Abstract
Lithium (Li+) affects the physiology of cells from a broad range of organisms including plants and both vertebrate and invertebrate animals. Although its effects result presumably from changes in gene expression elicited by its interaction with intracellular signal transduction pathways, the molecular mechanisms of Li+ action are not well understood. The biflagellate green alga Chlamydomonas reinhardtii is an ideal genetic model for the integration of the effects on Li+ on signal transduction, gene expression, and aspects of flagellar biogenesis. Li+ causes C. reinhardtii flagella to elongate to ∼1.4 times their normal length and blocks flagellar motility (S. Nakamura, H. Tabino, and M. K. Kojima, Cell Struct. Funct. 12:369-374, 1987). We report here that Li+ treatment increases the abundance of several flagellar mRNAs, including α- and β-tubulin and pcf3-21. Li+-induced flagellar gene expression occurs in cells pretreated with cycloheximide, suggesting that the abundance change is a response that does not require new protein synthesis. Deletion analysis of the flagellar α1-tubulin gene promoter showed that sequences necessary for Li+-induced expression differed from those for acid shock induction and contain a consensus binding site for CREB/ATF and AP-1 transcription factors. These studies suggest potential promoter elements, candidate factors, and signal transduction pathways that may coordinate the C. reinhardtii cellular response to Li+.
Cilia and flagella are motile organelles used for the clearance of fluids by lung and oviduct tissue and for swimming by unicellular eukaryotes and sperm. Modified cilia also aid in the transduction of light, chemical, and mechanical signals by sensory epithelial cells. Recently, defects in ciliary structure and function have been linked to several human diseases and syndromes such as polycystic kidney disease, obesity, and diabetes (33, 34). Indeed, cilia and the primary cilium found on almost every vertebrate cell may be fundamental to sensing and transducing information from the extracellular environment into biochemical signals that affect cell behavior (44).
The green alga Chlamydomonas reinhardtii is central to research in this area (38). Characterization of several C. reinhardtii mutants has led to the identification of components of intraflagellar transport (IFT), the mechanism by which cilia and flagella are assembled and disassembled (3, 20, 30, 33, 47, 50). During its life cycle, C. reinhardtii alters the lengths of its flagella, disassembling the subunits, resorbing them in preparation for mitosis and meiosis, and regrowing them after the completion of these processes or after they are shed in response to extracellular cues (12). Several chemicals also alter the lengths of C. reinhardtii flagella: treatment with isobutylmethyl xanthine, sodium pyrophosphate, or low Ca2+/high Na+ concentrations causes flagella to shorten (22, 23, 32, 43, 45), whereas treatment with Li+ causes flagella to elongate beyond their normal length (28, 48, 50). Presumably, IFT shuttles flagellar protein subunits up and down the flagellum during both naturally occurring and chemically induced assembly and disassembly.
Changes in flagellar length are correlated with changes in the expression of a set of genes, many encoding flagellar protein subunits, collectively referred to as “the flagellar genes.” When flagella regenerate after loss, a large specific set of flagellar RNAs increases transiently (42) in a response regulated both transcriptionally (18) and posttranscriptionally (2). When flagella shorten during isobutylmethyl xanthine treatment, abundances of several flagellar RNAs decrease to below basal expression levels; when flagella elongate after release from treatment, those abundances rebound (23). Little is known about correlations between flagellar elongation and gene expression changes in response to Li+ treatment. Recently, Wilson and Lefebvre (50) showed that RNAs of two flagellar genes, RSP3 and PF20, decrease in abundance when flagella elongate during Li+ treatment, but several questions remain about the correlation of flagellar RNA abundance changes with changes in flagellar length. Unknowns include the kinetics of the abundance changes, their source (transcriptional, posttranscriptional, or a combination of mechanisms), the regulatory sequences involved, and the identities of the genes regulated, i.e., whether the entire set or only a subset of flagellar genes is coordinately up- or down-regulated in response to changes in flagellar length.
Aside from its effects on C. reinhardtii flagellar lengths, Li+ affects several signal transduction pathways in vertebrate and invertebrate cells and plants (51). One of the best-known effects is in the therapeutic control of bipolar disorder in humans (15). Although several pathways are implicated in humans and other systems, the signaling and gene regulatory mechanisms through which Li+ exerts its effects are still not yet fully understood (13, 14, 25).
We report here studies on Li+-induced flagellar elongation and the increases in abundance of three flagellar mRNAs encoding α1- and β2-tubulin and pcf3-21 in response to Li+. Their induction is a primary response to the addition of Li+ that does not require protein synthesis. In addition, we have identified regions of the α1-tubulin gene promoter that are necessary for its induction by Li+ and are distinct from promoter sites previously shown to be required for gene induction during flagellar regeneration after acid shock (35).
