Abstract
Cryptococcus neoformans is a pathogenic yeast that often causes devastating meningoencephalitis in immunocompromised individuals. We have previously identified the C. neoformans CPS1 gene, which is required for a capsular layer on the outer cell wall. In this report, we investigate the function of the CPS1 gene and its pathogenesis. We demonstrated that treatment of yeast with either 4-methylumbelliferone or hyaluronidase resulted in a reduction of the level of C. neoformans binding to human brain microvascular endothelial cells (HBMEC). Yeast extracellular structures were also altered accordingly in hyaluronidase-treated cells. Furthermore, observation of yeast strains with different hyaluronic acid contents showed that the ability to bind to HBMEC is proportional to the hyaluronic acid content. A killing assay with Caenorhabditis elegans demonstrated that the CPS1 wild-type strain is more virulent than the cps1Δ strain. When CPS1 is expressed in Saccharomyces cerevisiae and Escherichia coli, hyaluronic acid can be detected in the cells. Additionally, we determined by fluorophore-assisted carbohydrate electrophoretic analysis that hyaluronic acid is a component of the C. neoformans capsule. The size of hyaluronic acid molecules is evaluated by gel filtration and transmission electron microscopy studies. Together, our results support that C. neoformans CPS1 encodes hyaluronic acid synthase and that its product, hyaluronic acid, plays a role as an adhesion molecule during the association of endothelial cells with yeast.
Cryptococcus neoformans is an encapsulated pathogenic fungus that primarily afflicts immunocompromised individuals. Patients with T-cell deficiencies, including AIDS, lymphoma, corticosteroid therapy, and idiopathic CD4 lymphocytopenia, are the most susceptible to C. neoformans infection. Rates of infection by C. neoformans have increased considerably over the past few years (31, 32). The increasingly expanded use of immunosuppressive drugs and the onset of AIDS have contributed to this epidemic. The organisms show a remarkable propensity to spread to the brain and meninges, where meningoencephalitis develops (19, 22). C. neoformans meningoencephalitis causes extremely high mortality and morbidity in patients without adequate T-cell-dependent immune function. The existing arsenal of antifungal agents is limited. Toxic side effects and the emergence of drug resistance are significant impediments to effective therapy. In order to cause meningitis, C. neoformans must penetrate the blood-brain barrier (BBB) (13, 24). The BBB is a barrier between blood circulation and the brain parenchyma and consists mainly of specialized capillary endothelium, comprising brain microvascular endothelial cells (BMEC) (22, 35). It is characterized by the presence of tight junctions between cells and extremely low phagocytosis. It is responsible for maintaining the biochemical homeostasis within the central nervous system. The underlying mechanism(s) whereby C. neoformans crosses the BBB is largely unknown. We have previously studied the invasion of C. neoformans with human BMEC (HBMEC) by using in vitro binding and transcytosis assays (13). C. neoformans induces considerable morphological changes and actin reorganization in HBMEC through the phosphorylation regulation of cofilin, LIMK, and ROCK-linked pathways. The yeast cells were able to traverse across the BBB via a transcellular pathway through HBMEC (12).
The major virulence factors of C. neoformans are the polysaccharide capsular components (1, 3, 8, 21, 31). The main component of the C. neoformans polysaccharide capsule is glucuronoxylomannan; there are also minor components (galactoxylomannan, mannoproteins), and other polysaccharide derivatives (14). The capsule has the ability to exert a broad spectrum of influences on the immune response. Acapsular mutants are typically avirulent, whereas encapsulated isolates have differing degrees of virulence (21). Some capsule-associated genes, such as CAP10 (10), CAP59 (8), CAP60 (9), and CAP64 (11), have been identified. CAP64 was found to have six homologues in the C. neoformans genome that play an important role in determining the position and linkage of xylose and/or O-acetyl residues on the mannose backbone of the glucuronoxylomannan. CAP59 and CAP60 have a putative transmembrane domain, and CAP60 protein is localized primarily to the nuclear membrane. The functions of these genes are currently unknown. It seems that any genes directly or indirectly associated with capsule formation are crucial for C. neoformans virulence. It is also possible that an intact capsule structure may retain an essential virulence component(s) that is required for adhesion and/or invasion. In addition to these capsule genes, several genes involved in the biosynthesis of a polysaccharide capsule precursor and the process of polymerization have been characterized, such as UDP-glucuronic acid (GlcUA) decarboxylase (2, 27), α-1,3-mannosyltransferase (39), and phosphomannose isomerase (45). A missing link here is whether there is any capsular component playing a role as a ligand to adhere or bind to the host cells to precipitate the invasion process.
We have previously identified and investigated a C. neoformans gene, CPS1 (7). Deletion of CPS1 from C. neoformans resulted in alterations of several phenotypes important for the virulence of C. neoformans, including modification of the ultrastructure between the cell wall and the capsule, temperature sensitivity, and a reduced ability to associate with HBMEC in vitro. These results suggest that CPS1 plays an important role in the pathobiology of C. neoformans. The CPS1 gene shares similarity with the type 3 polysaccharide synthase gene, CAP3B, of Streptococcus pneumoniae (7). CAP3B is essential for the capsular lipopolysaccharide synthesis as well as for the virulence of S. pneumoniae. The type 3 synthase from S. pneumoniae belongs to the β-glycosyltransferase superfamily, including the hyaluronic acid synthases from prokaryotes and eukaryotes, the cellulose synthases from plants and bacteria, the chitin synthases from yeast, and the Nod factor synthases from Rhizobium (18, 26). We have demonstrated that the yeast wild-type CPS1 strain contains hyaluronic acid molecules, while the cps1Δ strain does not (7). Hyaluronic acid is a linear unbranched molecule with repeating disaccharide units of GlcUA (β1→3) and GlcNAc (β1→4). The molecular size of hyaluronic acid is usually between 1 and 10 million Da. Whether the hyaluronic acid molecule plays any role during C. neoformans infection remains to be demonstrated. In this report, we provide several lines of evidence to demonstrate that CPS1 encodes a hyaluronic acid synthase. It synthesizes cell surface hyaluronic acid, which is important for yeast infection in vitro and in vivo. Its role in the pathogenesis of cryptococcal central nervous system infection is discussed.
MATERIALS AND METHODS
Strains, media, and cultures.
C. neoformans strains have been described in detail previously (7, 13). Briefly, strains B-4500FO2 and C559 are isogenic strains with the wild-type CPS1 gene and a deleted cps1Δ gene, respectively. Strain C588 is C559 transformed with pYCC662; the CPS1 gene was cloned into the pCIP3 vector. Strain 602 is a cap64 mutant strain, and strain TYCC38-602 is an encapsulated transformant of 602 with the wild-type CAP64 gene. Strain TYCC33 is generated by deletion of the CAP59 gene and is an isogenic strain of B4476FO5 (CAP59). Yeast cells were grown aerobically at 30°C in 1% yeast extract, 2% peptone, and 2% dextrose (YPD broth) or Sabouraud medium (Difco Laboratories, Detroit, MI). Cells were harvested at early-log phase, washed with phosphate-buffered saline (PBS), and resuspended in Ham's F-12 medium-M199 (1:1, vol/vol), 5% heat inactivated fetal bovine serum (experimental medium), and 1% human serum. Cryptococcus yeast cell numbers were determined by direct counting using a hemocytometer. Saccharomyces cerevisiae strain BJ2168 (a prc1-407 prb1-1122 pep4-3 leu2 trp1 ura3-52) (42) and Escherichia coli strain BL21(DE3) were used for heterogenous expression of the CPS1 gene. All chemicals were ordered from Sigma Chemical Co. (St. Louis, MO), and the restriction enzymes were obtained from New England Biolabs (Beverly, MA).
