Malaria arises from the infection of red blood cells by protozoan parasites of the genus Plasmodium that are transmitted by anopheline mosquitoes. More than 400 species of Anopheles mosquitoes are known, of which about 40 species are characterized as important disease vectors for human malaria transmission (29). It is estimated that 300 to 500 million cases of malaria and over 1 million deaths from the disease occur each year (49).
The Plasmodium parasite must complete its development in the mosquito before it can be transmitted to the vertebrate host and cause malaria. Each stage of parasite development in the mosquito offers potential targets to interfere with malaria transmission. Development of the malaria parasite in the mosquito begins when the gametocyte forms of the parasite are picked up by the mosquito in the blood meal from an infected human and quickly develop into extracellular gametes in the mosquito midgut. After fertilization, round zygotes form and transform into banana-shaped ookinetes. The ookinetes are motile and must exit the gut by crossing the peritrophic membrane and midgut epithelium. On the basal side of the epithelium, surviving ookinetes lodge against the basal lamina and transform into spherical oocysts. In the oocyst, the parasite develops into several thousand sporozoites, which then exit the oocyst and are carried by the hemolymph to the mosquito's salivary glands to infect another host (22).
There is ongoing research to develop antiparasite vaccines against each stage of the complicated life cycle of Plasmodium (17, 24). Liver-stage vaccines are intended to reduce infection rates, and asexual-blood-stage vaccines will reduce disease severity and the risk of death during infection. Transmission-blocking vaccines would prevent the spread of disease by targeting antigens expressed in the mosquito stage on the surfaces of the gametocyte, gamete, zygote, and ookinete forms of the parasite (6, 61). These vaccines induce antibodies in the human host that inhibit parasite development in the mosquito midgut and thereby block parasite transmission to another person.
This article reviews the biology and structural knowledge of the Plasmodium P25 and P28 proteins and their contributions to transmission-blocking vaccine development.
TARGET ANTIGENS AND TRANSMISSION-BLOCKING IMMUNITY
Target antigens.
The target antigens for transmission-blocking vaccines are divided into two groups, namely, prefertilization and postfertilization parasite surface proteins. Prefertilization antigens are proteins expressed on the surfaces of male and female gametocytes and gametes, for example, the P48/45 and P230 proteins (41). These antigens have a unique repeated six-cysteine disulfide-bonded structure (54). Monoclonal antibodies (MAbs) against either of these proteins can block the infectivity of the gametes to the mosquito (7, 40, 63), and their blocking activities are enhanced by complement (39).
Postfertilization antigens are proteins expressed on the surfaces of zygotes and the maturing ookinete form of the parasite (32, 36, 63). The P25 and P28 proteins have been cloned from several Plasmodium species (14, 15, 27, 28, 36, 53, 58-60). Low-level expression of P25 is detectable in early gametogenesis, and the expression level dramatically increases after fertilization (63). Anti-P25 antibodies bind specifically to the surfaces of parasites ranging from zygotes to ookinetes. P28 is expressed slightly later in development, as anti-P28 antibodies stain mainly the retort and mature forms of ookinetes (21). P25 and P28 are distributed evenly and abundantly over the entire ookinete surface, as seen by immunofluorescent antibody staining (66) and immunogold electron microscopy (15, 48). Plasmodium P25 and P28 proteins are the targets of effective transmission-blocking antibodies that inhibit oocyst development in the mosquito gut. When a mixture of infected blood and antisera against Plasmodium P25 and P28 proteins is fed to mosquitoes through a laboratory membrane feeder, a significant reduction in oocyst numbers is observed (9, 21).
In addition to the P25 and P28 proteins, other ookinete proteins that are important in ookinete-to-oocyst development have been identified. These proteins are (i) parasite-produced chitinase, a potential target of malaria transmission-blocking interventions (43), as chitinase-disrupted Plasmodium falciparum parasites are significantly impaired in the ability to form oocysts in the mosquito gut (57); (ii) circumsporozoite protein and thrombospondin-related adhesive protein (CTRP), present in the ookinete micronemes and essential for ookinete invasion and oocyst formation in the mosquito midgut epithelium (12, 55, 69); (iii) Pbsub2, a subtlisin-like protease (19); (iv) von Willebrand factor A domain-related protein, a secreted protein with adhesive properties of unknown function (70); (v) membrane attack ookinete protein, which contains a perforin-related domain (23); and (vi) secreted ookinete adhesive protein (SOAP), which contains two unique cysteine-rich domains and interacts with laminin (13). Ookinetes that were deficient in SOAP exhibited significantly reduced midgut invasion and oocyst formation (13).
Transmission-blocking immunity.
Transmission-blocking immunity can be mediated by antibodies against parasite surface proteins, which act in the midgut of a blood-fed mosquito. The P25 and P28 proteins are expressed only in the mosquito. These proteins normally do not encounter the human immune system, but antibodies raised against recombinant P25 and P28 proteins, when taken up by mosquitoes, stop parasite development in the mosquito gut.