MATERIALS AND METHODS
Cell cultures.
The C. reinhardtii arg7 strain (CC-1931) and strain 137c (CC-124) were obtained from E. Harris at the C. reinhardtii Genetics Center (http://www.chlamy.org). Cells were grown in liquid medium I (39), with continuous aeration, at 25°C on a cycle of 14 h of light/10 h of dark. For growth of the arg7 strain, l-arginine (50 μg/ml; Sigma) was added to medium I (12). For promoter mapping studies, pooled and individual arg7::ARG7 cell lines were grown in medium I. Cell cultures were grown to a density of 1 ×106 to 4 ×106 cells/ml. Cell density was determined with a Neubauer counting chamber (Clay-Adams, Parsippany, NJ).
Cell treatments and flagellar length measurements.
Cells were collected by centrifugation (1,000 × g for 5 min), resuspended to 2 ×107 to 4 ×107 cells/ml in fresh medium I, and allowed to recover from collection for 2 h at room temperature with gentle mixing at ∼100 rpm on a rotary platform shaker. Lithium was then added from a 5 M LiCl (Sigma) aqueous stock to a final concentration of 50 mM. Control treatments of 50 mM NaCl, KCl, or Li acetate were made by dilution from 5 M, 1 M, and 1 M stocks, respectively. Cycloheximide (Sigma) was added from a 2-mg/ml aqueous stock to a final concentration of 10 μg/ml, which blocks >95% of protein synthesis in C. reinhardtii (2).
Flagella on cells fixed at the indicated times by the addition of an equal volume of 2% glutaraldehyde were measured with an ocular micrometer mounted on a Zeiss phase-contrast light microscope. Differential interference contrast images of bulbed flagella were produced with a Zeiss Axioplan II microscope using 63× or 10× 1.4-numerical-aperture Axioplan objectives.
Total RNA isolation.
Cells in 1 ml of concentrated culture were collected by centrifugation at 8,000 × g for 10 s. Pellets were lysed, and RNA was isolated by an acid-guanidine thiocyanate-phenol-chloroform method as described previously (6, 35). Concentration and quality of the RNA were determined by A260-280 measurement using a UV spectrophotometer (Beckman).
RNase protection assay.
RNA abundances in control and experimentally treated cells were measured by RNase protection assays performed as previously described (35). Total C. reinhardtii RNA (5 μg) was hybridized overnight with 3 to 5 ng of in vitro-transcribed, [α-32P]UTP-labeled, antisense RNA probes. After RNase digestion, protected RNA fragments were dissolved in 5 μl of loading buffer (8% sucrose and 0.025% each bromphenol blue and xylene cyanol) and run at 0.6 mA/cm (constant current) on 4% nondenaturating polyacrylamide gels (1, 40), which preserve the integrity of long, protected fragments and reduce background. For denaturing gels, protected RNA fragments were resuspended in 5 μl of standard formamide loading buffer, heated to 95°C for 5 min, immediately chilled in ice water, and run on 6% polyacrylamide-8 M urea gels (40). Dried gels were exposed to X-ray film (Fuji). Autoradiographs were scanned, and band optical densities were measured with Quantity One version 2.2 software from Protein+Dna ImageWare Systems (Huntington Station, NY).
The change in RNA abundance was expressed as the factor by which RNA abundance in treated cells changed from that in untreated cells. Measured optical densities for untreated samples at all time points for a single experimental set were averaged to yield an average untreated optical density. To derive relative RNA abundance, we divided the optical density for each time point by the average untreated optical density. Relative RNA abundances for each time point in different biological replicates were averaged, and standard deviations were calculated. To express the RNA abundance change (n-fold) after treatment as the change over that in untreated samples, we divided the average relative RNA abundance of each time point after treatment by the average relative RNA abundance of untreated cells.
Probes.
