔPRD appears to localize normally to the endosome, where it interacts with Snf7 and Vps23. The PRD domain of Bro1 is thus not required for interaction between Bro1 and the ESCRT-I and ESCRT-III complexes.
FIG. 3.
The C-terminal PRD of Bro1 mediates recruitment of Doa4 to the endosome in wild-type cells. (A) Localization of GFP-Bro1 (EN035 cells), GFP-Bro1ΔPRD/Npi3 (EN040 cells) (left, wild type), and GFP-Bro1 (pEN009) in doa4Δ (OS20-3) and doa4Δ vps4Δ (36329a) cells. The cells were visualized after a 2-h induction by galactose. (B) Anti-HA immunoprecipitates (IP: α-HA) from lysates of cells expressing Doa4-13Myc (EN155) transformed with empty control (pYeF1), HA-Bro1 (pEN001), or HA-Bro1ΔPRD (pEN079) plasmid were immunoblotted with anti-HA (WB: α-HA) to detect Bro1 and Bro1ΔPRD or with anti-c-myc antibodies (WB: α-c-myc) to detect Doa4. Lysate samples were collected before immunoprecipitation. (C) Localization of Doa4-GFP (pEN124) in wild-type (23344c), bro1Δ (27092a), snf7Δ (ME029), bro1ΔPRD/npi3 (27086c), vps4Δ (ME027), vps4Δ bro1Δ (EN079), and snf7Δ bro1Δ (EN082) cells and of Doa4W782R-GFP (pEN127) in wild-type (23344c) and vps4Δ (ME027) cells. The cells were visualized after a 3-h induction by galactose.
The PRD of Bro1 participates in recruitment of the Doa4 ubiquitin hydrolase to the endosome.
We previously proposed that Bro1 might function in conjunction with the Doa4 deubiquitinating enzyme in the MVB sorting pathway (48). In a more recent study, Bro1 was shown to interact with Doa4 in coimmunoprecipitation experiments (34). A clear interaction between Bro1 and Doa4 was also observed in the mbSUS assay (Fig. 1B). Interestingly, in contrast to Snf7 and Vps23, Bro1 interaction with Doa4 was dependent on the PRD domain of Bro1, as growth of cells on selective medium was not observed when the Bro1ΔPRD mutant was used instead of full-length Bro1. This was true in both construct combinations, i.e., NubG-Bro1ΔPRD with Doa4-Cub and Bro1ΔPRD-Cub with NubG-Doa4 (Fig. 1B). Furthermore, the PRD domain of Bro1 (residues 702 to 844) is sufficient to interact with Doa4 but not with Bro1 or Bro1ΔPRD (see Fig. S1 in the supplemental material). For further demonstration, we performed anti-HA immunoprecipitations from lysates of cells expressing a Doa4-13Myc fusion protein, together with HA-Bro1 or HA-Bro1ΔPRD. Immunoblotting with anti-c-myc antibodies clearly revealed the association between Doa4 and full-length Bro1, but this association was strongly altered (although not completely) when the truncated Bro1ΔPRD form was used (Fig. 3B). As the truncated Bro1ΔPRD form still localizes to the endosome, and since localizations of GFP-Bro1 are comparable in doa4Δ and wild-type cells (Fig. 3A), as previously reported (34), recruitment of Bro1 to the endosome does not depend on its interaction with Doa4. Consistently, GFP-Bro1 accumulated in the endosome similarly in vps4Δ cells, defective in dissociation of class E Vps factors from the endosomal membrane (5), and in doa4Δ vps4Δ double-mutant cells (Fig. 3A and data not shown) (34, 51).
We next tested whether the reduced ability of Bro1ΔPRD to interact with Doa4 would affect recruitment of Doa4 to the endosome. In wild-type cells, Doa4-GFP localizes to the cytoplasm and also to endosomes, the latter appearing as punctate structures (Fig. 3C) (2, 34). In contrast, in the bro1ΔPRD mutant cells, no Doa4-GFP fluorescence was observed as distinct intracellular dots (Fig. 3C), indicating that the PRD of Bro1 is important for the partial endosomal localization of Doa4. This cytoplasmic localization of Doa4-GFP is also observed in bro1Δ cells and snf7Δ cells (Fig. 3C), as previously reported (34).
Loss of Doa4 function in the npi2/doa4 mutant cells isolated in our laboratory (17) is due to replacement of tryptophan 782 by an arginine (W782R) (64). This residue is located between the catalytically important Cys and His boxes in a region that we refer to as the ELC box because of invariant glutamate, leucine, and cysteine residues (64) (Fig. 1D). A comparison between the versions of Doa4 and Doa4W782R with GFP attached revealed that the mutant protein is not correctly recruited to the endosome, and as opposed to what occurred with Doa4-GFP, the fluorescence associated with Doa4W782R-GFP was solely cytoplasmic (Fig. 3C). This defect in endosomal localization of Doa4W782R likely explains why vacuolar sorting of the endocytic Gap1 (64) and biosynthetic CPS cargoes (Fig. 4) is impaired in the npi2/doa4W782R mutant, as in the doa4Δ strain. Interestingly, Doa4W782R proved unable to interact with Bro1 in the mbSUS (Fig. 1B). Hence, integrity of the ELC box of Doa4 is required for both interaction with Bro1 and normal endosomal localization. The simplest interpretation of these observations is that Bro1 contributes to endosomal recruitment of Doa4 via an interaction between its PRD and a region of Doa4 encompassing the ELC box.