Binding of yeast cells to HBMEC.
The in vitro assay of the binding of C. neoformans to HBMEC has been described previously (7, 13). Briefly, HBMEC were grown until confluence in collagen-coated 24-well tissue culture plates (Costar, Corning, NY). An inoculum of 106 yeast cells suspended in 0.5 ml experimental medium was added to the HBMEC monolayer (multiplicity of infection, 10) for 2 h at 37°C. Yeast cells unattached to the HBMEC monolayer were removed by washing the monolayer four times with experimental medium. HBMEC were lysed with 0.5% Triton, diluted, and plated onto a blood or YPD agar plate to determine the number of CFU that were associated with HBMEC. Results are presented as percentages of the level of adhesion of the inoculum, calculated as [(number of Cryptococcus cells recovered)/(number of Cryptococcus cells inoculated)] × 100.
Hyaluronidase treatment of C. neoformans.
Yeast cells were grown in a rich medium (YPD) at 30°C. Fresh overnight cultures (1 ml) were washed with 30 mM potassium acetate (pH 5.5) once, then resuspended in 100 μl potassium acetate buffer with 10 μl hyaluronidase, and shaken at 30°C for 1 h. After enzymatic treatment, the cells were washed with PBS three times. These cells were used for our in vitro HBMEC binding assay or for transmission electron microscopy (TEM) study.
TEM.
C. neoformans cells were fixed in buffer P (0.2 M sodium phosphate, pH 7.2) with 2% paraformaldehyde and 2% glutaraldehyde for 2 h at 37°C in a vacuum oven. Cells were washed three times with buffer P and then incubated with 8 ml of 50% normal human serum in GVB buffer (5 mM sodium Veronal-buffered 142 mM saline, pH 7.3, containing 0.1% gelatin, 1 mM MgCl2, and 0.15 mM CaCl2) for 30 min at 37°C. Cells were then washed with buffer P five times, and 0.5 ml was incubated with a 1/25 dilution of biotinylated hyaluronic acid-binding protein at 0°C for 30 min. After four washes with buffer P, cells were incubated with 1 ml of a 1/50 dilution of a streptavidin-horseradish peroxidase (HRP) conjugate at 0°C for 30 min. Cells were washed three times with buffer P and suspended in a substrate solution of diaminobenzidine (1 mg/ml) and hydrogen peroxide (0.01%) in ice-cold 0.05 M Tris, pH 7.0, for 5 min at 0°C. The cell pellet was washed with 0.144 M cacodylate buffer, pH 7.4, and resuspended in 10% warm gelatin (in cacodylate buffer). Solidified gelatin was cut into 1- to 2-mm blocks and sectioned (thickness, ∼30 nm), and the grid was stained with 5% aqueous uranyl acetate for 15 min, followed by Satos' lead for 1 min. The resulting sections were observed and photographed with an FEI Tecnai Twin TEM.
ELISAs for hyaluronic acid.
The hyaluronic acid enzyme-linked immunosorbent assay (ELISA) kit obtained from Corgenix, Inc. (Denver, CO), was used to assay hyaluronic acid. Yeast cells (106 cells) in the exponential-growth phase were incubated in individual wells at room temperature to trap the surface polysaccharide. After 30 min, the wells were washed with washing buffer according to the manufacturer's instructions. A second solution containing a hyaluronic acid-binding protein-HRP conjugate was added to the wells. After incubation, the wells were rinsed, and a chromogenic substrate (tetramethyl benzidine/H2O2) was added to develop a color reaction. The intensity of the resulting color was measured in optical density units with a spectrophotometer at 450 nm. The concentrations of hyaluronic acid were calculated by comparing the absorbance of the sample against a reference curve prepared from the reagent blank and hyaluronic acid reference solutions.
Expression of CPS1 in Saccharomyces cerevisiae and E. coli.
Coexpression of genes in Saccharomyces cerevisiae has been described previously (42). In this study, C. neoformans CPS1 cDNA was subcloned into plasmid YGp181-HA (selection marker, Leu2), and C. neoformans uridine diphosphoglucose dehydrogenase (UDPG DH) cDNA (GenBank accession no. AF405548) was subcloned into YGp123-HA (selection marker, Trp1). Both vectors are multicopy plasmids with a hemagglutinin (HA) tag under the control of the Gal1,10 promoter. The yeast media YPD and CSM medium were purchased from Bio 101, Inc. The constructed plasmids were transformed, individually or together, into S. cerevisiae strain BJ2168. For the expression experiments, 500 ml of cultures was induced with 2% galactose for 4 h. The cell pellets were disrupted by a Bead-Beater in PBS supplemented with 20 mM benzamidine, 20 mM phenylmethylsulfonyl fluoride, and 1 mM DNase. The expressed HA-tagged Cps1p and UDPG DH proteins were probed with an anti-HA antibody on protein blots. A monoclonal antibody against the HA tag was purchased from Roche Life Sciences (Indianapolis, IN).
For the expression of CPS1 in E. coli, a 1.35-kb CPS1 cDNA fragment was amplified from a λZap C. neoformans cDNA library using primers EP-5 (CAAGAGTCCATGGTATATCTCACAGCAGACAAA) and EP-3 (CGCAAGCTTTGCCTTTCTGCGCGCAGCTGG). The DNA fragment was subcloned into the pET22b expression vector (Novagen, Madison, WI) at the NcoI and HindIII sites. The Cps1 protein was expressed and purified from E. coli strain Rosetta-gami B(DE3) (Novagen, catalog no. 71136) according to the manufacturer's instructions. Briefly, the induced culture (250 ml) was harvested, and the cell pellet was resuspended in 10 ml buffer LY (50 mM sodium phosphate buffer [pH 7.0], 5% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml pepstatin A, 1 mM benzamidine, and 0.5% n-dodecyl-β-maltoside [Sigma D-4641]) and homogenized with a membrane disrupter (model 60 Sonic Dismembrator; Fisher Scientific) at 15 W for 1 min in ice. The resultant supernatant was mixed with nickel-nitrilotriacetic acid resin for the purification of Cps1p as described by the manufacturer. The bound proteins were eluted with 0.2 ml PBS-20% glycerol-0.25 M imidazole. For the hyaluronic acid synthase assay, 20 μl purified proteins was added to the 100-μl reaction mixture, containing 50 mM Tris (pH 7.6), 20 mM MaCl2, 1 mM β-mercaptoethanol, 100 μM UDP-GlcUA, and 100 μM UDP-GlcNAc. The reaction proceeded at 30°C for 2 h, and results were analyzed with the hyaluronic acid ELISA kit (Corgenix, Inc., Denver, CO).
FACE and gel filtration.
C. neoformans capsule polysaccharides were fractionated prior to fluorophore-assisted carbohydrate electrophoresis (FACE) analysis. Briefly, the growth of a C. neoformans culture is terminated by adding 5% trichloroacetic acid. This step can effectively separate capsule polysaccharides from cell debris (based on reference 2a). The supernatant is filtered through a 0.2-μm-pore-size filter and then mixed with 3 volumes of ethanol to precipitate the polysaccharides. The resulting crude preparation is resuspended in PBS and subjected to Dowex 1 (2X) chromatography (37). After application of the sample, elution is carried out using a 0.1 to 2.0 M gradient of NaCl. Based on the uronic acid assay, the fractions eluting at 0.5 to 0.65 M NaCl are first collected (6) and then subjected to FACE analysis, as described previously (33). For size fractionation, 1 ml of each Dowex 1 (2X)-purified fraction was applied to a Bio-Rad Bio-gel SEC-1000 high-performance liquid chromatography (HPLC) column with a flow rate of 1 ml/s PBS. The elution was monitored with a model R403 differential refractometer, showing negative peaks, and 1 ml per fraction was collected. Some fractions were used for a hyaluronic acid ELISA (see Fig. 7B, upper panel).