All probes were antisense RNA that protected parts of the α1-tubulin, β2-tubulin, pcf3-21, and pcf8-13 RNAs from RNase cleavage. Tubulin gene products are localized in both flagella and the cell body; localization of the pcf3-21 gene product is unknown. Expression of the pcf3-21 gene was assayed because, like tubulin, its mRNA product is strongly induced after acid shock (5, 42). The pcf8-13 gene encodes a G-protein-like β subunit that is expressed at constant levels after acid shock (5, 41) and also during Li+ treatment, as reported here; its localization in the cell is unknown. Full-length α1-tubulin RNA probes were 742 nucleotides (nt) (for promoter mapping experiments shown in Fig. 5 and 6) and 323 nt (for RNase protection assays shown in Fig. 2 and 3), whereas the protected RNA fragments after RNase treatment were 710 and 170 nt, respectively. The β2-tubulin probe (232 nt) protected 217 nt of β2-tubulin RNA. The 390-nt-long pcf3-21 probe protected 350 nt of pcf3-21 RNA. The full-length 380-nt pcf8-13 probe protected 320 nt at the 3′ end of the pcf8-13 RNA. All probes contained sequences from the cloning vector that distinguish them in size from the protected RNA fragments.
FIG. 5.
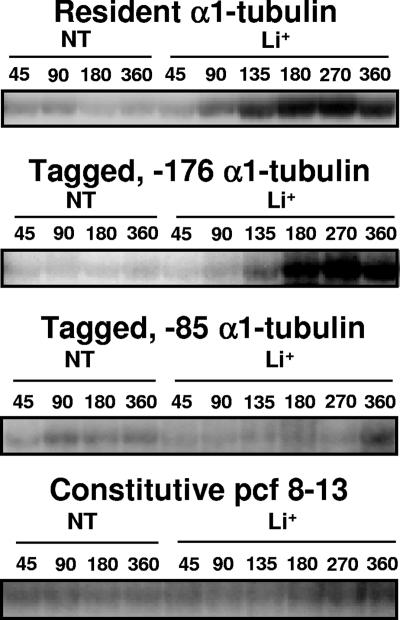
Autoradiograph of a representative RNase protection assay. Bands show protected fragments of α1-tubulin resident and tagged RNAs and pcf8-13 RNA isolated from pooled cell lines transformed with −176 α1-tubΔ/ARG7 plasmids and tagged RNA isolated from a single −85 α1tubΔ/ARG7 transformed cell line. RNA samples were isolated from nontreated (NT) and Li+-treated (Li+) cells at the indicated times after the addition of LiCl to 50 mM.
FIG. 6.
Expression of tagged −176, −276Δ, −122, −85, and −56 α1-tubulin genes during Li+ treatment. RNA was isolated from cell lines at the indicated times after the addition of LiCl. RNA was also isolated from nontreated cells at 45, 90, 180, and 360 min after the start of the experiment. The average of the four nontreated RNA levels is designated NT. RNA abundances of Li+-treated samples were compared to the average RNA abundances of control nontreated samples. Data for the induction of the resident (open bars) and the tagged (black bars) α1-tubulin genes and the constitutive pcf8-13 gene (hatched bars) are shown. The brackets show the standard deviations for each time point. (A) Pool of 82 arg7 cell lines transformed with the −176 α1-tubΔ/ARG7 plasmid. (B) Pool of 74 arg7 cell lines transformed with the −276Δ α1-tubΔ/ARG7 plasmid. (C) Pool of 76 arg7 cell lines transformed with the −122 α1-tubΔ/ARG7 plasmid. One-half of each pool was Li+ treated, and one-half was left untreated. Total RNA was isolated, and each set was tested twice by RNase protection assays as described in the text. Graphs in A, B, and C represent the averages of data from four RNase protection assays. (D) α1-Tubulin expression in a single cell line transformed with the −85 α1-tubΔ/ARG7 plasmid, which was previously shown to induce the tagged α1-tubulin gene after acid shock (35). The graph represents the averages of data from three RNase protection assays performed on control and Li+-treated samples. (E) The graph represents two RNase protection assays performed on control and Li+-treated RNA samples isolated from a cell line transformed with the −56 α1-tubΔ/ARG7 plasmid, which was previously shown to not induce the tagged α1-tubulin gene after acid shock (35). The same result was obtained with another cell line transformed with the −56 α1-tubΔ/ARG7 plasmid.
FIG. 2.