Several transmission-blocking vaccine formulations are being developed using Plasmodium vivax and P. falciparum P25 and P28 proteins produced in Saccharomyces cerevisiae. Antisera from mice immunized with recombinant Pvs25 (P25 from P. vivax parasites) completely prevented the appearance of oocysts in mosquitoes that had ingested the antisera with P. vivax parasites (21). In a phase I vaccine trial of Pvs25 bound to aluminum hydroxide, the levels of antibodies that were generated correlated with transmission-blocking activity (34). Antibodies obtained after immunization of mice and monkeys with yeast-produced Pfs25 (P25 from P. falciparum parasites) (73) showed significant transmission-blocking activity in experiments in which a mixture of antisera, blood, and parasite cultures were fed to the mosquitoes through a membrane feeding apparatus (22). In humans, priming with a Pfs25 gene-containing vaccinia virus and boosting with Pfs25 protein yielded antisera with significant transmission-blocking activity (25). Covalent conjugation of P25 proteins by chemical cross-linking to carrier proteins is a promising strategy, as it yields strong and sustained antibody responses (31, 37, 67).
INTERACTIONS OF P25 AND P28 WITH MOSQUITO MIDGUT PROTEINS
The interaction of ookinetes with the basal lamina is important for ookinete invasion and oocyst development in the mosquito. Plasmodium P25 and P28 proteins play an important role in parasite recognition of and attachment to the mosquito midgut (45, 46, 56). The Plasmodium berghei P25 and P28 proteins were shown, by yeast two-hybrid experiments, to interact with laminin, a major constituent of the basal lamina surrounding the midgut of Anopheles gambiae mosquitoes (64). The Plasmodium gallinaceum P25 and P28 proteins interact with the midgut basement membrane in order to attach the parasite to its surface (1). The P25 protein of P. berghei binds to laminin and collagen IV, and the binding is involved in the transformation of ookinetes into oocysts (3).
A study that combined knowledge of the sequenced genomes of Drosophila melanogaster and A. gambiae identified annexin proteins, which bind to P. berghei ookinetes during invasion of the mosquito midgut and play important roles in mosquito infection (30). When a blood meal containing a mixture of P. berghei parasites and anti-annexin serum was fed to mosquitoes in membrane feeding experiments, the number of observed oocysts was considerably reduced compared to that of the control. Confocal analysis of dissected midguts with anti-anopheles annexin mouse serum and the 13.1 MAb recognizing P. berghei P28 revealed that the staining of P28 and annexin overlapped and so the two proteins colocalized (30).
GENE DISRUPTION STUDIES
Gene disruptions of P25 and P28 revealed that the two proteins have partially redundant functions in parasites and are involved in ookinete survival in the midgut, penetration of the midgut epithelium, and the transformation of ookinetes to oocysts (56). When blood infected with P. berghei having either the P25 or the P28 gene disrupted was fed to mosquitoes through a membrane feeder, oocyst formation was slightly affected compared to oocyst formation in mosquitoes infected with wild-type P. berghei. However, when both genes were knocked out at the same time, almost no oocysts were formed (56). The ookinetes of the double-knockout parasite were swollen in appearance and did not cluster together in the gut as wild-type parasites do (56). A recent study of midgut epithelium invasion by double-knockout P. berghei ookinetes implied that the loss of P25/P28 proteins greatly reduced, but did not entirely prevent, the entry of ookinetes into midgut epithelial cells (5).
STRUCTURAL STUDIES
Primary structure.
The Plasmodium P25 and P28 proteins are evolutionarily conserved and are comprised of a predicted signal sequence at their N termini, followed by four epidermal growth factor (EGF)-like domains and a C-terminal glycosylphosphatidylinositol moiety that anchors the proteins to the parasite surface (24, 27). Sequence analysis shows that P25 proteins contain 22 cysteine residues held together with 11 disulfide bonds and that P28 proteins contain 20 cysteine residues with 10 disulfide bonds. EGF-like domains are found predominantly in extracellular proteins of eukaryotes, where they participate in adhesion and signaling (2). A typical EGF-like domain contains 40 to 50 residues, including six cysteines that form disulfide bonds in the pattern 1-3, 2-4, and 5-6. EGF domains contain a variable number of residues between the cysteines, except for a single residue between cysteines 4 and 5.
X-ray crystal structure of Pvs25.
The structure determination of Pvs25 used the same yeast-produced recombinant protein as that used for vaccine trials (34, 42). The Pvs25 structure was the first structure of a Plasmodium surface protein from the mosquito stage and revealed the unprecedented arrangement of the four EGF-like domains of Pvs25 to form a compact triangular prism. In the Pvs25 crystal, triangular prisms are arranged as layers of sheets. Pvs25 residues that form interdomain contacts within the molecule and intermolecular contacts involved in sheet formation in the crystal are highly conserved in P25 and P28 proteins from all Plasmodium species (42).