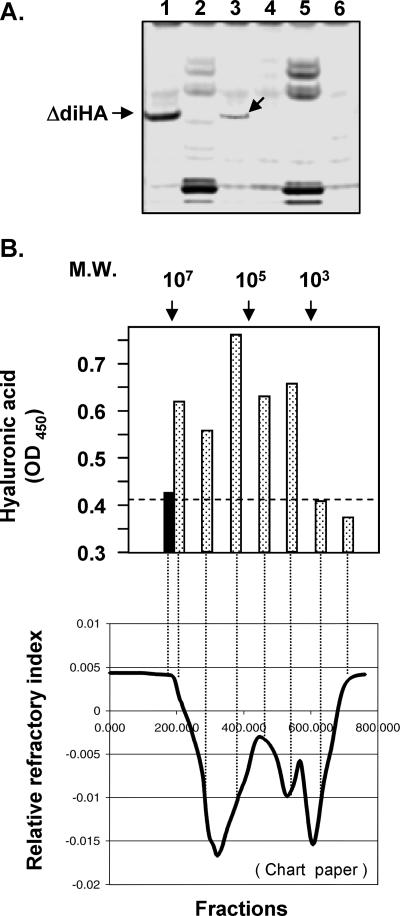
FIG. 7.
Characterization of C. neoformans capsule polysaccharide fractions. (A) FACE analysis. 2-Aminoacridone-derivatized products from C. neoformans capsular polysaccharide samples were resolved by electrophoresis. Lane 1, 5 μg hyaluronic acid as a standard marker; lane 2, B-4500FO2 crude capsular polysaccharide preparation; lane 3, B-4500FO2 Dowex 1 (2X) elution fraction (0.5 to 0.65 M NaCl); lane 4, enzyme/buffer control; lane 5, C559 (Δcps1) crude capsular polysaccharide preparation; lane 6, C559 Dowex 1 (2X) fraction (0.5 to 0.65 M NaCl fraction). An arrow in lane 3 indicates the band comigrating with a digested hyaluronic acid disaccharide unit (ΔdiHA). (B) Gel permeation using a Bio-Rad SEC-1000 size fractionation column to fractionate partially purified hyaluronic acid, followed by a hyaluronic acid ELISA. (Upper panel) Molecular weight (M.W.) markers are indicated at the top. Solid bar, blank control; stippled bars, aliquots (100 μl) from different fractions subjected to hyaluronic acid ELISA analysis. (Lower panel) Fractions corresponding to points on the curve on the refractor chart paper are shown (lower panel).
Caenorhabditis elegans killing assays.
Cryptococcal yeasts were grown as a lawn for C. elegans killing assays (28). In brief, C. neoformans strains (B-4500FO2 and C559) were inoculated into 2 ml of YPD medium and grown at 25°C for 48 h. Cells from each culture were counted and adjusted to 108 cells per ml. Thirty microliters of the culture was spread on NGM (nematode growth medium, containing NaCl, agar, peptone, cholesterol, CaCl2, MgSO4, potassium phosphate, and 75 μg/ml carbenicillin to prevent bacterial growth) agar in 35-mm-diameter tissue culture plates. The plates were incubated at 25°C overnight. Worms from the wild-type C. elegans strain N2 were transferred to the yeast plates. These worms were reared on E. coli OP50 on NGM until they were at the L4 developmental stage, and then they were moved to the yeast plates at a density of 10 to 15 nematodes per plate. At transfer, worms were dipped in an ampicillin solution (100 μg/ml) to prevent bacterial growth carried over from the E. coli OP50 plates. The experimental plates and a set of control plates on which the worms ate E. coli OP50 were incubated at 20°C and examined for viability at 24-h intervals under a stereomicroscope. The experimental N2 worms were fertile, but their hatchling progeny were unable to eat C. neoformans and thus did not grow. The control worms fed OP50 required frequent transfer to fresh plates during their fertile period to remove them from their growing progeny. The mean life span of E. coli OP50 was about 18 days under our experimental conditions. The nematodes were considered dead when they did not respond to touch with a platinum wire pick. The percentage of worms that were still alive was plotted against time by using SPSS (Chicago, IL) 11.0 statistical software.
Statistical analysis.
The basic method of statistical analysis of the data from our in vitro studies involved analysis of variance, as well as analysis of covariance when there was a continuous covariate such as time or titration. The dependent variable was the percentage of adherent cells or CFU, while the independent fixed factor was the treatment (CAP versus cap mutant, or CPS1 isogenic strains, etc.). In addition, the experiment number was used as a blocking factor. When the grouping variable had several levels, we made a multiple-comparison adjustment. Transformation of the dependent variable was considered, if necessary, to stabilize variance. Raw data were entered into Excel files and automatically converted to statistical packages. Analysis of variance and covariates, followed by a multiple-comparison test such as the Newman-Keuls test, was used to determine the statistical significance of differences between the control and treatment groups. A P value of <0.05 is considered to be significant.
RESULTS
Effect of 4-MU on C. neoformans adhesion.
We previously developed an in vitro C. neoformans binding assay that detects both adherent and invading yeast cells in HBMEC (7, 13). In order to test whether any hyaluronic acid is synthesized in C. neoformans and whether there is a role for hyaluronic acid in HBMEC binding, we examined the effect of 4-methylumbelliferone (4-MU) on the binding of C. neoformans to HBMEC. 4-MU is a reagent that can extract cardiolipin from hyaluronic acid synthase complex, leading to the loss of hyaluronic acid synthase activity (41). It has been demonstrated that hyaluronic acid production in Streptococcus can be blocked by 4-MU without affecting bacterial growth (up to 2 mM) (25). Initially, we did a control experiment to ensure that there was no detectable effect on C. neoformans growth (up to 6 mM 4-MU) (data not shown). We then examined the effect of 4-MU on C. neoformans association by using our in vitro binding assay. A range of concentrations of 4-MU was added to examine the consequences of the treatments. As shown in Fig. 1, the binding activity of strain B-4500FO2 (wild-type CPS1) decreased as the 4-MU concentration increased; presumably, blockage by 4-MU of the formation of hyaluronic acid resulted in a decrease in the ability to bind to HBMEC. In parallel, the binding ability of strain C559 (cps1Δ) was low, but did not differ, at all 4-MU concentrations. The results suggest that there is an intimate correlation between the hyaluronic acid content and C. neoformans binding to HBMEC in strain B-4500FO2 but no difference in strain C559.
FIG. 1.
Effect of 4-MU on C. neoformans binding activity to HBMEC. An in vitro binding assay was performed and analyzed as described in Materials and Methods. A range of concentrations of 4-MU (0, 0.5, 1.0, and 2.0 mM) was used to treat C. neoformans B-4500FO2 (solid bars) or C559 (open bars) cells for 2 h; then cells were washed with PBS three times prior to the in vitro binding assay. Statistical analysis, using linear regression, shows P values of 0.01 and 0.004 in comparison to the 0 mM group for strains B-4500FO2 and C559, respectively.
Effect of hyaluronidase treatment on the in vitro binding of C. neoformans to HBMEC.