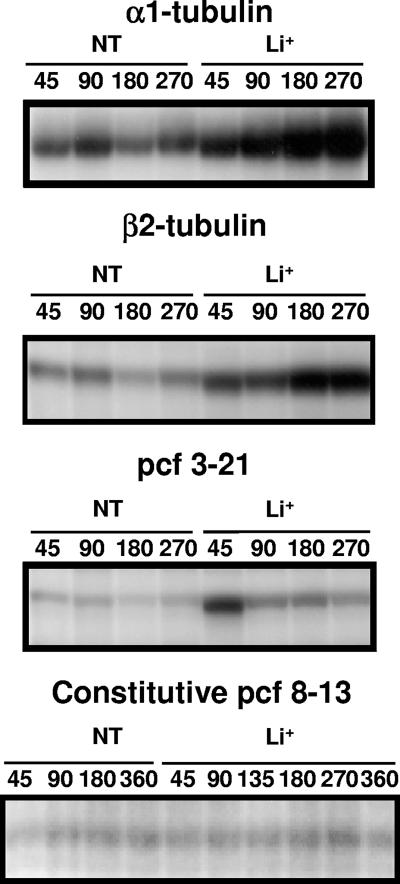
Autoradiograph of a representative RNase protection assay. Bands represent protected RNA fragments of α1- and β2-tubulin, pcf3-21, and pcf8-13 RNAs. Times in minutes at which total RNA was isolated from nontreated (NT) and Li+-treated (Li+) cells after the addition of Li+ are indicated by numbers (45 to 360). Cells were treated, and RNA was isolated and analyzed as described in Materials and Methods.
FIG. 3.
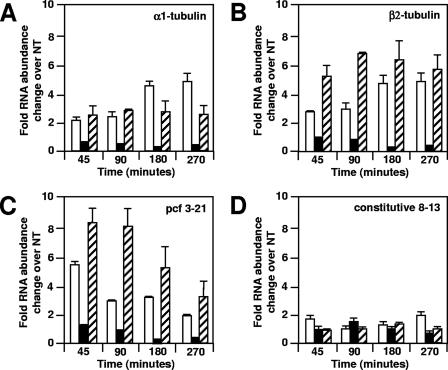
Effect of cycloheximide on Li+-treated wild-type (ARG7) cells. Cells were treated with Li+ (open bars), cycloheximide (black bars), or both Li+ and cycloheximide (hatched bars) or not treated (NT). RNA was isolated and analyzed by RNase protection assays as described in Materials and Methods. The average of two independent experiments is shown with brackets. Average RNA abundances of treated samples were normalized to an average of RNA abundances of four nontreated samples. (A) α1-tubulin RNA; (B) β2-tubulin RNA; (C) pcf3-21 RNA; (D) pcf8-13 RNA.
Plasmid constructions for promoter mapping.
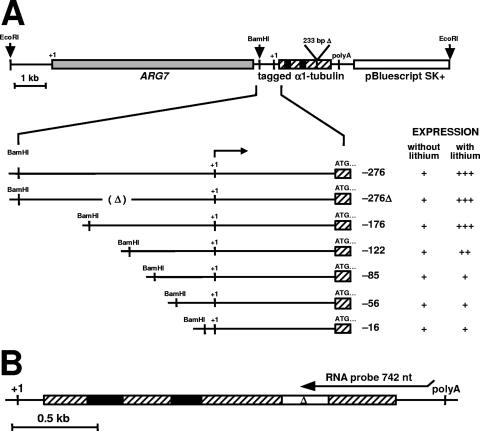
Plasmid constructions of the α1-tubulin gene were described previously (35). In short, we tagged the α1-tubulin gene by a 233-bp deletion between the EcoRV and MluI restriction sites in the coding region of the α1-tubulin gene to produce α1-tubΔ. In addition to the tag, different lengths of 5′-flanking sequence were deleted from α1-tubΔ to generate progressively shorter constructs containing from −276 bp to −16 bp upstream of the transcription start site. The 5′ end of each deletion construct was blunted, and a BamHI linker was ligated into it. The ARG7 selectable marker gene was inserted in a 5′-to-3′orientation upstream of the 5′ end of the tagged α1-tubulin genes (see Fig. 4). In one construct (−276Δ α1-tubΔ/ARG7), the promoter sequences between bp −156 and −122 upstream of the α1-tubulin transcription start site were also deleted. The set of cyc6/ars constructs was prepared by the insertion of α1-tubulin promoter fragments at the SalI restriction enzyme site upstream of the cytochrome c6 promoter and the arylsulfatase reporter gene (35, 37).
FIG. 4.
Tagged α1-tubulin gene constructions (α1-tubΔ/ARG7). (A) An XbaI-BamHI restriction fragment containing the ARG7 gene was inserted at the BamHI restriction enzyme site upstream of the 5′ end of the tagged α1-tubulin gene. The tagged α1-tubulin genes had various lengths of 5′ sequence upstream of the transcription start site, indicated by negative numbers. (B) Structure of the resident α1-tubulin gene. The 742-nt antisense RNA probe used in RNase protection assays hybridizes to sequences in the 3′ end of both resident and tagged α1-tubulin RNAs, as indicated above the gene in B, and protects fragments 710 nt from the resident α1-tubulin gene and 554 nt from the tagged α1-tubulin gene. Symbols: +++ to ++, relative magnitudes of the tagged-gene and resident-gene inductions; +, basal level of expression; +1, transcription start site; ATG . . . , translation start site; 233bpΔ and open box with Δ, deletion that tags all α1-tubulin gene constructions; (Δ), deletion between bp −156 and −122 of the −276Δ α1-tubΔ/ARG7 construct; hatched boxes, α1-tubulin gene exons; black boxes, α1-tubulin gene introns; gray box, ARG7 gene.