Examination of the P. falciparum P25 sequence revealed that the Pfs25 protein likely assumes the same triangular structure as Pvs25. There is complete conservation of the residues forming the contacts among EGF-like domains 1, 3, and 4 that bring the four domains into their shape. The overall sequence identity between Pfs25 and Pvs25 is 46%, yet Pfs25 has been predicted to be similar to Pvs25 due to the disulfide-bonding similarities of the EGF-like domains. Pvs25 and Pvs28 are related, exhibiting 41% amino acid sequence identity over 157 residues of Pvs28. The residues that interact between domain 1 and domains 3 and 4 in Pvs25 are conserved throughout the sequences of the P28 family. Therefore, the structure of Pvs25 will likely be a valid model for the structures of all P28 family members (42).
Sequence polymorphisms.
There are relatively few sequence polymorphisms in P25 and P28 proteins isolated from P. falciparum and P. vivax populations in the field, presumably because the P25 and P28 proteins are not expressed in the vertebrate host and thus are not exposed to selection pressure from the vertebrate immune system (8). In Pfs25, two conserved amino acid substitutions and two silent changes were found, while in the Pfs28 protein a Lys-to-Arg change at position 72 had been found and, recently, a new nonsynonymous substitution (Asp to Ala at position 104) was found through a genome-wide single nucleotide polymorphism analysis (18, 28, 44; www.plasmodb.org [accession no. PF10_0302]). A study conducted on P. vivax strain SalI, using one isolate from India and two isolates from Bangladesh, indicated that the Pvs25 protein contains only 3 point mutations that would result in amino acid substitutions while the Pvs28 gene has 22 point mutations, but all are conserved substitutions (60). The most striking variation was detected in an Indian isolate of Pvs28 that contains four C-terminal tandem repeats of Gly-Ser-Gly-Gly-Glu/Asp instead of the usual six repeats found elsewhere (60).
BINDING OF TRANSMISSION-BLOCKING ANTIBODIES
In the mosquito gut, parasites exit red blood cells and thus become vulnerable to antiparasite antibodies in the blood meal. Studies with mice, rabbits, and rhesus monkeys demonstrated that yeast-expressed Pvs25 formulated on aluminum hydroxide gel induces antibodies that block the development of P. vivax in mosquitoes, as demonstrated in the ex vivo membrane feeding assay (6, 21). When antisera from mice immunized with individual domains of P. falciparum P25 were mixed with infected blood and fed to mosquitoes in the laboratory, EGF-like domain 2 antisera had the highest transmission-blocking activity (51). For P. berghei, the transmission-blocking MAb 13.1 against P28 was mapped using deletions and overlapping peptides to the sequence Gly-Leu-Glu-Lys-Ala-Phe-Val-Cys on the B loop of domain 2 (50).
EGF-like domain 3 is the target of other transmission-blocking MAbs. The MAbs 4B7 and 1D2 recognized domain 3 in enzyme-linked immunosorbent assays using the individually expressed domains of P. falciparum P25 (51). The 4B7 binding site was mapped to the sequence Leu-Asp-Thr-Ser-Asn-Pro-Val-Lys at the apex of the B loop of EGF-like domain 3, using overlapping synthetic peptides from the P. falciparum P25 sequence (52). By similar methods, the independently generated MAb 32F81 against P. falciparum P25 was mapped to the same site on the B loop of domain 3 (62).
To investigate the place and mode of binding of a transmission-blocking antibody to Pvs25, the structure of a Fab fragment of a transmission-blocking antibody bound to Pvs25 was determined (42). MAbs were generated in mice by using yeast-produced Pvs25 as an immunogen and were shown to bind to parasites in immunofluorescence experiments (72). In the structure, the Fab fragment of 2A8 binds to the B loop of domain 2 of Pvs25 (Fig. 1). MAbs 1H10 and 1A5 also bind near or at the B loop of domain 2, as they were unable to bind Pvs25 that had been prebound with saturating amounts of Fab 2A8 (42).
FIG. 1.
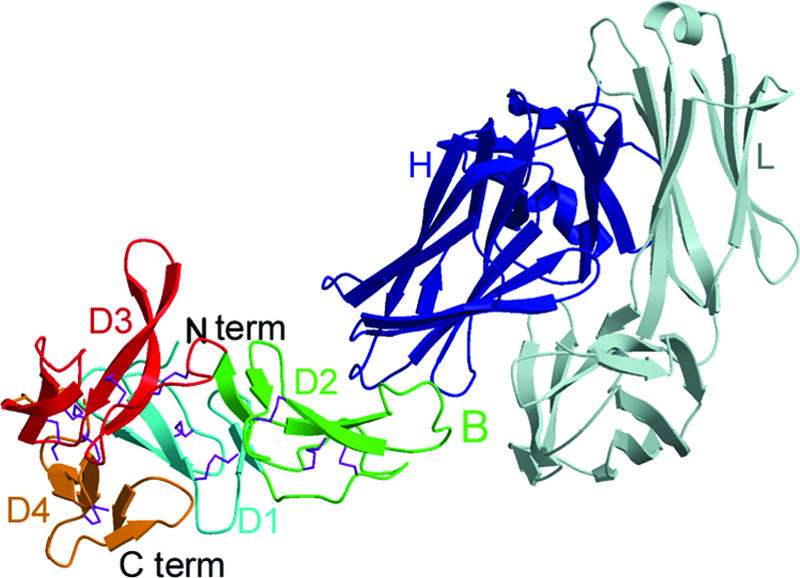
Fab of the transmission-blocking MAb 2A8 bound by its heavy chain (H; blue) to the B loop (B; green) of domain 2 of Pvs25 (D2; green). The light chain of 2A8 Fab (L; gray) does not contact Pvs25, appearing to play no role in direct binding. The Pvs25 triangular prism is formed by domain 1 (D1; cyan), domain 2 (D2; green), domain 3 (D3; red), and domain 4 (D4; gold) (PDB accession code 1Z3G). (Reprinted from reference 42 with permission of the publisher).