To further test whether hyaluronic acid was involved in C. neoformans adhesion, we used a specific enzyme, hyaluronidase, to treat different C. neoformans strains. Treated yeast cells were washed extensively with PBS to remove the enzyme prior to the in vitro binding assay. The control, without hyaluronidase treatment, was assigned a value of 100% binding for each individual C. neoformans strain. The results showed that, regardless of the strain, the binding activity for HBMEC decreased in a dose-dependent manner (Fig. 2). No effect could be observed when chitinase or heparinase was used (data not shown). Thus, the results suggest that the binding of C. neoformans to HBMEC is a hyaluronic acid-dependent process.
FIG. 2.
Effect of hyaluronidase on C. neoformans binding activity to HBMEC. A titration amount of hyaluronidase (0. 0.5, 1, and 5 U/ml) was used to treat different C. neoformans strains. An in vitro binding assay was then performed. For the purpose of comparison, we assigned the no-treatment control for each individual Cryptococcus strain a binding level of 100%. Strains used were B-3501 (wild type), TYCC33 (cap59), B4476FO5 (CAP59), 602 (cap64), and TYCC38-602 (CAP64). Data, expressed as percentages of the binding activity of the inoculum, are means of triplicates. Error bars, standard deviations. Statistical analysis of covariance using each individual strain as a grouping variance and titration as a continuous variable revealed a significant linear concentration effect (P = 0.001), with no statistical difference between the strains (P > 0.05). Thus, the level of binding of C. neoformans cells to HBMEC decreased after hyaluronidase treatment.
Detection of hyaluronic acid from various C. neoformans strains.
Based on the hyaluronic acid ELISAs (Corgenix, Inc.), we have previously demonstrated that the yeast cells of the wild-type strain contain hyaluronic acid molecules, while a cps1Δ strain does not (7). To further explore the role of hyaluronic acid in the binding of C. neoformans cells to HBMEC, we examined several isogenic CAP versus capΔ strains, in addition to CPS1 wild-type and disrupted strains. CPS1 wild-type strain B-4500FO2, used as a control, contains a much stronger hyaluronic acid signal than the deletion strain C559 (cps1Δ) (Fig. 3A, lane 1 versus lane 2). The abilities of these strains to bind to HBMEC are also proportional to their hyaluronic acid contents (Fig. 3B, lane 1 versus lane 2). Encapsulated strains (CAP64 and CAP59) always have a higher binding activity (Fig. 3B, lanes 3 and 5) than their isogenic mutants (Fig. 3B, lanes 4 and 6). Interestingly, they also have higher hyaluronic acid contents (Fig. 3A, lanes 3 and 5) than their isogenic cap mutants (Fig. 3A, lanes 4 and 6). The results indicate that the HBMEC binding activities are proportional to the hyaluronic acid contents of different strains. This raises the interesting point that acapsular mutant strains have an incomplete capsule structure, which may fail to retain hyaluronic acid molecules on their surfaces, resulting in a decreased ability to bind to HBMEC. The hyaluronic acid ELISA kit used here does not detect other glucosaminoglycans, such as chitin, heparin, and chondroitin sulfate (data not shown). It is clear that C559 (cps1Δ) has the weakest hyaluronic acid signal of all the strains used. In addition, the CPS1 protein sequence shares the highest homology to known hyaluronic acid synthases (see Discussion). We concentrated on the CPS1 gene in subsequent experiments.
FIG. 3.
C. neoformans ability to bind to HBMEC and the relationship to their hyaluronic acid contents. (A) Hyaluronic acid ELISA. The hyaluronic acid molecules from the surfaces of C. neoformans cells were detected using a hyaluronic acid ELISA kit (Corgenix, Inc.). (B) An in vitro binding assay was performed to examine the ability of C. neoformans cells to associate with HBMEC. The CFU of C. neoformans cells binding to HBMEC in vitro was analyzed. Columns in both panels: 1, strain B-4500FO2 (wild-type CPS1); 2, strain C559 (cps1Δ); 3, strain TYCC38-602 (wild-type CAP64); 4, strain 602 (cap64); 5, strain B4476FO5 (wild-type CAP59); 6, strain TYCC33 (cap59Δ).
Pathogenesis of CPS1 by a C. elegans longevity assay.
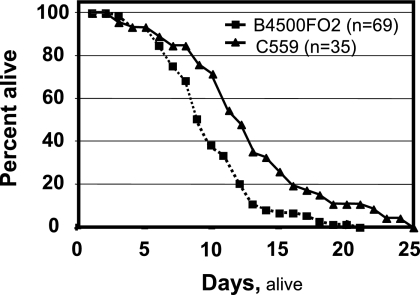
C559 (cps1Δ) is a temperature-sensitive mutant strain. It does not grow well at 37°C, preventing study of its pathogenesis in a mouse model. On the other hand, an in vivo pathogenesis assay for C. neoformans has been established, based on the yeast killing rate on C. elegans (28). This assay is particularly useful for the temperature-sensitive C559 (cps1Δ) study, because C. elegans grows and multiplies at room temperature. Here worms were exposed to the yeast strains after they had reached the L4 developmental stage, and their longevity reflects, in part, the virulence of the yeast strains. The survival curves plotted in Fig. 4 show that the nematodes had a longer life span when feeding on C. neoformans cps1Δ lawn cells. The 50% lethal time (time for half of the worms to die) was calculated with SPSS 11.0 statistical software. The average life span is greater when worms are exposed to C559 (cps1Δ) (∼12.8 days) than when they are exposed to B-4500FO2 (wild-type CPS1) (∼8.8 days) (P < 0.026). Our results demonstrate that CPS1 deletion decreases the virulence of yeast in the C. elegans animal model (Fig. 4).
FIG. 4.
C. neoformans pathogenesis assay using a C. elegans in vivo animal model. Survivorship curves of nematodes in the presence of yeast strains B-4500FO2 (▪) and C559 (▴).
Expression of CPS1 in Saccharomyces cerevisiae.
In order to demonstrate the CPS1 gene function, we subcloned and expressed C. neoformans CPS1 cDNA in S. cerevisiae. Hyaluronic acid biosynthesis requires hyaluronic acid synthase (presumably Cps1p) and two precursor enzymes, UDPG DH and glucosamine-6-phosphate acetyltransferase, to provide the substrates. The baker's yeast S. cerevisiae contains only the gene encoding glucosamine-6-phosphate acetyltransferase. We therefore coexpressed the genes encoding UDPG DH and Cps1p in baker's yeast to build the C. neoformans hyaluronic acid biosynthesis pathway.
The cDNAs of CPS1 and UDPGDH were isolated and subcloned into the yeast expression vectors YGp181 (selection marker LEU2) and YGp123 (selection marker Trp1) to obtain YGp181-CPS1 and YGp123-UDPG.DH, respectively. These vectors are all under the control of a strong Gal1,10 inducible promoter. The expressed proteins were detected by an anti-HA antibody on the protein blot (Fig. 5A). The result showed that C. neoformans UDPGDH, CPS1, and both genes together could be expressed in S. cerevisiae strain B2168, a protease-deficient strain (42). Hyaluronic acid synthase is a membrane protein, and its product, hyaluronic acid, extrudes outside the plasma membrane during hyaluronic acid biosynthesis. We detected the hyaluronic acid contents on the surfaces of S. cerevisiae cells expressing C. neoformans CPS1 by using the hyaluronic acid ELISA kit. Our results showed that only coexpression of CPS1 and UDPGDH in S. cerevisiae displayed a high level of hyaluronic acid (Fig. 5B). The result strongly supported the hypothesis that the CPS1 gene encodes hyaluronic acid synthase.