Transformation and screening.
The argininosuccinate lyase-deficient strain (CC-1931) of C. reinhardtii (11) was used as a recipient for 0.8 μg of linearized α1-tubΔ/ARG7 plasmids or 4 μg and 2 μg of circular α1tub/cyc6/ars and pARG7.8/gem plasmids, respectively (35). Before transformation, the α1-tubΔ/ARG7 plasmids were linearized with the restriction enzyme EcoRI such that the tagged α1-tubulin gene was flanked at its 5′ end by the ARG7 gene and at its 3′ end by vector (Fig. 4). Cells, at 1 × 107 to 3 × 107 cells/ml, were transformed by the glass bead method (19) and spread onto SGII medium plates lacking arginine (8, 39). Colonies appeared 7 to 10 days after transformation. Transformed cell lines were cultured and analyzed either as pools or as individual cell lines that showed basal expression of the tagged α1-tubulin gene, as previously described (35). Our previous results indicate that pooled and individual cell lines can be used interchangeably in α1-tubulin gene induction analyses (35).
Sequence analysis of the α1-tubulin promoter.
We analyzed sequences between bp −122 and −85 of the α1-tubulin promoter with the Transfac (v7.0) database (http://www.gene-regulation.com), which consists of a large number of nucleotide distribution matrices, each containing aligned binding sequences for a known transcription factor (36). MatInspector 6.2 computer software was used to compare to the matrices in the Transfac database.
RESULTS
Li+ causes flagellar elongation and loss of motility.
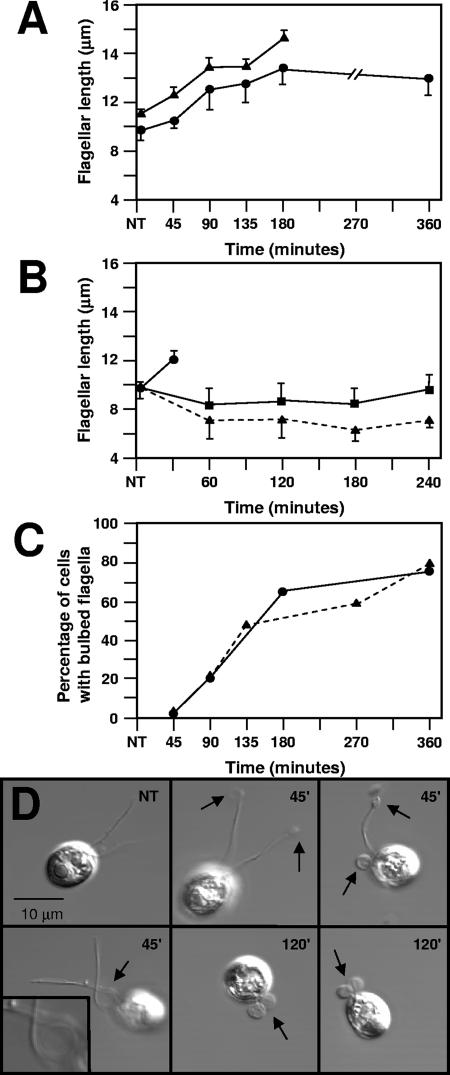
C. reinhardtii strains treated with 50 mM Li+ elongated their flagella from about 9 μm to 13 μm (about 1.4 times the length of control flagella) (Fig. 1A) and stopped swimming, confirming previous reports (9, 28, 50). Flagella were not elongated on control cells treated with 50 mM NaCl or 50 mM KCl (Fig. 1B).
FIG. 1.
Effect of Li+ on flagellar length and morphology. (A) Lengths of 25 straight flagella on randomly chosen cells treated with LiCl (circles) or lithium acetate (triangles) per time point were measured with an ocular micrometer and averaged. Bars show the standard deviations of the samples. NT, not treated. (B) Average lengths of 25 flagella on cells randomly chosen to be treated with 50 mM LiCl (circles), 50 mM KCl (squares), or 50 mM NaCl (triangles). Bars show the standard deviations of each sample. In this experimental series, flagella on LiCl-treated cells formed bulbs after 45 min and were not measured at later times. (C) Percentage of cells with bulbed flagella determined by scoring between 58 and 164 cells per time point for rolled or straight flagella. For each time point, the total number of cells with bulbed flagella was divided by the total number of cells counted. Circles indicate measurements in experiment 1; triangles represent measurements in experiment 2. (D) Differential interference contrast images of C. reinhardtii cells untreated (NT) or treated with Li+ for the times indicated on each panel. Arrows point to flagellar bulbs. The inset in lower left panel shows a ×4 enlargement of a flagellum in the process of coiling.