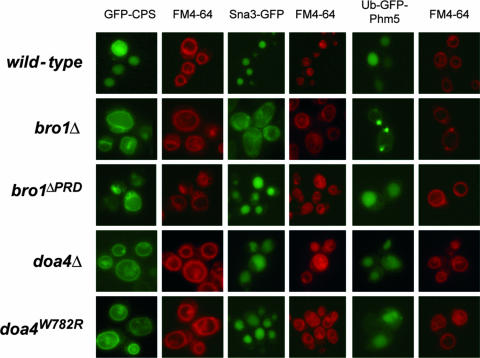
FIG. 4.
The proline-rich C terminus of Bro1 is required for MVB sorting of CPS, but not for that of Sna3 or Ub-Phm5. Localization of the biosynthetic cargoes GFP-CPS, Sna3-GFP, and Ub-GFP-Phm5 in wild-type (23344c), bro1Δ (27092a), bro1ΔPRD/npi3 (27086c), doa4Δ (OS20-3), and doa4W782R/npi2 (27040d) cells is shown. The cells were stained with the lipophilic dye FM4-64 in order to visualize the vacuolar membrane and class E compartments.
Doa4 is important for ubiquitin recycling, and this is marked by the reduced pool of monomeric ubiquitin in doa4Δ cells (2, 68). The fact that the endosome-associated Doa4 contributes to ubiquitin homeostasis is in line with our previous observation that the amount of monomeric ubiquitin is also reduced in the doa4W782R/npi2 mutant (64). The pool of monomeric ubiquitin is also clearly reduced in the bro1ΔPRD strain (data not shown), which is fully consistent with an important role for the PRD domain of Bro1 in endosomal recruitment of Doa4. A recent study by Amerik and collaborators similarly reported a reduction of the ubiquitin level in cells totally devoid of Bro1 (1). Interestingly, this study also showed that recruitment of Doa4 to the class E compartments of vps4 cells is not dependent on Bro1. An alternative recruitment mechanism of Doa4 to endosomes, which was further shown to depend on short N-terminal motifs in Doa4, thus seems to exist in vps4 (1). We also observed that Doa4-GFP localizes to strong fluorescent dots of vps4Δ cells independently of Bro1 (Fig. 3C). This localization is also observed in vps4Δ snf7Δ double-mutant cells, in which Bro1 is not recruited to endosomes (34). As localization of Doa4 to the class E compartments of vps4 cells is not dependent on Bro1, we expected that the Doa4W782R form, unable to interact with Bro1, would also localize to the typical intracellular dots of vps4 cells, and this was indeed the case (Fig. 3C). Why Bro1 is not required for localization of Doa4 to endosomes in a vps4 or snf7 mutant while it is in a wild type remains unclear. It may be indicative of an alternative recruitment mode of Doa4 when the late endosome is transformed into a class E compartment. This alternative recruitment mechanism might also be exacerbated by overproduction of the Doa4-GFP fusion protein. Most importantly, the cellular pool of ubiquitin is reduced in a bro1Δ mutant, as in a doa4Δ mutant (64) (our unpublished data), thus confirming the crucial role of Bro1 in Doa4 recruitment and function in a wild-type background (Fig. 3C).
Collectively, these data show that in wild-type cells, Bro1, through its C-terminal PRD, plays an essential role in Doa4 recruitment to the endosomal membrane, thereby enabling it to catalyze deubiquitination late in the MVB sorting pathway and to recycle ubiquitin. A region in Doa4 apparently important for interaction with Bro1 encompasses the so-called ELC box located between the Cys and His boxes. Interestingly, the ELC box is conserved in a group of deubiquitinating enzymes, including the mammalian UBPY recruited to the endosome via the Hrs/STAM complex (23, 59).
The conserved C-terminal PRD of Bro1 is required for MVB sorting of Doa4-dependent cargo proteins.
Previous studies have shown that Bro1 is essential to MVB sorting of endocytic and biosynthetic cargoes, such as the Gap1 permease and CPS, respectively (48, 51). Accordingly, in bro1Δ cells, GFP-CPS is missorted to the peripheral membrane of the vacuole (51) and internalized Gap1 permease is recycled to the cell surface instead of being targeted for vacuolar degradation (48). Both Gap1 and CPS correspond to cargo proteins whose MVB sorting is dependent on their own ubiquitination and Doa4 (25, 38, 62, 64). Furthermore, the essential role of Doa4 in MVB sorting of these cargoes is not solely due to the role of Doa4 in maintaining a normal level of ubiquitin in the cell (47). In contrast, at least two proteins were reported to be normally sorted in the MVB pathway independently of Doa4. One is the vacuolar lumen resident Sna3 protein (55). This protein is naturally ubiquitinated, but it can still be sorted to the vacuolar lumen when its ubiquitination is defective; this mode of sorting is dependent on its ability to interact with the Rsp5 ubiquitin ligase (38, 52). The other cargo is the polyphosphatase Phm5 fused at its N terminus to ubiquitin (55). We first examined the localizations of GFP-CPS, Sna3-GFP, and Ub-GFP-Phm5 in wild-type, doa4Δ, and doa4W782R cells. As expected, all three proteins localized to the vacuolar lumen in the wild type. In contrast, in doa4Δ and doa4W782R cells, while GFP-CPS was missorted to the vacuolar membrane, both Sna3-GFP and Ub-GFP-Phm5 cells were correctly sorted to the lumen of the vacuole, in keeping with previous findings (38, 52, 55). We next compared the localizations of the three proteins in bro1Δ and bro1ΔPRD mutant cells. In keeping with Bro1 being an integral component of the MVB pathway, the three proteins were missorted to the vacuolar membrane in bro1Δ cells. In the case of Ub-GFP-Phm5, highly fluorescent dots next to the vacuole, likely corresponding to class E compartments, were also observed. Similarly, the three proteins were missorted to the vacuolar membrane in vps23Δ and snf7Δ cells defective in components of the ESCRT-I and -III complexes, respectively (data not shown). In contrast, in bro1ΔPRD cells, GFP-CPS was missorted to the vacuolar membrane whereas Sna3-GFP and Ub-GFP-Phm5 were normally sorted to the lumen of the vacuole, just like in doa4Δ and doa4W782R cells (Fig. 4). Altogether, these results indicate that, although Bro1 contributes to MVB sorting of all cargo proteins thus far tested, the integrity of its PRD region is important only for proteins requiring Doa4 for correct MVB sorting.