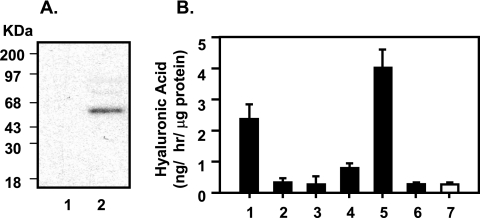
FIG. 5.
Expression of C. neoformans UDPG DH and CPS1 cDNA in S. cerevisiae and detection of their ability to synthesize hyaluronic acid. C. neoformans UDPG DH and CPS1 cDNA were subcloned into the S. cerevisiae expression vectors YGp181 and YGp123 with an HA tag, respectively. The cloned cDNA was induced by galactose. (A) Protein blots show the expected HA-tagged proteins: expressed UDPG DH (lane a), Cps1p (lane b), and both (lane c) from S. cerevisiae. The expressed proteins were detected by an antibody against the HA tag. (B) The hyaluronic acid contents from induced yeast cells were subjected to the hyaluronic acid assay (Corgenix hyaluronic acid kits). Lane 1, buffer only; lane 2, standard control with 50 ng hyaluronic acid (no yeast); lane 3, hyaluronic acid contents from coexpression of UDPG DH and CPS1 cDNA in S. cerevisiae; lane 4, hyaluronic acid contents from CPS1 expression alone in S. cerevisiae; lane 5, expression of UDPG DH cDNA alone in S. cerevisiae. Each value is the average of triplicate experiments.
Characterization of hyaluronic acid synthase activities from E. coli-purified Cps1p.
The Streptococcus HAS gene has been expressed in E. coli with hyaluronic acid synthase activity. The C-terminal His6 tag of the recombinant hyaluronic acid synthase protein does not affect its enzymatic activity (40). We used a similar expression system (the pET22b vector) to express C. neoformans CPS1 cDNA with a His6 tag to facilitate its purification. In parallel, E. coli cells with the pET22b vector only were used as a control. The expressed cells were lysed, extracted with n-dodecyl β-maltoside (0.5%), and subjected to nickel-nitrilotriacetic acid affinity purification. The purified fraction was detected with an anti-His6 monoclonal antibody. As shown in Fig. 6A, a single band of ∼55 kDa was found in CPS1-expressing cells but was absent in the control. The affinity-purified Cps1p-His6 fraction was subjected to a hyaluronic acid synthase enzymatic assay using UDP-GlcUA and UDP-GlcNAc as the substrates. After a 2-h reaction, the synthesized molecule was measured with the hyaluronic acid ELISA kit (Corgenix, Inc.). The results in Fig. 6B show that E. coli-purified Cps1p was able to synthesize hyaluronic acid in the presence of substrates (lane 1) and that the reaction could be enhanced in the presence of cardiolipin, which is known as a cofactor of hyaluronic acid synthase (lane 5). Only background readings were observed under control conditions, such as the use of one substrate only (Fig. 6B, lanes 2 and 3), the use of EDTA to chelate magnesium ions (lane 4), hyaluronidase treatment (lane 6), and the control derived from extracts containing vector only without the CPS1 gene (lane 7). Thus, E. coli-expressed Cps1p possesses hyaluronic acid synthase activity.
FIG. 6.
Expression and hyaluronic synthase activity of C. neoformans CPS1 cDNA in E. coli. (A) Approximately 5 μg of total protein purified from cells transformed with either vector pET22b only (lane 1) or pET22b-CPS1 (lane 2) was probed with an anti-His6 monoclonal antibody. A single band of ∼54 kDa was identified in lane 2. (B) A standard hyaluronic acid synthase assay mixture contains ∼2.5 μg purified Cps1 protein from E. coli in a 100-μl solution of 50 mM Tris (pH 8), 10 mM MgCl2, 1 mM dithiothreitol, and 0.5 mM UDP-GlcUA and UDP-GlcNAc. Lane 1 shows results for a standard reaction. Lanes 2 to 7 are also based on the standard hyaluronic acid synthase assay with the following modifications: lane 2, absence of one substrate, UDP-GlcUA; lane 3, absence of the other substrate, UDP-GlcNAc; lane 4, addition of 25 mM EDTA; lane 5, addition of 2 mM cardiolipin; lane 6, treatment with 2 U of hyaluronidase; lane 7, a standard reaction using the protein fraction from the vector sample (no CPS1 insert) as the control.
Identification of C. neoformans hyaluronic acid by FACE analysis.
One fundamental question here is whether hyaluronic acid is present in C. neoformans. In addition to the hyaluronic acid ELISA kit (Corgenix, Inc.), an alternative hyaluronic acid detection method was used to confirm our observation. To identify hyaluronic acid molecules among the bulky capsule polysaccharides is a challenging task. However, a recently developed advanced methodology, FACE (6, 33), allowed us to detect hyaluronic acid in a rather straightforward way. The unique feature of FACE is the use of specific enzymes to cleave glycosaminoglycan into small products, usually disaccharide units. Each cleavage exposes a new reducing terminus that is fluoro-tagged by reductive amination with 2-aminoacridone. The tagged products are then displayed by electrophoresis, identified by their characteristic migration, and quantitated by their molar fluorescence. This is a very sensitive method allowing detection in the nanogram range. More importantly, it can leave the bulk of C. neoformans capsule polysaccharides invisible (large molecules, not fluoro-tagged) and specifically detect hyaluronic acid disaccharide units (small molecules, fluoro-tagged) when present (6). To examine C. neoformans samples, a partial purification step is necessary to remove small sugar molecules derived from the culture medium (see Materials and Methods).
The partially fractionated C. neoformans capsule polysaccharides and the pooled fractions were subjected to FACE analysis (Fig. 7A). Approximately 10 μg (wet weight) per sample from various fractions was used for each experiment. As a positive control, 5 μg pure hyaluronic acid was treated in parallel to produce the hyaluronic acid disaccharide unit (ΔdiHA) as a marker (Fig. 7A, lane 1). No corresponding band could be clearly observed in a crude capsule preparation, and some nonspecific bands may be derived from reducing sugars from the culture medium (lanes 2 and 5); however, after being enriched by the anion-exchange resin, the FACE gel showed a ΔdiHA band in the Dowex 1 (2X)-eluted fraction of B-4500FO2 (wild-type CPS1 strain) (lane 3). The enzyme-buffer mixture was used as a negative control (lane 4). The result demonstrated that hyaluronic acid was present in the C. neoformans capsule polysaccharide fraction. In parallel, no signal could be detected in the corresponding fractions from cps1Δ capsule polysaccharide (lanes 5 and 6).
C. neoformans capsular components were extracted from B-4500FO2 (wild-type CPS1 strain) and fractionated by HPLC gel filtration. The hyaluronic acid contents from different fractions were detected with the hyaluronic acid ELISA kit (Fig. 7B, top panel). Hyaluronic acid molecules were detected from several fractions. The highest molecular size (void volume) is around 107 Da, corresponding to ∼30,000 disaccharide subunits of approximately 3 μm. However, this high-molecular-weight fraction is most likely derived from hyaluronic acid complex, in which hyaluronic acid molecules are associated with each other or with other components, instead of a single long molecule (see Fig. 8). The peak of the hyaluronic acid signal was in the range of ∼105 Da, corresponding to ∼300 disaccharide subunits (approximately 30 nm long). Little hyaluronic acid (or a background reading) was detected below the size of 103 Da (Fig. 7B).