In addition, Li+-treated cells showed kinked flagella or flagella with small “bulbs” located at the tips or along the flagella (Fig. 1D). In extreme cases, the axonemes were presumably completely coiled inside the flagellar membrane to form one or two large bulbs. The number of kinked and bulbed flagella increased with the duration of Li+ treatment (Fig. 1C). Elongated, kinked, and bulbed flagella were observed in both C. reinhardtii strains examined and also on cells treated with Li acetate rather than LiCl.
Li+ increased RNA abundances of flagellar genes.
RNA abundances of α1- and β2-tubulin and pcf3-21 increased in cells treated with Li+, whereas pcf8-13 RNA abundance was unchanged. After a lag of 45 to 90 min, tubulin RNA abundances increased about fivefold to a plateau by 180 min (Fig. 2 and 3A and B, open bars). The pcf3-21 RNA abundance increased about sixfold to reach its highest levels in the first 45 min after the addition of Li+ and then decreased at later times (Fig. 2 and 3C, open bars). The abundance of pcf8-13 RNA did not change significantly during the experiment (Fig. 2 and 3D, open bars).
Flagellar RNA abundances increased in cells treated with Li+ plus cycloheximide.
In cells treated with Li+ plus cycloheximide (Fig. 3A, hatched bars), the increase in abundance of α1-tubulin RNA showed kinetics that were similar to those in cells treated with Li+ only (Fig. 3A, open bars). The increases in β2-tubulin and pcf3-21 RNA abundance were larger in cells treated with Li+ plus cycloheximide (Fig. 3B and C, hatched bars) than in cells treated only with Li+ (Fig. 3B and C, open bars). Cells treated with cycloheximide but not with Li+ showed a 50% decrease in flagellar RNA abundances (Fig. 3A to C, black bars). Flagellar length increased, and bulbs appeared in Li+ treatments, with and without cycloheximide, but not in the cycloheximide-only treatment (not shown). The abundance of pcf8-13 RNA did not change significantly during treatment with Li+, cycloheximide, or both (Fig. 3D). These results demonstrate that new protein synthesis is not required for the Li+-induced flagellar RNA abundance increase, although blocking protein synthesis does affect the magnitude of the flagellar RNA abundance change.
Mapping of Li+ response elements in the α1-tubulin promoter.
RNase protection assays measure RNA levels, which are the combined product of both transcriptional and posttranscriptional activity. To investigate the role of transcriptional activity in the Li+-induced increases in the abundance of RNA, we created a set of tagged α1-tubulin constructs with progressively shorter promoter sequences (Fig. 4) and introduced them into cells by transformation. Abundance levels of the resident and tagged α1-tubulin RNAs were measured simultaneously in the same RNA samples isolated from stably transformed cell lines by RNase protection (representative autoradiographs are shown in Fig. 5), and inductions were calculated for both the tagged and resident α1-tubulin genes at the indicated times (Fig. 6). This experimental design ensures that all tagged gene transcripts were subjected to the same posttranscriptional regulation as resident gene transcripts so that differences in abundance reflect differences in transcriptional activity from the different promoters. In this experimental series, expression of the control pfc8-13 gene (Fig. 6A to E, hatched bars) was constant in all experiments, varying less than twofold between time points in all cell lines examined (Fig. 5 and 6).
The resident and tagged −276, −276Δ, and −176 α1-tubulin genes are induced similarly during Li+ treatment.
Pooled arg7::ARG7 cell lines transformed with −176 α1-tubΔ/ARG7 and −276Δ α1-tubΔ/ARG7 plasmids induced the tagged α1-tubulin gene (black bars) and the resident α1-tubulin genes (open bars) to the same levels (Fig. 6A and B). The same results were obtained by analysis of an individual cell line transformed with plasmid −276 α1-tubΔ/ARG7 (constructs 1 to 3) (Table 1 and Fig. 6B). Although the −176 tagged gene was induced somewhat less than the resident gene at 135 and 180 min in Li+, this difference was not statistically significant, as determined by a two-tailed t test. These results suggest that regulatory elements necessary for the response to the Li+ are located within 176 nt upstream of the α1-tubulin transcription start site.