Bro1/Alix interacts with the ubiquitin ligase Rsp5/Nedd4.
The ubiquitin ligase Rsp5 is recruited to the endosomal membrane, and members of the Rsp5/Nedd4 family have been shown to interact with components of the Vps27/Hse1 complex (8, 13, 35, 56, 72). Accordingly, Rsp5 interacted detectably with Vps27 in the mbSUS assay (Fig. 1C). The fact that both Rsp5 and Bro1 localize to the endosome prompted us to test whether they interact. Interestingly, Rsp5 was also found to interact detectably with full-length Bro1 in the mbSUS (Fig. 1C). It did not interact with Snf7, however, nor with Doa4. For further demonstration, wild-type cells were transformed with HA-Bro1 or HA-Bro1ΔPRD plasmids, and anti-HA precipitates were immunoblotted to detect Rsp5 (Fig. 5A). Rsp5 coimmunoprecipitated with HA-Bro1, confirming the result obtained in the mbSUS. It is noteworthy that on immunoblots Rsp5 was detected as a doublet and that, although the lower form was clearly predominant, Bro1 preferentially bound the upper form of Rsp5. Interestingly, binding of Rsp5 in the coimmunoprecipitation experiments was compromised when the truncated HA-Bro1ΔPRD form was used (Fig. 5A). In line with this, no interaction was observed between Rsp5 and Bro1ΔPRD in the mbSUS assay (Fig. 1C). These observations suggest that the C-terminal PRD of Bro1 contributes to the interaction with the ubiquitin ligase Rsp5, as is the case with the ubiquitin hydrolase Doa4.
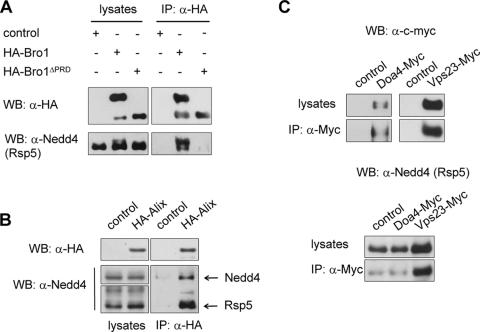
FIG. 5.
Bro1/Alix interacts with Rsp5/Nedd4. (A) Anti-HA immunoprecipitates (IP: α-HA) from lysates of wild-type (23344c) cells transformed with control (pYeF1), HA-Bro1 (pEN001), or HA-Bro1ΔPRD (pEN079) plasmid were immunoblotted with anti-HA (WB: α-HA) or anti-Nedd4 (WB: α-Nedd4) antibodies. Lysate samples were collected before immunoprecipitation. (B) Anti-HA immunoprecipitates from lysates of cells of the 27061b strain transformed with pKT10-Nedd4 plasmid alone (control) or together with HA-Alix plasmid (pEN187) were immunoblotted with anti-HA or anti-Nedd4 antibodies. Lysate samples were collected before immunoprecipitation. (C) Anti-c-myc immunoprecipitates (IP: α-MYC) from lysates of cells expressing Doa4-13Myc (EN155) or Vps23-13Myc (EN156) or from control cells (23344c) were immunoblotted with anti-c-myc antibodies (WB: α-c-myc) to detect Doa4 or Vps23 (top) or with anti-Nedd4 antibodies to detect Rsp5 (bottom).
Given the high conservation of protein complexes along the MVB sorting pathway, we wondered whether the mammalian homologues of yeast Rsp5 and Bro1 also interact. To answer this question, we expressed mouse Nedd4 and an HA-tagged form of mouse Alix in yeast. Neither Nedd4 nor the HA-Alix fusion protein could complement the Gap1 down-regulation defect of npi1/rsp5 or bro1Δ cells, respectively (our unpublished data), showing that these proteins are unable to fulfill the role of their yeast counterparts in MVB sorting. Anti-HA precipitates were immunoblotted with anti-Nedd4 and anti-HA antibodies (Fig. 5B). It clearly appeared that Nedd4 binds to HA-Alix, suggesting that like Vps23-Bro1 and Snf7-Bro1, the Rsp5-Bro1 interaction is also conserved in yeast and mammalian cells. Furthermore, yeast Rsp5 also coimmunoprecipitated with HA-Alix (Fig. 5B), suggesting that the protein regions of Rsp5/Nedd4 involved in interaction with Alix/Bro1 are conserved. These regions might encompass the central WW domains of Rsp5/Nedd4, known to mediate interactions with proline-rich sequences (29).