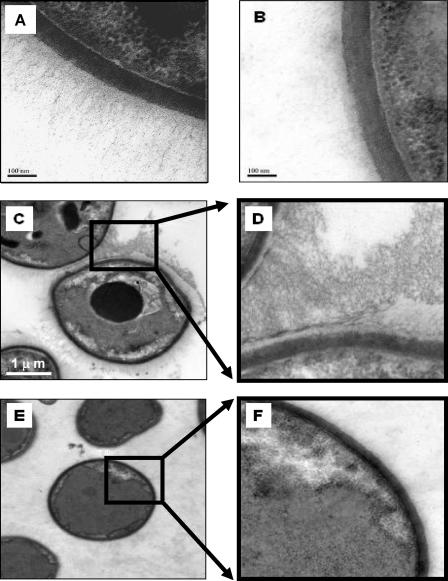
FIG. 8.
Staining of hyaluronic acid fibrous structures outside the C. neoformans cell wall. Yeast strain B-4500FO2 (wild-type CPS1) and its isogenic cps1Δ strain (C559) were probed with biotinylated hyaluronic acid-binding protein and a streptavidin-HRP conjugate. Treated samples were subjected to TEM studies to determine their morphological features. (A) Strain B-4500FO2 shows fibrous structures radiating from the cell wall. Bar, 100 nm. (B) Strain C559 shows only a light patch background near the cell wall (no fibrous structure). Bar, 100 nm. (C) Strain B-4500FO2 cells show a high-density fibrous structure between cells. Bar, 1 μm. (D) Higher magnification of boxed area in panel C. (E) In contrast, strain C559 cells do not show any fibrous structure. (F) Higher magnification of boxed area in panel E.
Staining of hyaluronic acid fibrous structures outside the C. neoformans cell wall.
The CPS1 gene is predicted to encode a hyaluronic acid synthase in C. neoformans, as indicated in Fig. 5 and 6. Since hyaluronic acid from other species is accumulated extracellularly, it would be of interest to locate hyaluronic acid on C. neoformans cells. We therefore used biotinylated hyaluronic acid-binding protein to locate hyaluronic acid molecules and then probed with a streptavidin-HRP conjugate. The typical morphology of the wild-type CPS1 strain (B-4500FO2), with numerous fibrous structures radiating from the cell wall, is displayed in Fig. 8A. However, no such structure was observed adjacent to the walls of the cps1Δ cells (Fig. 8B). Neither the HRP conjugate nor biotinylated marker proteins alone showed any nonspecific staining on HBMEC in our control experiments (data not shown). More TEM images were used to further illustrate the differences between the wild-type CPS1 and cps1Δ cells. In Fig. 8C, one can observe fibrous structures, mostly 300 to 500 nm long, surrounding the wild-type CPS1 cells. Figure 8D, a higher magnification of part of Fig. 8C, shows the area between the capsules of two adjacent yeast cells, presented as interlocking fiber structures. In limited regions, the hyaluronic acid molecules can extend longer than 1 μm, corresponding to ∼1,000 disaccharide units. In parallel, no obvious fibrous structure was observed in the cps1Δ strain (Fig. 8E and F); only some lightly shadowed background (presumably other capsular components) appeared in the corresponding regions. These results (Fig. 8), together with the result presented in Fig. 3, allow us to conclude that C. neoformans hyaluronic acid, presumably the product synthesized by CPS1, extends from the cell wall to the exterior part of the yeast cells. The presence of hyaluronic acid, therefore, appears to play a major role in the binding of C. neoformans yeast cells to HBMEC in vitro (Fig. 1, 2, and 3) and is important for virulence in C. elegans (Fig. 4).
DISCUSSION
Capsular polysaccharides are a major virulence factor of C. neoformans. The genetic approach to the isolation and characterization of capsule-deficient strains revealed several important genes in capsule biosynthetic pathways, encoding either precursors or molecules associated with the transport of essential components. However, it is still not clear whether there is any capsule component on the yeast cell surface that plays a role as an adhesion molecule during invasion. One direct way to identify potential adhesion molecules would be to search for candidate genes based on their sequences from genomic databases. This can be verified by deleting the candidate genes and examining the phenotypic consequences of the deletions. C. neoformans CPS1 was the one gene identified through this reverse genetic approach (7).
The C. neoformans Cps1 protein was homologous to Streptococcus pneumoniae type 3 β-glycosyltransferase CAP3B (39.4% similarity), and the two proteins displayed similar patterns in the hydrophilicity plot. The type 3 synthase from S. pneumoniae is a β-glycosyltransferase that functions in the assemblage of the type 3 polysaccharide [3)-β-d-glucuronic acid-(1→4)-β-d-glucose-(1→] (7). A further database search using NCBI BLAST alignment showed that the Cps1 protein sequence aligned with COG1215, a group of β-glycosyltransferases. The first 50 highest homologies are summarized in Fig. 9A in accordance with their annotated functions (36 proteins). The hypothetical proteins, or proteins with unknown function, were not included (14 proteins). Of the proteins with annotated functions, 16 out of 36 (or 44%) are hyaluronic acid synthases (Fig. 9A). Note that both hyaluronic acid synthase and chitin synthase are N-acetylglucosamine transferases, because they use N-acetylglucosamine as the substrate, and that β-glycosyltransferase is a generic term that includes all of these enzymes. The sequence alignments have identified invariant residues within the conserved multiple transmembrane domains and the predicted intracellular loop, the putative binding site of hyaluronic acid synthase (Fig. 9B and C). These motifs are the structural features of the hyaluronic acid synthase proteins (23, 40, 43). In contrast, multiple transmembrane domains are usually not found in chitin synthase. In general, the hyaluronic acid synthase family contains six transmembrane domains, and the C-terminal transmembrane domain is less conserved. Indeed, C. neoformans Cps1 protein also contains five conserved transmembrane domains, and the sixth one, at the C-terminal end, is less conserved (Fig. 9C) (43). Finally, the amino acid sequence of the encoded Cps1 protein shares the highest homology (28% identity and 42% similarity) with the Streptococcus hyaluronic acid synthase, HasA (Fig. 9D). Based on this sequence information, C. neoformans CPS1 most likely encodes a hyaluronic acid synthase. The questions remaining now are whether the sequence homology represents its functional conservation and whether CPS1 plays any role in the pathogenesis of C. neoformans.
FIG. 9.
Molecular features of the Cryptococcus CPS1 gene, encoding a putative hyaluronic acid synthase. (A) The number of homologues to CPS1 is listed. Based on their annotated functions, hyaluronic acid (HA) synthase has the highest count (16/36 [44%]), followed by other enzymes as listed. (B) Predicted protein sequence of CPS1 deduced from the cDNA sequence. The putative transmembrane domains are boldfaced and underlined. The conservative GDD motif (substrate site), presumably involving the metal ion and UDP-sugar binding, is shaded. (C) Diagram of the predicted protein sequence shows the five conserved transmembrane domains (stippled boxes 1 to 5) and a large intracellular loop containing the hyaluronic acid synthase substrate sites. The lightly stippled box 6 shows the less conserved transmembrane domain. Asterisks above the diagram indicate the conserved amino acid residues among hyaluronic acid synthases. (D) Sequence homology comparison by the ClusterLw program shows that Cryptococcus CPS1 is evolutionarily closest to Streptococcus hyaluronic acid synthase (HAS). Mus, mouse; Equ, horse; Hum, human; Crypto, Cryptococcus; Strepto, Streptococcus.
In this report, we first demonstrated that treatment of yeast with 4-MU, a specific inhibitor of hyaluronic acid synthase, resulted in a reduction of the level of C. neoformans binding to HBMEC (Fig. 1). The ability of the hyaluronidase-treated yeast cells to bind to HBMEC was also reduced (Fig. 2). Furthermore, the hyaluronic acid contents of different yeast strains are proportional to their abilities to bind to HBMEC (Fig. 3). These data suggested the existence of C. neoformans hyaluronic acid on the yeast cell surface.