TABLE 1.
Comparison of the α1-tubulin promoter requirements for induction during lithium treatment and after acid shock
| Transforming constructa | Promoter requirement | Inductionb
|
|
|---|---|---|---|
| Lithium | Acid shock | ||
| α1-tubulinΔ/ARG7 | |||
| 1 | −276 | +++ | +++ |
| 2 | −276Δ | +++ | +++ |
| 3 | −176 | +++ | +++ |
| 4 | −122 | ++ | ++ |
| 5 | −85 | + | ++ |
| 6 | −56 | + | + |
| 7 | −16 | + | + |
| cyc6/ars | |||
| 8 | −276-−75 | + | ND |
| 9 | −276-−16 | + | ++/+++c |
| 10 | −85-−16 | + | ++ |
Constructs were described in Materials and Methods and in the legend of Fig. 4.
Induction was determined as described in Materials and Methods. +, basal expression; ++ and +++, expression induced by the indicated treatment; ND, not determined.
Qualitative estimate.
The tagged −122 α1-tubulin gene shows reduced induction during Li+ treatment.
Deleting the α1-tubulin promoter region to contain −122 bp upstream of the transcription start site decreased the induction of the tagged α1-tubulin gene (black bars) to about 55% of induction levels of the resident α1-tubulin gene (construct 4) (Fig. 6C, open bars, and Table 1). This difference between tagged and resident gene induction is statistically significant for time points between 45 and 270 min, as determined by a two-tailed t test (P < 0.05), but was not significantly different at 360 min of Li+ treatment.
Tagged −85, −56, and −16 α1-tubulin genes are not induced by Li+ but are expressed at basal levels.
Cell lines transformed with −85 and −56 α1tubΔ/ARG7 plasmids showed an increase in resident RNA abundance but no increase in tagged α1-tubulin RNA abundance during Li+ treatment (constructs 5 to 7) (Fig. 5 and 6D and E and Table 1). Instead, the tagged RNA abundances in Li+-treated cells from 45 to 270 min (Fig. 6D and E, black bars) were decreased to about 50% of those in control samples (Fig. 6D and E). At 360 min, the abundance of tagged RNA in the Li+-treated sample was either the same as that in the nontreated sample, as in −56 α1tubΔ/ARG7 transformed cell lines, or somewhat elevated, as in −85 α1tubΔ/ARG7 transformed cell lines (constructs 5 and 6) (Fig. 6D and E, black bars, and Table 1). Analysis of an individual cell line transformed with the −16 α1-tubΔ/ARG7 plasmid also revealed basal expression but no induction in Li+-treated samples (construct 7) (Table 1). These results suggest that additional sequences upstream of bp −85 and contained within bp −122 and −85 are necessary for α1-tubulin gene induction by Li+.
None of constructs 8 to 10 in Table 1, including one with the α1-tubulin promoter sequence from bp −276 to +75, conferred Li+ inducibility on the cyc6/ars reporter gene, although all constructs were expressed at basal levels. Therefore, although promoter sequences upstream of bp −85 are necessary for the α1-tubulin abundance change, other sequences in addition to the −122 and −85 sequence must also be required for Li+-induced activation of the α1-tubulin gene.
Basal expression of tagged α1-tubulin genes.
Control, pooled cell lines transformed with −176, −276Δ, or −122 α1-tubΔ/ARG7 plasmids expressed tagged α1-tubulin RNA at low but detectable basal levels that are comparable to resident gene expression levels (Fig. 5 and 6A to C) and similar to previously determined tagged gene basal expression levels in pooled cell lines transformed with −276, −85, −56, or −16 α1-tubΔ/ARG7 plasmids (35).
Sequence comparisons of tubulin promoters.
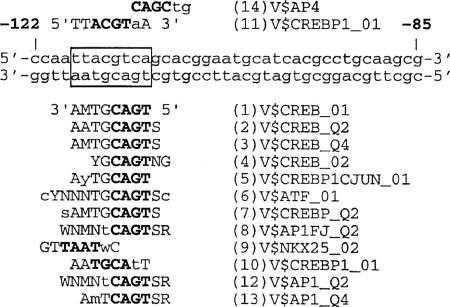
Transfac database analyses revealed several potential binding sites for known transcription factors located in the examined region. Most strikingly, in the region between bp −118 and −110, we found a significant number of matches to matrices containing binding sequences for the transcription factors CREB/ATF and AP-1 (Fig. 7). Most matches occurred on the minus strand and were homologous to the imperfect palindrome sequence 5′-TGACGTAA-3′ (Fig. 7). Criteria for the selection of these matrices were a core similarity of 1 and a matrix similarity of >0.85. To determine whether potential binding sites for those transcription factors were present in other C. reinhardtii tubulin or flagellar gene promoters, we compared DNA sequences from the C. reinhardtii genome database (http://www.jgi.doe.gov/chlamy) to those from the Transfac database. Similarly positioned sites in the upstream promoter of the pcf3-21 gene and each α- and β-tubulin gene were found to be homologous to matrices for CREB/ATF and AP-1. The presence of these sites suggests possible mechanisms for the control of expression of these genes in response to Li+ treatment.