We then carried out anti-c-Myc immunoprecipitations from lysates of Vps23-13Myc- or Doa4-13Myc-expressing cells, followed by immunoblotting with anti-Nedd4 and anti-c-Myc antibodies (Fig. 5C). Consistent with the data of the split-ubiquitin two-hybrid assay (Fig. 1C), no interaction between Doa4 and Rsp5 was detected. Rsp5, however, clearly coimmunoprecipitated with the ESCRT-I complex component Vps23 (Fig. 5C). However, whether the apparent Vps23-Rsp5 association reflects a direct interaction between the two proteins remains uncertain: Rsp5 binding to Vps23 might indeed be indirect, with the gap being bridged by Bro1 and/or Vps27, as these factors interact with both Vps23 and Rsp5. We could not study the putative Rsp5-Vps23 interaction in the mbSUS because of spontaneous cleavage of the PLV reporter from both the Vps23-Cub and the Rsp5-Cub fusion proteins, resulting in positive readout with the empty control plasmid (NubG) as well (Fig. 1C). A faster growth above background, however, allowed detection of an apparent interaction of Vps23 with itself (Fig. 1C). This Vps23-Vps23 interaction is likely relevant, since Tsg101, the mammalian homologue of Vps23, has been shown to self-oligomerize (36, 71).
Thus, these data reveal that Bro1 interacts with the ubiquitin ligase Rsp5, a result consistent with the previously reported genetic interaction between the npi1/rsp5 and bro1 mutations (15, 65). Furthermore, the Bro1-Rsp5 interaction is apparently conserved in higher cells, since the corresponding mouse homologous proteins, Alix and Nedd4, coimmunoprecipitate in yeast. Importantly, the interaction of Bro1 with Rsp5 depends on its intact C-terminal PRD.
DISCUSSION
The Bro1/Alix protein contributes importantly to the MVB sorting pathway, but the precise mechanisms involved remain unknown (31, 36, 48, 51, 67, 71). Previous studies of yeast have shed light on a contribution of Bro1 in recruitment to the endosome of the Doa4 deubiquitinating enzyme (34) and in deubiquitination of cargo proteins, such as the Gap1 permease (48). Genetic-epistatis data also indicate that Bro1's contribution to the MVB pathway is dependent on normal functioning of various factors of the ESCRT-I, -II, and -III complexes (48). Furthermore, recruitment of Bro1 to the endosome is dependent on Snf7, a component of the ESCRT-III complex (31, 51).
In this study, we used for the first time the split-ubiquitin two-hybrid assay to explore interactions between proteins involved in membrane trafficking at the endosome level. In principle, this method allows protein-protein interactions to be analyzed at their natural locations in the cell (66). It was recently improved so that the assay is suitable for large-scale analyses (49). Using the improved method coupled with coimmunoprecipitation experiments, we have shown that the Bro1-Doa4 interaction involves the C-terminal PRD of Bro1 and a conserved ELC box motif in Doa4. The question of whether this interaction is direct or mediated by one or several bridging factors could not be addressed in this study. However, the PRD of Bro1 is clearly essential for endosomal association of Doa4, and conversely, a Doa4 form with a mutated ELC motif fails to be recruited to the endosome. Although the C-terminal PRD of Bro1 is conserved in mammalian Alix (41, 65, 70), no evidence has been presented so far for its eventual role in mediating an interaction with any deubiquitinating enzyme. Interestingly, the ELC box—located between the catalytically important Cys and His boxes—is conserved in a group of deubiquitinating enzymes, including the mammalian UBPY recruited to the early endosome via the Vps27-Hse1/Hrs-STAM complex (23, 59). As the present study was being reviewed, Richter et al. also reported that the C-terminal domain of Bro1 (residues 388 to 844) interacts with the catalytic domain of Doa4 (57). These authors also reported the interesting observation that Bro1 is required, not only for recruitment of Doa4 to endosomes, but also for intrinsic activation of the deubiquitinating enzyme, and that this activation involves an interaction between the C terminus of Bro1 and a YPFL sequence in the catalytic domain of Doa4 (57). The study also concludes that the interaction between the Bro1 C-terminal domain and the catalytic region of Doa4 is not needed for localization of Doa4 to endosomes, which contradicts our observations. Further work will thus be needed to solve this apparent discrepancy.
In yeast, ubiquitinated cargo proteins are supposed to undergo Doa4-mediated deubiquitination just before their incorporation into the endosomal vesicles. This enables recycling of ubiquitin, which otherwise would be targeted to the vacuolar lumen and degraded (2, 14). In doa4 mutant cells, the cellular pool of monomeric ubiquitin is thus reduced (64, 68), and this is also true for bro1ΔPRD cells. Although the reduced ubiquitin pool of doa4Δ cells has been proposed to be the main cause of the defective MVB sorting of many cargoes (14, 33, 55, 64), we recently demonstrated that the requirement for Doa4 in MVB sorting of cargoes like Gap1 and CPS is not solely due to its role in maintaining a normal cellular pool of ubiquitin (47). Hence, Doa4—and thus indirectly Bro1—plays a more direct role in MVB sorting, and although the mechanism involved remains unknown, it was shown to depend on Doa4's catalytic site. Intriguingly, the requirement for Doa4 in MVB sorting is not general, i.e., cargoes like Sna3-GFP and Ub-GFP-Phm5 correctly reach the vacuolar lumen in the doa4Δ mutant (55), and we showed that this is also true in the bro1ΔPRD and doa4W782R mutants, where the Bro1-Doa4 interaction is disrupted. Although a particular feature of these cargoes is that they do not need to be ubiquitinated to be sorted in the MVB pathway (38, 52, 55), this is in principle also true for the Ub-GFP-CPS chimera, which nevertheless requires Doa4 for MVB sorting (47). Further experiments are thus clearly required to determine the exact role of Doa4 in MVB sorting of specific cargoes.