These results support the hypothesis that the function of CPS1 is closely associated with the biosynthesis of hyaluronic acid in C. neoformans cells, i.e., either CPS1 encodes the hyaluronic acid synthase or it enhances hyaluronic acid biosynthesis and/or accumulation. In either case, the presence of hyaluronic acid molecules is closely related to the ability of C. neoformans to bind to HBMEC in vitro and its pathogenicity in C. elegans (Fig. 3 and 4). It is known that capsule genes are required for capsule formation and virulence. Mutation or deletion of any capsule gene significantly reduces the size of the C. neoformans capsule (9, 10, 11). In this scenario, an incomplete capsule structure may fail to retain hyaluronic acid molecules on its surface, resulting in decreased ability to bind to HBMEC, as shown in Fig. 3. Since C559 (cps1Δ) is a temperature-sensitive mutant strain, we used a C. elegans model of the in vivo pathogenesis assay for C. neoformans. Since C. elegans grows at room temperature, it is suitable for characterization of temperature-sensitive mutants such as the cps1Δ strain. The studies clearly demonstrated that the wild-type CPS1 strain is more virulent than the cps1Δ strain in this experimental animal model.
To further determine whether C. neoformans CPS1 encodes hyaluronic acid synthase, we expressed CPS1 in S. cerevisiae (Fig. 5) and in E. coli (Fig. 6). We were able to detect a hyaluronic acid signal on the baker's yeast cell surface, indicating that the hyaluronic acid biosynthesis pathway can be constituted in baker's yeast. This result is interesting in view of the fact that S. cerevisiae is a nonpathogenic yeast. It exhibits a very clean background in terms of virulence and hyaluronic acid synthesis. Another line of study is that recombinant Cps1p expressed in E. coli possesses the ability to incorporate UDP-GlcUA and UDP-GlcNAc into a product, which can be detected by the hyaluronic acid ELISA kit (Fig. 6). Taking these results together, our findings strongly support the idea that CPS1 is the C. neoformans hyaluronic acid synthase gene, which plays a major role in the binding of C. neoformans to HBMEC. The importance of CPS1 for polysaccharide metabolism and potential virulence warrants further investigation. On the other hand, one should not rule out the possibility that the binding of C. neoformans to HBMEC is not directly mediated by hyaluronic acid. Instead, lack of hyaluronic acid may induce some changes in the fungal cell, resulting in altered ability to bind to HBMEC. Identification of a hyaluronic acid receptor on HBMEC would solve this issue.
We have also characterized the product encoded by CPS1, presumably hyaluronic acid, via a biochemical approach. We used FACE analysis to demonstrate the presence of hyaluronic acid isolated from C. neoformans capsule polysaccharides. Initially, we used trichloroacetic acid to precipitate capsule polysaccharides from C. neoformans in the presence of ethanol. The resultant materials were further purified through a Dowex 1 (2X) column and eluted at 0.5 to 0.65 M NaCl fractions. This column chromatographic pattern of the C. neoformans hyaluronic acid is similar to that of bovine vitreous-body hyaluronate (37). FACE has been used to detect hyaluronic acid from tissue samples, such as cartilage and heart valve (4, 5, 20), but it has never been applied to the C. neoformans capsule polysaccharides. Our studies reveal that FACE can be used to investigate another dimension of C. neoformans capsule structures, complemented by conventional methodologies. It is difficult to accurately measure the amount of hyaluronic acid relative to other capsule components. However, the recovery of purified hyaluronic acid from FACE analysis accounts for less than 1% of the total crude capsule preparation, suggesting that hyaluronic acid is a minor component in C. neoformans. An HPLC gel filtration study indicated that C. neoformans hyaluronic acid could consist of very high molecular weight molecules or complexes, up to 107 Da. The peak of hyaluronic acid content was in the range of ∼105 Da, corresponding to ∼300 disaccharide subunits, or a length of approximately 30 nm. Our TEM study (Fig. 8) revealed that yeast hyaluronic acid molecules extend from 0.3 to ∼1 μm, corresponding to 300 to 1,000 disaccharide units. All these hyaluronic acid molecules could be detected in the wild type CPS1 strain but not in the cps1Δ strain. Taken together, C. neoformans CPS1 encodes a hyaluronic acid synthase, which generates a long hyaluronic acid molecule.
Hyaluronic acid not only is an important structural component in vertebrates but also plays an important role in many biological processes. Recent observations indicated that the association of hyaluronic acid with cell surface receptors such as CD44 and RHAMM (receptor for hyaluronic acid-mediated motility) influences cellular proliferation, differentiation, migration, and adhesion of eukaryotic cells (29, 30). In addition, some previous studies revealed that bacterial hyaluronic acid is important for pathogenesis, i.e., it protects the microbes from host defenses, including complement and phagocytosis. Using genetically defined acapsular mutants of Streptococcus pyogenes, an increase in phagocytosis of bacteria by granulocytes and a decrease in virulence in mice were reported (44). Loss of the hyaluronic acid capsule in bacteria typically reduces virulence >90% (36). Transforming Streptococcus hasA (the hyaluronic acid synthase gene) back into either acapsular Streptococcus strains or Enterococcus faecalis conferred the ability to synthesize hyaluronic acid so as to form a capsule and regain virulence (16, 17). Pasteurella multocida uses its surface hyaluronic acid as a ligand to bind to the target cells of the host (34). Other studies have shown that group A streptococci also use their hyaluronic acid as a ligand to CD44 on human keratinocytes, suggesting that the Streptococcus hyaluronic acid plays a direct role in microbial adhesion. This interaction between hyaluronic acid and host CD44 may lead to the transduction of signals that participate in cytoskeletal rearrangement during pathogen entry (15, 38). We have previously observed that C. neoformans was able to induce significant morphological alterations and cytoskeletal reorganization in HBMEC (13). Plausibly, this response might take place via interaction between hyaluronic acid and the host receptor on HBMEC.
In summary, our current data demonstrate that C. neoformans CPS1 encodes hyaluronic acid synthase. Hyaluronic acid on the yeast cell surface may act as a ligand that interacts with a putative receptor on HBMEC during the entry process. This is the first demonstration that hyaluronic acid in yeast might function as a ligand facilitating adhesion to host cells, as is the case for Streptococus or Enterococcus. An understanding of the yeast-host interaction could serve as an important starting point in dissecting the complicated mechanism of endothelial cell invasion by C. neoformans that causes meningoencephalitis. We are currently investigating the potential receptor of C. neoformans hyaluronic acid, which would allow us to determine the yeast-host interaction triggering yeast entry into HBMEC.
Acknowledgments
We thank A. Tang for critical reading.
This project is financially supported by Public Health Service grants R01-NS047599 (to A.J.) and R01-AI40635 (to S.-H.H.).
Footnotes
Published ahead of print on 1 June 2007.
REFERENCES
- 1.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J. Infect. Dis. 180:915-919. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Peled, M., C. L. Griffith, and T. L. Doering. 2001. Functional cloning and characterization of a UDP-glucuronic acid decarboxylase: the pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc. Natl. Acad. Sci. USA 98:12003-12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bracke, J. W., and K. Thacker. 14 May 1985. Hyaluronic acid from bacterial culture. U.S. patent 4,517,295.