POSSIBLE FUNCTIONS IN MOSQUITO MIDGUT
Protection.
Plasmodium P25 and P28 proteins may play an important role in protecting the parasite from the harsh proteolytic environment of the midgut and the mosquito immune system. When a mosquito takes a blood meal from an infected person, the P25 and P28 proteins are expressed early and coat the parasite surface. Interactions between P25/P28-coated cell surfaces (46) may mediate parasite clustering, as these proteins are extremely abundant on ookinete surfaces (48). P25/P28 double-knockout and antibody-treated ookinetes did not cluster together (46, 47, 56) in the blood meal as do wild-type and untreated ones (68). Lack of clustering arising from lessened interactions between adjacent parasites may expose even the inner ookinetes of the cluster to the damaging proteolytic conditions of the midgut (16).
The Pvs25 crystal structure revealed a triangular prism-shaped structure that could tile the parasite surface (42). In the crystal, Pvs25 is packed as tightly arranged sheets that could also occur on the parasite surface to form a protective coat (Fig. 2). P25 and P28 can substitute for each other in single-knockout parasites (56). Perhaps either one alone can form an effective surface sheet, but a more protective one might be formed when both proteins are present. Yeast two-hybrid experiments (46) showed that Pvs25 has a tendency to form dimers, a requirement for forming a sheet.
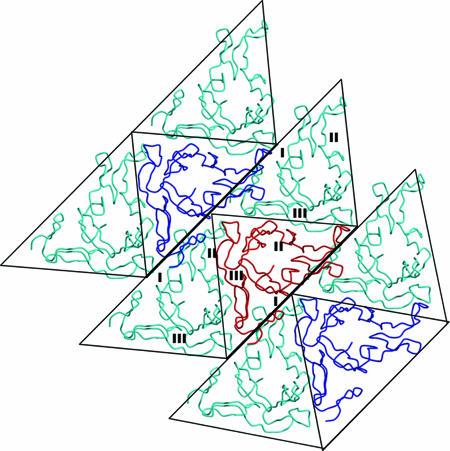
FIG. 2.
Observed sheets of Pvs25 molecules in the Pvs25 crystal. The reference molecule (red) contacts four symmetry mates (cyan) and two molecules related by crystal lattice translations (blue). The six Pvs25 molecules (cyan) have the same triangular face up, where three other molecules (red and blue) have the opposite face up. Edge I of Pvs25 packs against edge I of the neighboring ribbon, edge II packs against edge III, and edge III packs against edge II to form sheets in crystal (PDB accession code 1Z1Y). (Reprinted from reference 42 with permission of the publisher).
In five independent structural views of Pvs25, the position of the C-terminal half of domain 4 pivots in the plane of the triangle so that the angle it makes with the rest of domain 4 varies (A. K. Saxena and D. N. Garboczi, unpublished data). This variation would be useful for adjusting a molecule's fit in a sheet of other cell surface molecules.
Involvement with ookinete entry into midgut epithelial cells.
The migration of P25/P28 double-knockout parasites into and across the midgut epithelium is significantly reduced, but not abolished, compared to that of wild-type parasites (56). A study using wild-type and double-knockout P. berghei indicated that double-knockout parasites may migrate through the midgut epithelium via an intercellular route rather than the intracellular route used by wild-type parasites (5). P25 and P28 may play an important role in mediating ookinete entry into midgut epithelial cells (5). The P28 protein is shed from the ookinete and is found at the site of ookinete penetration of the midgut epithelial cell, and tracks through the invaded cell's cytoplasm are seen (10, 20). The presence of the P28 protein on and in cells suggests a function in an aspect of the traversal of the epithelium, though the conclusion that P25 and P28 do not play critical roles in recognition or penetration of the midgut epithelium came from the double-knockout studies (46, 56). During invasion of the epithelium, wild-type P. berghei ookinetes cause significant damage to cells (10, 20, 35, 65, 71), which show the loss of microvilli, extension into the midgut lumen, and increased expression of nitric oxide synthase (20, 33) and particular serpin molecules (10, 11). Midgut cells that were invaded by P25/P28 double-knockout P. berghei do not show the abnormal characteristics observed during and after invasion by wild-type P. berghei ookinetes (5, 10).