Although Sna3-GFP and Ub-GFP-Phm5 are normally sorted in the MVB pathway in the bro1ΔPRD mutant, they are not in cells entirely devoid of Bro1. The same is true for other tested cargoes, like Gap1 and CPS. Thus, besides its role in endosomal recruitment of Doa4, Bro1 appears to perform another function that is essential to MVB sorting of cargo proteins, whether they require Doa4 or not. Furthermore, this other function is normally performed by Bro1 largely lacking its PRD region. Although further experiments will be required to precisely define this function, we have observed that Bro1 associates with Vps23 and that this interaction, unlike the one with Doa4, does not depend on the integrity of Bro1's PRD region. The same is true for the interaction between Bro1 and Snf7, an observation fully consistent with recent reports showing the essential role of the conserved N-terminal “Bro1 domain” in interaction with Snf7 and recruitment to the endosome (31, 74). The domains in Vps23 involved in interaction with Bro1, however, remain to be determined. The Bro1-Vps23 and/or Bro1-Snf7 interactions are thus likely important for the MVB pathway, and this is not related to the role of Bro1 in recruitment of Doa4 to the endosome. The above-mentioned interactions have also been observed in mammalian cells, i.e., Alix interacts with Tsg101 and Chmp4 (36, 67, 71). These results have been interpreted in terms of a role of Alix as a bridge between the ESCRT-I and -III complexes, with the resulting interaction being important for the proper functioning of the MVB pathway (36, 67, 71). According to the current view, cargo proteins are first recognized by the ESCRT-0 (Vps27/Hse1 or Hrs/STAM) complex and are passed on to ESCRT-I, then to ESCRT-II, and finally to the ESCRT-III complex. Efficient sorting of cargo proteins thus implies a close connection between the four protein complexes. The putative role of Bro1/Alix in bridging ESCRT-I and -III might therefore be important in order to strengthen an association of these protein complexes to allow efficient sorting of cargo proteins. However, two comments are worth making at this stage. First, current knowledge, including the genetic-epistasis data concerning the bro1Δ and class E vps mutations (48), the requirement for Snf7 in recruitment of Bro1 to the endosome (51), and the fact that Bro1 is not needed for formation of the ESCRT-III complex at the endosome (51), are not consistent with an essential role of Bro1 in assembly of the core ESCRT machinery. Rather, Bro1 seems to intervene only once the ESCRT complexes have been recruited to the endosome and when the cargo proteins are engaged in the MVB pathway. Secondly, although the Bro1/Alix interactions with Snf7/Chm4 and Vps23/Tsg101 could be indicative of a role for Bro1/Alix in bridging the ESCRT-I and -III complexes, another possibility is that these interactions are exclusive, i.e., Bro1 might interact with factors involved early and late in the MVB pathway without contributing to any linkage between the corresponding ESCRT complexes. Further work is clearly needed to confirm and clarify the functional implications of the depicted Bro1 interactions in MVB sorting.
Finally, our data have also revealed a possible interaction between Bro1 and Rsp5 that is also dependent on the PRD region of Bro1, yet we could not detect any interaction between Rsp5 and Doa4, possibly suggesting that the Bro1-Doa4 and Bro1-Rsp5 associations are exclusive. Whether the detected Bro1-Rsp5 association is direct or indirect (i.e., whether other proteins serve as a bridge) remains to be investigated. We have also shown, however, that mouse Alix and Nedd4 coimmunoprecipitate in yeast lysates, suggesting that the Bro1-Rsp5 association could be conserved. Interestingly, our data are also consistent with preferential binding of Bro1 to a modified version of Rsp5. The functional implications of the apparent Bro1/Alix-Rsp5/Nedd4 association will require further examination. Previous studies have clearly shown that Rsp5 plays a central role in MVB sorting of biosynthetic cargo proteins by mediating their ubiquitination (13, 27, 43). Furthermore, in the case of CPS, this modification occurs before the cargo reaches the late endosome (i.e., before Pep12) (25). In the case of endocytic cargoes, Rsp5-dependent ubiquitination has been shown to be required for early steps of endocytosis, and it is generally assumed that this modification is also required for subsequent MVB sorting of the protein at the late-endosome level (58). Rsp5 was recently found to associate with Hse1, a protein component of the ESCRT-0 complex (56), and disruption of this interaction impaired MVB sorting of CPS, but not that of CPS fused in frame to ubiquitin. The Rsp5-dependent ubiquitination of CPS at an early step of the MVB pathway thus appears crucial for correct ubiquitination of the protein and for subsequent delivery to the vacuolar lumen. Interestingly, the data from our mbSUS assay indicate an interaction between Rsp5 and Vps27, the other protein component of the ESCRT-0 complex. This is reminiscent of the interaction depicted between the mammalian Rsp5-related Itch ligase and the Vps27-related Hrs protein (35). The question of whether Rsp5 also intervenes downstream from the Vps27/Hse1 complex in the MVB sorting process itself is not readily addressable with normal cargo proteins, since they are not correctly ubiquitinated in rsp5 mutants and are thus not correctly engaged in the MVB pathway. As indicated above, Rsp5 is at least dispensable for MVB sorting of cargoes fused in frame to ubiquitin, but this situation might not be representative of the one involving naturally ubiquitinated proteins. The latter proteins might indeed be faced with the action of deubiquitinating enzymes, and thus, their progression along the MVB pathway might well require several rounds of Rsp5-dependent reubiquitination (56). Hence, our observation that Bro1 binds to Rsp5 via its PRD domain suggests a possible additional role of the ubiquitin ligase at the ESCRT-I and -III levels, at least when ubiquitin is potentially removable by deubiquitinating enzymes. In fact, the reduced ubiquitination of Gap1 observed in extracts of bro1ΔPRD mutant cells (64) and the fact that the permease is nevertheless internalized in a manner dependent on its own ubiquitination but inefficiently sorted into the MVB pathway, resulting in its recycling to the cell surface (48), are perhaps the consequences of an inability to maintain Gap1 in a sufficiently ubiquitinated state during its transit through the MVB pathway.