- 3.Buchanan, K. L., and J. W. Murphy. 1998. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 4:71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabro, A., M. Benavides, M. Tammi, V. C. Hascall, and R. J. Midura. 2000. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 10:273-281. [DOI] [PubMed] [Google Scholar]
- 5.Calabro, A., V. C. Hascall, and R. J. Midura. 2000. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology 10:283-293. [DOI] [PubMed] [Google Scholar]
- 6.Calabro, A., R. Midura, A. Wang, L. West, A. Plaas, and V. C. Hascall. 2001. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage 9(Suppl. A):S16-S22. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y. C., A. Jong, S. Huang, P. Zerfas, and K. J. Kwon-Chung. 2006. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans. Infect. Immun. 74:3930-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. C., and K. J. Kwon-Chung. 1998. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 66:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. C., and K. J. Kwon-Chung. 1999. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 181:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 1996. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 64:1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y. C., M. F. Stins, M. J. McCaffery, G. F. Miller, D. R. Pare, T. Dam, M. Paul-Satyaseela, K. S. Kim, and K. J. Kwon-Chung. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, S. H., M. F. Stins, S. H. Huang, Y. H. Chen, K. J. Kwon-Chung, Y. Chang, K. S. Kim, K. Suzuki, and A. Y. Jong. 2003. Crytptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J. Med. Microbiol. 52:961-970. [DOI] [PubMed] [Google Scholar]
- 14.Cherniak, R., and J. B. Sundstrom. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect. Immun. 62:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cywes, C., and M. R. Wessels. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648-652. [DOI] [PubMed] [Google Scholar]
- 16.DeAngelis, P. L., J. Papaconstantinou, and P. H. Weigel. 1993. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J. Biol. Chem. 268:14568-14571. [PubMed] [Google Scholar]
- 17.DeAngelis, P. L., J. Papaconstantinou, and P. H. Weigel. 1993. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J. Biol. Chem. 268:19181-19184. [PubMed] [Google Scholar]
- 18.Dillard, J. P., M. W. Vandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottfredsson, M., and J. R. Perfect. 2000. Fungal meningitis. Semin. Neurol. 20:307-322. [DOI] [PubMed] [Google Scholar]
- 20.Grande-Allen, K. J., B. P. Griffin, A. Calabro, N. B. Ratliff, D. M. Cosgrove III, and I. Vesely. 2001. Myxomatous mitral valve chordae. II. Selective elevation of glycosaminoglycan content. J. Heart Valve Dis. 10:325-332. [PubMed] [Google Scholar]
- 21.Hamilton, A. J., and J. Goodley. 1996. Virulence factors of Cryptococcus neoformans. Curr. Top. Med. Mycol. 7:19-42. [PubMed] [Google Scholar]
- 22.Huang, S. H., and A. Y. Jong. 2001. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell. Microbiol. 3:277-287. [DOI] [PubMed] [Google Scholar]
- 23.Itano, N., and K. Kimata. 1996. Molecular cloning of human hyaluronan synthase. Biochem. Biophys. Res. Commun. 222:816-820. [DOI] [PubMed] [Google Scholar]
- 24.Jong, A. Y., M. F. Stins, S. H. Huang, S. H. Chen, and K. S. Kim. 2001. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 69:4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakizaki, I., K. Takagaki, Y. Endo, D. Kudo, H. Ikeya, T. Miyoshi, B. A. Baggenstoss, V. L. Tlapak-Simmons, K. Kumari, A. Nakane, P. H. Weigel, and M. Endo. 2002. Inhibition of hyaluronan synthesis in Streptococcus equi FM100 by 4-methylumbelliferone. Eur. J. Biochem. 269:5066-5075. [DOI] [PubMed] [Google Scholar]
- 26.Keenleyside, W. J., and C. Whitfield. 1996. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J. Biol. Chem. 271:28581-28592. [DOI] [PubMed] [Google Scholar]
- 27.Moyrand, F., B. Klaproth, U. Himmelreich, F. Dromer, and G. Janbon. 2002. Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol. Microbiol. 45:837-849. [DOI] [PubMed] [Google Scholar]
- 28.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura, H., and H. Ozawa. 1996. Immunolocalization of CD44 and the ERM family in bone cells of mouse tibiae. J. Bone Miner. Res. 11:1715-1722. [DOI] [PubMed] [Google Scholar]
- 30.Oliferenko, S., I. Kaverina, J. V. Small, and L. A. Huber. 2000. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J. Cell Biol. 148:1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. N. Am. 16:837-874. [DOI] [PubMed] [Google Scholar]
- 32.Perfect, J. R., B. Wong, Y. C. Chang, K. J. Kwon-Chung, and P. R. Williamson. 1998. Cryptococcus neoformans: virulence and host defences. Med. Mycol. 36(Suppl. 1):79-86. [PubMed] [Google Scholar]
- 33.Plaas, A. H., L. West, R. J. Midura, and V. C. Hascall. 2001. Disaccharide composition of hyaluronan and chondroitin/dermatan sulfate. Analysis with fluorophore-assisted carbohydrate electrophoresis. Methods Mol. Biol. 171:117-128. [DOI] [PubMed] [Google Scholar]
- 34.Pruimboom, I. M., R. B. Rimler, and M. R. Ackermann. 1999. Enhanced adhesion of Pasteurella multocida to cultured turkey peripheral blood monocytes. Infect. Immun. 67:1292-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22:11-28. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, K. H., E. Gunther, and H. S. Courtney. 1996. Expression of both M protein and hyaluronic acid capsule by group A streptococcal strains results in a high virulence for chicken embryos. Med. Microbiol. Immunol. (Berlin) 184:169-173. [DOI] [PubMed] [Google Scholar]
- 37.Schmut, O., and H. Hofmann. 1981. A method for the purification of bovine vitreous body hyaluronic acid. Biochim. Biophys. Acta 673:192-196. [PubMed] [Google Scholar]
- 38.Schrager, H. M., S. Alberti, C. Cywes, G. J. Dougherty, and M. R. Wessels. 1998. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Investig. 101:1708-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer, U., H. Liu, and T. L. Doering. 2003. An α-1,3-mannosyltransferase of Cryptococcus neoformans. J. Biol. Chem. 278:47724-47730. [DOI] [PubMed] [Google Scholar]
- 40.Tlapak-Simmons, V. L., B. A. Baggenstoss, K. Kumari, C. Heldermon, and P. H. Weigel. 1999. Kinetic characterization of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis. J. Biol. Chem. 274:4246-4253. [DOI] [PubMed] [Google Scholar]
- 41.Tlapak-Simmons, V. L., E. S. Kempner, B. A. Baggenstoss, and P. H. Weigel. 1998. The active streptococcal hyaluronan synthases (HASs) contain a single HAS monomer and multiple cardiolipin molecules. J. Biol. Chem. 273:26100-26109. [DOI] [PubMed] [Google Scholar]
- 42.Wang, B., L. Feng, Y. Hu, S. H. Huang, C. P. Reynolds, L. Wu, and A. Y. Jong. 1999. The essential role of Saccharomyces cerevisiae CDC6 nucleotide-binding site in cell growth, DNA synthesis, and Orc1 association. J. Biol. Chem. 274:8291-8298. [DOI] [PubMed] [Google Scholar]
- 43.Weigel, P. H., V. C. Hascall, and M. Tammi. 1997. Hyaluronan synthases. J. Biol. Chem. 272:13997-14000. [DOI] [PubMed] [Google Scholar]
- 44.Wessels, M. R., J. B. Goldberg, A. E. Moses, and T. J. DiCesare. 1994. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect. Immun. 62:433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills, E. A., I. S. Roberts, P. M. Del, J. Rivera, A. Casadevall, G. M. Cox, and J. R. Perfect. 2001. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol. Microbiol. 40:610-620. [DOI] [PubMed] [Google Scholar]