VACCINE DEVELOPMENT
Transmission-blocking vaccines against the two major species of human malaria parasite, P. falciparum and P. vivax, are under development (6, 61). Both P25 and P28 are encoded by single-copy genes that are highly conserved among parasite isolates. This feature simplified the vaccine design, as a vaccine based on a single target gene sequence will be effective for all parasite isolates in various geographic locations. On the other hand, a lack of expression in the human host indicated that this vaccine would not be boosted by natural malaria infection. As a result, the vaccine has to be highly potent to induce adequate antibody levels that are sustained for at least one transmission season. Vaccination of mice, rabbits, and monkeys with yeast-produced Pfs25, with aluminum hydroxide as an adjuvant, induces transmission-blocking antibodies (4, 26, 38). Yeast-produced Pvs25 formulated with aluminum hydroxide as an adjuvant was evaluated in a phase I clinical trial (34). However, antibody titers produced after Pvs25 immunization are low, and more potent adjuvant formulations are required for inducing high antibody titers. Yeast-produced Pfs25 formulated with Montanide ISA51 is in a phase I clinical trial. Despite the evidence of Pfs25 being an effective vaccine target, the protein requires potent adjuvant formulations to increase the immunogenicity to sustain high antibody titers. A covalent chemical conjugate of yeast-produced Pfs25 linked to OMPC (the outer membrane protein complex of Neisseria meningitidis serogroup B) and formulated with aluminum hydroxyphosphate was more effective in generating anti-Pfs25 enzyme-linked immunosorbent assay reactivity than Pfs25 alone in Montanide ISA720 at the same dose (67). The conjugate vaccine Pfs25-OMPC has shown sustained high immunogenicity in rhesus monkeys (67). Conjugating Pfs25 to a nontoxic recombinant Pseudomonas aeruginosa exoprotein A (37) or to Pfs25 itself to form multimeric molecules (31) also significantly increased the immunogenicity of Pfs25.
CONCLUDING REMARKS
Vaccines targeting the Plasmodium P25 and P28 proteins are promising strategies for malaria control, as they induce human antibodies that inhibit the parasite in the mosquito midgut. Gene knockouts show that P25 and P28 share multiple functions during ookinete-to-oocyst development. The structure of Pvs25 from P. vivax is the first of a mosquito-stage surface protein and has a novel arrangement of EGF domains. Pvs25 forms triangular prism structures, and residues forming the triangles are highly conserved in all other Plasmodium spp.; thus, Pvs25 is a good template for predicting the structures of other P25 and P28 proteins. P25 and P28 interactions with transmission-blocking antibodies indicate that antibodies bind to the B loops of the second and third EGF-like domains of P25 and P28 proteins. The complex of Pvs25 with a Fab fragment showed how one transmission-blocking antibody binds P25; the generation of such antibodies by inexpensive and simple transmission-blocking vaccines should play an important role in the control of malaria transmission.
Plasmodium surface proteins play key roles in host cell invasion. The completion of the P. falciparum and A. gambiae genomes has provided information about important molecular events involved in parasite-insect interactions. Knockout studies of many genes expressed in the mosquito stages have given hints of their biological functions and survival strategies of the parasite in the mosquito gut. The three-dimensional structural analysis of parasite surface proteins will be indispensable in understanding the structure-function relationship that will contribute to the development of therapeutic and vaccine strategies against malaria.
Acknowledgments
We thank Carole A. Long, Louis H. Miller, and the staff of the Malaria Vaccine Development Branch, National Institute of Allergy and Infectious Diseases (NIAID), for collaboration and discussions.
Ajay K. Saxena is supported by a grant from the Council of Scientific and Industrial Research (CSIR), New Delhi, India. Yimin Wu and David N. Garboczi are supported by the intramural research program of the NIAID, NIH.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Adini, A., and A. Warburg. 1999. Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology 119:331-336. [DOI] [PubMed] [Google Scholar]
- 2.Appella, E., I. T. Weber, and F. Blasi. 1988. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 231:1-4. [DOI] [PubMed] [Google Scholar]
- 3.Arrighi, R. B., and H. Hurd. 2002. The role of Plasmodium berghei ookinete proteins in binding to basal lamina components and transformation into oocysts. Int. J. Parasitol. 32:91-98. [DOI] [PubMed] [Google Scholar]