Studies of mammalian cells have shown that Hrs and Eps15 factors are subject to monoubiquitination and that Nedd4 and AIP4/Itch (orthologues of Rsp5) account for these modifications (24, 35, 40, 54). Furthermore, this monoubiquitination apparently inhibits the function of Hrs in the MVB pathway (20, 24), and the same is apparently true for the endocytic proteins Sts1, Sts2, and Eps15 (20). The fact that the Nedd4-dependent monoubiquitination of components of the MVB machinery has a negative, rather than a positive, influence on lysosomal/vacuolar sorting of cargoes may thus account for the apparent inability of the rsp5 mutations to negatively interfere with MVB sorting of cargoes fused in frame to ubiquitin. In other words, what might be expected is, instead, more efficient sorting of these cargoes in rsp5 mutant strains. Hence, the Bro1/Alix protein, through its ability to interact simultaneously with factors of the MVB sorting machinery (a function not dependent on its intact PRD region) and with ubiquitinating and deubiquitinating enzymes (via its PRD), may not only influence the ubiquitination of cargoes, but also promote successive rounds of ubiquitination and deubiquitination of specific Vps factors along the MVB pathway. For instance, Doa4-dependent deubiquitination of a Vps factor (i.e., activation) might be needed for binding to the ubiquitinated cargo, and subsequent Rsp5-dependent reubiquitination (i.e., inhibition) might be required for dissociation of the cargo and translocation to the next Vps factor. This might ensure efficient, coordinated sorting of the cargo protein through the successive steps of the MVB pathway. This model, however, suffers from a lack of evidence that any yeast Vps factor is regulated by monoubiquitination, i.e., the action of ubiquitin ligases and peptidases might be restricted to cargoes. Further experimentation is required to resolve these issues and to further investigate a putative role of Bro1 in coordinating the functions of Rsp5 and Doa4 at ESCRT-I and -III levels of the MVB pathway.
Supplementary Material
Acknowledgments
We are grateful to Sandra Lecomte and Catherine Jauniaux for excellent technical assistance. We also thank Scott Emr, Hugh Pelham, Stow Kumar, and Rémy Sadoul for providing plasmids and cDNA clones. We thank Anne-Marie Marini for discussions and comments on the manuscript.
E.N. was the recipient of a predoctoral fellowship from the Université Libre de Bruxelles and of a grant from the Finnish Cultural Foundation. This work was supported by grant FRSM 3.4.605.05.F from the National Funds for Scientific Research, Belgium; the Belgian Forton funds; and grant no. QLG2-CT-2001-01297 from the EU (Associoport Project, FPV).
Footnotes
Published ahead of print on 18 May 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Amerik, A., N. Sindhi, and M. Hochstrasser. 2006. A conserved late endosome-targeting signal required for Doa4 deubiquitylating enzyme function. J. Cell Biol. 175:825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amerik, A. Y., J. Nowak, S. Swaminathan, and M. Hochstrasser. 2000. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell 11:3365-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amit, I., L. Yakir, M. Katz, Y. Zwang, M. D. Marmor, A. Citri, K. Shtiegman, I. Alroy, S. Tuvia, Y. Reiss, E. Roubini, M. Cohen, R. Wides, E. Bacharach, U. Schubert, and Y. Yarden. 2004. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 18:1737-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babst, M. 2005. A protein's final ESCRT. Traffic 6:2-9. [DOI] [PubMed] [Google Scholar]
- 5.Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. [DOI] [PubMed] [Google Scholar]
- 6.Bilodeau, P. S., S. C. Winistorfer, W. R. Kearney, A. D. Robertson, and R. C. Piper. 2003. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 163:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blot, V., F. Perugi, B. Gay, M. C. Prevost, L. Briant, F. Tangy, H. Abriel, O. Staub, M. C. Dokhelar, and C. Pique. 2004. Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking towards the multivesicular body pathway prior to virus budding. J. Cell Sci. 117:2357-2367. [DOI] [PubMed] [Google Scholar]
- 8.Bowers, K., J. Lottridge, S. B. Helliwell, L. M. Goldthwaite, J. P. Luzio, and T. H. Stevens. 2004. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic 5:194-210. [DOI] [PubMed] [Google Scholar]
- 9.Chatellard-Causse, C., B. Blot, N. Cristina, S. Torch, M. Missotten, and R. Sadoul. 2002. Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J. Biol. Chem. 277:29108-29115. [DOI] [PubMed] [Google Scholar]
- 10.Cullin, C., and L. Minvielle-Sebastia. 1994. Multipurpose vectors designed for the fast generation of N- or C-terminal epitope-tagged proteins. Yeast 10:105-112. [DOI] [PubMed] [Google Scholar]
- 11.Demirov, D. G., and E. O. Freed. 2004. Retrovirus budding. Virus Res. 106:87-102. [DOI] [PubMed] [Google Scholar]
- 12.Di Fiore, P. P., S. Polo, and K. Hofmann. 2003. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat. Rev. Mol. Cell Biol. 4:491-497. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, R., D. A. Klos, A. S. Adler, and L. Hicke. 2004. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J. Cell Biol. 165:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupre, S., and R. Haguenauer-Tsapis. 2001. Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol. Cell. Biol. 21:4482-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsberg, H., M. Hammar, C. Andreasson, A. Moliner, and P. O. Ljungdahl. 2001. Suppressors of ssy1 and ptr3 null mutations define novel amino acid sensor-independent genes in Saccharomyces cerevisiae. Genetics 158:973-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 17.Grenson, M. 1983. Study of the positive control of the general amino-acid permease and other ammonia-sensitive uptake systems by the product of the NPR1 gene in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 133:141-144. [DOI] [PubMed] [Google Scholar]
- 18.Hein, C., J. Y. Springael, C. Volland, R. Haguenauer-Tsapis, and B. Andre. 1995. NPl1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol. Microbiol. 18:77-87. [DOI] [PubMed] [Google Scholar]
- 19.Hicke, L., H. L. Schubert, and C. P. Hill. 2005. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 6:610-621. [DOI] [PubMed] [Google Scholar]
- 20.Hoeller, D., N. Crosetto, B. Blagoev, C. Raiborg, R. Tikkanen, S. Wagner, K. Kowanetz, R. Breitling, M. Mann, H. Stenmark, and I. Dikic. 2006. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 8:163-169. [DOI] [PubMed] [Google Scholar]
- 21.Hurley, J. H., and S. D. Emr. 2006. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 35:277-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs, P., J. C. Jauniaux, and M. Grenson. 1980. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J. Mol. Biol. 139:691-704. [DOI] [PubMed] [Google Scholar]
- 23.Kato, M., K. Miyazawa, and N. Kitamura. 2000. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J. Biol. Chem. 275:37481-37487. [DOI] [PubMed] [Google Scholar]
- 24.Katz, M., K. Shtiegman, P. Tal-Or, L. Yakir, Y. Mosesson, D. Harari, Y. Machluf, H. Asao, T. Jovin, K. Sugamura, and Y. Yarden. 2002. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic 3:740-751. [DOI] [PubMed] [Google Scholar]
- 25.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 26.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3:893-905. [DOI] [PubMed] [Google Scholar]
- 27.Katzmann, D. J., S. Sarkar, T. Chu, A. Audhya, and S. D. Emr. 2004. Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol. Biol. Cell 15:468-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katzmann, D. J., C. J. Stefan, M. Babst, and S. D. Emr. 2003. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 162:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay, B. K., M. P. Williamson, and M. Sudol. 2000. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14:231-241. [PubMed] [Google Scholar]
- 30.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, J., S. Sitaraman, A. Hierro, B. M. Beach, G. Odorizzi, and J. H. Hurley. 2005. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 33.Losko, S., F. Kopp, A. Kranz, and R. Kolling. 2001. Uptake of the ATP-binding cassette (ABC) transporter Ste6 into the yeast vacuole is blocked in the doa4 mutant. Mol. Biol. Cell 12:1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luhtala, N., and G. Odorizzi. 2004. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J. Cell Biol. 166:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchese, A., C. Raiborg, F. Santini, J. H. Keen, H. Stenmark, and J. L. Benovic. 2003. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev. Cell 5:709-722. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Serrano, J., A. Yarovoy, D. Perez-Caballero, and P. D. Bieniasz. 2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 100:12414-12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo, H., J. Chevallier, N. Mayran, B. I. Le, C. Ferguson, J. Faure, N. S. Blanc, S. Matile, J. Dubochet, R. Sadoul, R. G. Parton, F. Vilbois, and J. Gruenberg. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531-534. [DOI] [PubMed] [Google Scholar]
- 38.McNatt, M. W., I. McKittrick, M. West, and G. Odorizzi. 2006. Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol. Biol. Cell 18:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. P., R. S. Lo, A. Ben-Hur, C. Desmarais, I. Stagljar, W. S. Noble, and S. Fields. 2005. Large-scale identification of yeast integral membrane protein interactions. Proc. Natl. Acad. Sci. USA. 102:12123-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, S. L., E. Malotky, and J. P. O'Bryan. 2004. Analysis of the role of ubiquitin-interacting motifs in ubiquitin binding and ubiquitylation. J. Biol. Chem. 279:33528-33537. [DOI] [PubMed] [Google Scholar]
- 41.Missotten, M., A. Nichols, K. Rieger, and R. Sadoul. 1999. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 6:124-129. [DOI] [PubMed] [Google Scholar]
- 42.Morita, E., and W. I. Sundquist. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20:395-425. [DOI] [PubMed] [Google Scholar]
- 43.Morvan, J., M. Froissard, R. Haguenauer-Tsapis, and D. Urban-Grimal. 2004. The ubiquitin ligase Rsp5p is required for modification and sorting of membrane proteins into multivesicular bodies. Traffic 5:383-392. [DOI] [PubMed] [Google Scholar]
- 44.Mumberg, D., R. Muller, and M. Funk. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22:5767-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murk, J. L., W. Stoorvogel, M. J. Kleijmeer, and H. J. Geuze. 2002. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin. Cell Dev. Biol. 13:303-311. [DOI] [PubMed] [Google Scholar]