- 4.Barr, P. J., K. M. Green, H. L. Gibson, I. C. Bathurst, I. A. Quakyi, and D. C. Kaslow. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baton, L. A., and L. C. Ranford-Cartwright. 2005. Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malar. J. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 7.Carter, R., P. M. Graves, D. B. Keister, and I. A. Quakyi. 1990. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12:587-603. [DOI] [PubMed] [Google Scholar]
- 8.Carter, R., P. M. Graves, I. A. Quakyi, and M. F. Good. 1989. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 169:135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coban, C., M. T. Philipp, J. E. Purcell, D. B. Keister, M. Okulate, D. S. Martin, and N. Kumar. 2004. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infect. Immun. 72:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danielli, A., C. Barillas-Mury, S. Kumar, F. C. Kafatos, and T. G. Loukeris. 2005. Overexpression and altered nucleocytoplasmic distribution of Anopheles ovalbumin-like SRPN10 serpins in Plasmodium-infected midgut cells. Cell. Microbiol. 7:181-190. [DOI] [PubMed] [Google Scholar]
- 11.Danielli, A., F. C. Kafatos, and T. G. Loukeris. 2003. Cloning and characterization of four Anopheles gambiae serpin isoforms, differentially induced in the midgut by Plasmodium berghei invasion. J. Biol. Chem. 278:4184-4193. [DOI] [PubMed] [Google Scholar]
- 12.Dessens, J. T., A. L. Beetsma, G. Dimopoulos, K. Wengelnik, A. Crisanti, F. C. Kafatos, and R. E. Sinden. 1999. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 18:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dessens, J. T., I. Siden-Kiamos, J. Mendoza, V. Mahairaki, E. Khater, D. Vlachou, X. J. Xu, F. C. Kafatos, C. Louis, G. Dimopoulos, and R. E. Sinden. 2003. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol. Microbiol. 49:319-329. [DOI] [PubMed] [Google Scholar]
- 14.Duffy, P. E., and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy, P. E., P. Pimenta, and D. C. Kaslow. 1993. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 177:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gass, R. F., and R. A. Yeates. 1979. In vitro damage of cultured ookinetes of Plasmodium gallinaceum by digestive proteinases from susceptible Aedes aegypti. Acta Trop. 36:243-252. [PubMed] [Google Scholar]
- 17.Good, M. F., D. C. Kaslow, and L. H. Miller. 1998. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16:57-87. [DOI] [PubMed] [Google Scholar]
- 18.Hafalla, J. C., M. L. Santiago, M. C. Pasay, B. L. Ramirez, M. M. Gozar, A. Saul, and D. C. Kaslow. 1997. Minimal variation in the Pfs28 ookinete antigen from Philippine field isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 87:97-99. [DOI] [PubMed] [Google Scholar]
- 19.Han, Y. S., and C. Barillas-Mury. 2002. Implications of time bomb model of ookinete invasion of midgut cells. Insect Biochem. Mol. Biol. 32:1311-1316. [DOI] [PubMed] [Google Scholar]
- 20.Han, Y. S., J. Thompson, F. C. Kafatos, and C. Barillas-Mury. 2000. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 19:6030-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisaeda, H., A. W. Stowers, T. Tsuboi, W. E. Collins, J. S. Sattabongkot, N. Suwanabun, M. Torii, and D. C. Kaslow. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janse, C. J., and A. P. Waters. 2004. Sexual development of malarial parasites, p. 445-474. In A. P. Waters and C. J. Janse (ed.), Malaria parasites, genomes, and molecular biology. Caister Academic Press, Wymondham, Norfolk, England.
- 23.Kadota, K., T. Ishino, T. Matsuyama, Y. Chinzei, and M. Yuda. 2004. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc. Natl. Acad. Sci. USA 101:16310-16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow, D. C. 1997. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27:183-189. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow, D. C. 2002. Transmission-blocking vaccines. Chem. Immunol. 80:287-307. [DOI] [PubMed] [Google Scholar]
- 26.Kaslow, D. C., I. C. Bathurst, T. Lensen, T. Ponnudurai, P. J. Barr, and D. B. Keister. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaslow, D. C., I. A. Quakyi, C. Syin, M. G. Raum, D. B. Keister, J. E. Coligan, T. F. McCutchan, and L. H. Miller. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74-76. [DOI] [PubMed] [Google Scholar]
- 28.Kaslow, D. C., C. Syin, T. F. McCutchan, and L. H. Miller. 1989. Comparison of the primary structure of the 25 kDa ookinete surface antigens of Plasmodium falciparum and Plasmodium gallinaceum reveals six conserved regions. Mol. Biochem. Parasitol. 33:283-287. [DOI] [PubMed] [Google Scholar]
- 29.Kiszewski, A., A. Mellinger, A. Spielman, P. Malaney, S. E. Sachs, and J. Sachs. 2004. A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 70:486-498. [PubMed] [Google Scholar]
- 30.Kotsyfakis, M., L. Ehret-Sabatier, I. Siden-Kiamos, J. Mendoza, R. E. Sinden, and C. Louis. 2005. Plasmodium berghei ookinetes bind to Anopheles gambiae and Drosophila melanogaster annexins. Mol. Microbiol. 57:171-179. [DOI] [PubMed] [Google Scholar]
- 31.Kubler-Kielb, J., F. Majadly, Y. Wu, D. L. Narum, C. Guo, L. H. Miller, J. Shiloach, J. B. Robbins, and R. Schneerson. 2007. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc. Natl. Acad. Sci. USA 104:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar, N., and R. Carter. 1985. Biosynthesis of two stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinetes. Mol. Biochem. Parasitol. 14:127-139. [DOI] [PubMed] [Google Scholar]
- 33.Luckhart, S., Y. Vodovotz, L. Cui, and R. Rosenberg. 1998. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 95:5700-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malkin, E. M., A. P. Durbin, D. J. Diemert, J. Sattabongkot, Y. Wu, K. Miura, C. A. Long, L. Lambert, A. P. Miles, J. Wang, A. Stowers, L. H. Miller, and A. Saul. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23:3131-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meis, J. F., G. Pool, G. J. van Gemert, A. H. Lensen, T. Ponnudurai, and J. H. Meuwissen. 1989. Plasmodium falciparum ookinetes migrate intercellularly through Anopheles stephensi midgut epithelium. Parasitol. Res. 76:13-19. [DOI] [PubMed] [Google Scholar]
- 36.Paton, M. G., G. C. Barker, H. Matsuoka, J. Ramesar, C. J. Janse, A. P. Waters, and R. E. Sinden. 1993. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol. 59:263-275. [DOI] [PubMed] [Google Scholar]
- 37.Qian, F., Y. Wu, O. Muratova, H. Zhou, G. Dobrescu, P. Duggan, L. Lynn, G. Song, Y. L. Zhang, K. Reiter, N. MacDonald, D. L. Narum, C. A. Long, L. H. Miller, A. Saul, and G. E. D. Mullen. 2007. Conjugating recombinant proteins to Pseudomonas aeruginosa exoprotein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine 25:3923-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawlings, D. J., and D. C. Kaslow. 1992. Adjuvant-dependent immune response to malarial transmission-blocking vaccine candidate antigens. J. Exp. Med. 176:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read, D., A. H. Lensen, S. Begarnie, S. Haley, A. Raza, and R. Carter. 1994. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 16:511-519. [DOI] [PubMed] [Google Scholar]
- 40.Rener, J., P. M. Graves, R. Carter, J. L. Williams, and T. R. Burkot. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 158:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauerwein, R. W., and W. M. Eling. 2002. Sexual and sporogonic stage antigens. Chem. Immunol. 80:188-203. [DOI] [PubMed] [Google Scholar]
- 42.Saxena, A. K., K. Singh, H. P. Su, M. M. Klein, A. W. Stowers, A. J. Saul, C. A. Long, and D. N. Garboczi. 2006. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 13:90-91. [DOI] [PubMed] [Google Scholar]
- 43.Shahabuddin, M., T. Toyoshima, M. Aikawa, and D. C. Kaslow. 1993. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl. Acad. Sci. USA 90:4266-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi, Y. P., M. P. Alpers, M. M. Povoa, and A. A. Lal. 1992. Single amino acid variation in the ookinete vaccine antigen from field isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 50:179-180. [DOI] [PubMed] [Google Scholar]
- 45.Siden-Kiamos, I., and C. Louis. 2004. Interactions between malaria parasites and their mosquito hosts in the midgut. Insect Biochem. Mol. Biol. 34:679-685. [DOI] [PubMed] [Google Scholar]
- 46.Siden-Kiamos, I., D. Vlachou, G. Margos, A. Beetsma, A. P. Waters, R. E. Sinden, and C. Louis. 2000. Distinct roles for pbs21 and pbs25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J. Cell Sci. 113:3419-3426. [DOI] [PubMed] [Google Scholar]
- 47.Sieber, K. P., M. Huber, D. Kaslow, S. M. Banks, M. Torii, M. Aikawa, and L. H. Miller. 1991. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp. Parasitol. 72:145-156. [DOI] [PubMed] [Google Scholar]
- 48.Sinden, R. E., L. Winger, E. H. Carter, R. H. Hartley, N. Tirawanchai, C. S. Davies, J. Moore, and J. F. Sluiters. 1987. Ookinete antigens of Plasmodium berghei: a light and electron-microscope immunogold study of expression of the 21 kDa determinant recognized by a transmission-blocking antibody. Proc. R. Soc. Lond. B 230:443-458. [DOI] [PubMed] [Google Scholar]
- 49.Snow, R. W., M. Craig, U. Deichmann, and K. Marsh. 1999. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull. W. H. O. 77:624-640. [PMC free article] [PubMed] [Google Scholar]
- 50.Spano, F., H. Matsuoka, R. Ozawa, Y. Chinzei, and R. E. Sinden. 1996. Epitope mapping on the ookinete surface antigen Pbs21 of Plasmodium berghei: identification of the site of binding of transmission-blocking monoclonal antibody 13.1. Parassitologia 38:559-563. [PubMed] [Google Scholar]
- 51.Stowers, A. W., D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 68:5530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stura, E. A., A. C. Satterthwait, J. C. Calvo, R. S. Stefanko, J. P. Langeveld, and D. C. Kaslow. 1994. Crystallization of an intact monoclonal antibody (4B7) against Plasmodium falciparum malaria with peptides from the Pfs25 protein antigen. Acta Crystallogr. D 50:556-562. [DOI] [PubMed] [Google Scholar]