- 46.Nickas, M. E., and M. P. Yaffe. 1996. BRO1, a novel gene that interacts with components of the Pkc1p-mitogen-activated protein kinase pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikko, E., and B. Andre. 2007. Evidence for a direct role of the Doa4 deubiquitinating enzyme in protein sorting into the MVB pathway. Traffic 8:566-581. [DOI] [PubMed] [Google Scholar]
- 48.Nikko, E., A. M. Marini, and B. Andre. 2003. Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J. Biol. Chem. 278:50732-50743. [DOI] [PubMed] [Google Scholar]
- 49.Obrdlik, P., M. El-Bakkoury, T. Hamacher, C. Cappellaro, C. Vilarino, C. Fleischer, H. Ellerbrok, R. Kamuzinzi, V. Ledent, D. Blaudez, D. Sanders, J. L. Revuelta, E. Boles, B. Andre, and W. B. Frommer. 2004. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101:12242-12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odorizzi, G., M. Babst, and S. D. Emr. 1998. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95:847-858. [DOI] [PubMed] [Google Scholar]
- 51.Odorizzi, G., D. J. Katzmann, M. Babst, A. Audhya, and S. D. Emr. 2003. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci. 116:1893-1903. [DOI] [PubMed] [Google Scholar]
- 52.Oestreich, A. J., M. Aboian, J. Lee, I. Azmi, J. Payne, R. Issaka, B. A. Davies, and D. J. Katzmann. 2006. Characterization of multiple multivesicular body sorting determinants within Sna3: a role for the ubiquitin ligase Rsp5. Mol. Biol. Cell 18:707-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penalva, M. A. and H. N. Arst, Jr. 2004. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58:425-451. [DOI] [PubMed] [Google Scholar]
- 54.Polo, S., S. Sigismund, M. Faretta, M. Guidi, M. R. Capua, G. Bossi, H. Chen, C. P. De, and P. P. Di Fiore. 2002. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416:451-455. [DOI] [PubMed] [Google Scholar]
- 55.Reggiori, F., and H. R. Pelham. 2001. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 20:5176-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren, J., Y. Kee, J. M. Huibregtse, and R. C. Piper. 2006. Hse1, a component of the yeast Hrs-STAM ubiquitin sorting complex, associates with ubiquitin peptidases and a ligase to control sorting efficiency into multivesicular bodies. Mol. Biol. Cell 18:324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter, C., M. West, and G. Odorizzi. 2007. Dual mechanisms specify Doa4-mediated deubiquitination at multivesicular bodies. EMBO J 16:2454-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1-17. [DOI] [PubMed] [Google Scholar]
- 59.Row, P. E., I. A. Prior, J. McCullough, M. J. Clague, and S. Urbe. 2006. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J. Biol. Chem. 281:12618-12624. [DOI] [PubMed] [Google Scholar]
- 60.Segura-Morales, C., C. Pescia, C. Chatellard-Causse, R. Sadoul, E. Bertrand, and E. Basyuk. 2005. Tsg101 and Alix interact with murine leukemia virus Gag and cooperate with Nedd4 ubiquitin ligases during budding. J. Biol. Chem. 280:27004-27012. [DOI] [PubMed] [Google Scholar]
- 61.Slagsvold, T., K. Pattni, L. Malerod, and H. Stenmark. 2006. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 16:317-326. [DOI] [PubMed] [Google Scholar]
- 62.Soetens, O., J. O. De Craene, and B. Andre. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949-43957. [DOI] [PubMed] [Google Scholar]
- 63.Springael, J. Y., and B. Andre. 1998. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol. Biol. Cell 9:1253-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Springael, J. Y., J. M. Galan, R. Haguenauer-Tsapis, and B. Andre. 1999. NH4+-induced down-regulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. J. Cell Sci. 112:1375-1383. [DOI] [PubMed] [Google Scholar]
- 65.Springael, J. Y., E. Nikko, B. Andre, and A. M. Marini. 2002. Yeast Npi3/Bro1 is involved in ubiquitin-dependent control of permease trafficking. FEBS Lett. 517:103-109. [DOI] [PubMed] [Google Scholar]
- 66.Stagljar, I., C. Korostensky, N. Johnsson, and S. te Heesen. 1998. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA 95:5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 68.Swaminathan, S., A. Y. Amerik, and M. Hochstrasser. 1999. The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol. Biol. Cell 10:2583-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincent, O., L. Rainbow, J. Tilburn, J. H. Arst, Jr., and M. A. Penalva. 2003. YPXL/I is a protein interaction motif recognized by Aspergillus PalA and its human homologue, AIP1/Alix. Mol. Cell. Biol. 23:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vito, P., L. Pellegrini, C. Guiet, and L. D'Adamio. 1999. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J. Biol. Chem. 274:1533-1540. [DOI] [PubMed] [Google Scholar]
- 71.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 72.Wang, G., J. M. McCaffery, B. Wendland, S. Dupre, R. Haguenauer-Tsapis, and J. M. Huibregtse. 2001. Localization of the Rsp5p ubiquitin-protein ligase at multiple sites within the endocytic pathway. Mol. Cell. Biol. 21:3564-3575.11313482 [Google Scholar]
- 73.Xu, W., and A. P. Mitchell. 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu, W., F. J. Smith, Jr., R. Subaran, and A. P. Mitchell. 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell 15:5528-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yashiroda, H., T. Oguchi, Y. Yasuda, E. Toh, and Y. Kikuchi. 1996. Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.