- 53.Tachibana, M., T. Tsuboi, T. J. Templeton, O. Kaneko, and M. Torii. 2001. Presence of three distinct ookinete surface protein genes, Pos25, Pos28-1, and Pos28-2, in Plasmodium ovale. Mol. Biochem. Parasitol. 113:341-344. [DOI] [PubMed] [Google Scholar]
- 54.Templeton, T. J., and D. C. Kaslow. 1999. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol. Biochem. Parasitol. 101:223-227. [DOI] [PubMed] [Google Scholar]
- 55.Templeton, T. J., D. C. Kaslow, and D. A. Fidock. 2000. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol. Microbiol. 36:1-9. [DOI] [PubMed] [Google Scholar]
- 56.Tomas, A. M., G. Margos, G. Dimopoulos, L. H. van Lin, T. F. de Koning-Ward, R. Sinha, P. Lupetti, A. L. Beetsma, M. C. Rodriguez, M. Karras, A. Hager, J. Mendoza, G. A. Butcher, F. Kafatos, C. J. Janse, A. P. Waters, and R. E. Sinden. 2001. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 20:3975-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai, Y. L., R. E. Hayward, R. C. Langer, D. A. Fidock, and J. M. Vinetz. 2001. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 69:4048-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuboi, T., Y. M. Cao, D. C. Kaslow, K. Shiwaku, and M. Torii. 1997. Primary structure of a novel ookinete surface protein from Plasmodium berghei. Mol. Biochem. Parasitol. 85:131-134. [DOI] [PubMed] [Google Scholar]
- 59.Tsuboi, T., D. C. Kaslow, Y. M. Cao, K. Shiwaku, and M. Torii. 1997. Comparison of Plasmodium yoelii ookinete surface antigens with human and avian malaria parasite homologues reveals two highly conserved regions. Mol. Biochem. Parasitol. 87:107-111. [DOI] [PubMed] [Google Scholar]
- 60.Tsuboi, T., D. C. Kaslow, M. M. Gozar, M. Tachibana, Y. M. Cao, and M. Torii. 1998. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 4:772-782. [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuboi, T., M. Tachibana, O. Kaneko, and M. Torii. 2003. Transmission-blocking vaccine of vivax malaria. Parasitol. Int. 52:1-11. [DOI] [PubMed] [Google Scholar]
- 62.van Amerongen, A., R. W. Sauerwein, P. J. Beckers, R. H. Meloen, and J. H. Meuwissen. 1989. Identification of a peptide sequence of the 25 kD surface protein of Plasmodium falciparum recognized by transmission-blocking monoclonal antibodies: implications for synthetic vaccine development. Parasite Immunol. 11:425-428. [DOI] [PubMed] [Google Scholar]
- 63.Vermeulen, A. N., T. Ponnudurai, P. J. Beckers, J. P. Verhave, M. A. Smits, and J. H. Meuwissen. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vlachou, D., G. Lycett, I. Siden-Kiamos, C. Blass, R. E. Sinden, and C. Louis. 2001. Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol. Biochem. Parasitol. 112:229-237. [DOI] [PubMed] [Google Scholar]
- 65.Vlachou, D., T. Zimmermann, R. Cantera, C. J. Janse, A. P. Waters, and F. C. Kafatos. 2004. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell. Microbiol. 6:671-685. [DOI] [PubMed] [Google Scholar]
- 66.Winger, L. A., N. Tirawanchai, J. Nicholas, H. E. Carter, J. E. Smith, and R. E. Sinden. 1988. Ookinete antigens of Plasmodium berghei. Appearance on the zygote surface of an Mr 21 kD determinant identified by transmission-blocking monoclonal antibodies. Parasite Immunol. 10:193-207. [DOI] [PubMed] [Google Scholar]
- 67.Wu, Y., C. Przysiecki, E. Flanagan, S. N. Bello-Irizarry, R. Ionescu, O. Muratova, G. Dobrescu, L. Lambert, D. Keister, Y. Rippeon, C. A. Long, L. Shi, M. Caulfield, A. Shaw, A. Saul, J. Shiver, and L. H. Miller. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 103:18243-18248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoeli, M., and R. S. Upmanis. 1968. Plasmodium berghei ookinete formation in vitro. Exp. Parasitol. 22:122-128. [DOI] [PubMed] [Google Scholar]
- 69.Yuda, M., H. Sakaida, and Y. Chinzei. 1999. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J. Exp. Med. 190:1711-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuda, M., K. Yano, T. Tsuboi, M. Torii, and Y. Chinzei. 2001. von Willebrand factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol. Biochem. Parasitol. 116:65-72. [DOI] [PubMed] [Google Scholar]
- 71.Zieler, H., and J. A. Dvorak. 2000. Invasion in vitro of mosquito midgut cells by the malaria parasite proceeds by a conserved mechanism and results in death of the invaded midgut cells. Proc. Natl. Acad. Sci. USA 97:11516-11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zollner, G. E., N. Ponsa, R. E. Coleman, J. Sattabongkot, and J. A. Vaughan. 2005. Evaluation of procedures to determine absolute density of Plasmodium vivax ookinetes. J. Parasitol. 91:453-457. [DOI] [PubMed] [Google Scholar]
- 73.Zou, L., A. P. Miles, J. Wang, and A. W. Stowers. 2003. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 21:1650-1657. [DOI] [PubMed] [Google Scholar]