FIG. 7.
Analysis of the α1-tubulin promoter region from bp −122 to −85 using the Transfac database. The region between bp −118 and −110 (box) of the α1-tubulin promoter was shown to be homologous to at least 15 matrices. Matrix consensus sequences that are homologous to the lower or upper DNA strand of the α1-tubulin promoter are shown below or above the α1-tubulin sequence, respectively. Numbers 5′ and 3′ indicate sequence orientation. Capital letters designate matches between the α1-tubulin promoter sequence and a matrix sequence. Letters in boldface type represent the core sequence of the matrix. Listed to the right of the matrix consensus sequences are matrix names. “V” designates the origin of the matrix sequence (vertebrate), while the rest of the name describes a transcription factor that binds a matrix and the quality of the matrix. The numbers preceding the matrix sequence name describe the score similarity, with 1 being the highest score match between matrix and the α1-tubulin sequence and 14 being the lowest. Letter codes for nucleotide sequence ambiguity follow IUB recommendations.
Promoter sequences required for activation of the α1-tubulin gene by Li+ and by acid shock partially differ.
Two regions proved necessary for normal Li+-induced activation of the α1-tubulin gene. Table 1 shows a comparison of promoter requirements for acid shock (35) and Li+-induced activation. Deleting the sequences between bp −176 and −122 reduced the induction response to either stimulus (constructs 1 to 4) (Table 1). Further deletion of sequences between bp −122 and −85 completely abolished Li+-induced activation of the tagged α1-tubulin gene, whereas acid shock-induced activation remained unchanged (construct 5) (Table 1). Deleting the sequence downstream of bp −85 completely abolished induction by either stimulus (constructs 6 and 7) (Table 1). These results suggest that elements located between bp −176 and −122 may be common to both responses, while elements downstream of bp −122 are distinct for acid shock and Li+-induced responses.
An additional difference in sequence requirements for Li+ and acid shock induction of the tagged α1-tubulin gene is shown in Table 1. When α1-tubulin promoter fragments were inserted upstream of the cyc6/ars reporter gene, none of the constructs (constructs 8 to 10) (Table 1) containing sequences from bp −276 to +75 of the α1-tubulin promoter supported cyc6/ars reporter gene induction during Li+ treatment. In contrast, the same constructs did support α1-tubulin induction after acid shock (35). Resident genes were induced normally after treatment in all cell lines where expression was measured.
DISCUSSION
Cellular responses to Li+.
Wild-type cells treated with Li+ elongate their flagella to ∼1.4 times their original length over 6 h. This gradual flagellar elongation may arise from the inhibition of a flagellum-length control mechanism (28, 50), or, because IFT is involved in flagellar assembly, Li+ may target structural or regulatory components of the IFT system or the mechanism of cilium-generated signaling (9, 21, 49). Recently, Wilson and Lefebvre showed that Li+ inhibits C. reinhardtii glycogen synthase kinase 3 (GSK3), suggesting a role for this kinase in regulating flagellar assembly and length (50).
Flagella of Li+-treated cells also accumulated kinks along their length and sometimes formed large bulbs in which the axoneme was presumably coiled within the flagellar membrane. Bulbs were previously observed in Li+-treated cells (28) and may be similar to the flagellar “disks” observed in the loop-1 mutant (29). Some loop-1 mutant cells also had flagella up to three times the normal length (29). Li+ may therefore directly or indirectly affect the same genes and/or proteins that are defective in loop-1 mutants. Although both kinks (called bulbs by Wilson Lefebvre [50]) and bulbs (also called discs by Nakamura et al. [29]) have been reported previously, the mechanism by which Li+ induces these morphologies remains unknown.
In our studies, flagellar elongation correlated with a gradual increase, over 4.5 h, in abundance of three flagellar RNAs, α1- and β2-tubulin and pcf3-21, to levels five- to ninefold above basal levels in untreated cells. The kinetics of increase are similar for α- and β-tubulin, and the abundance of pcf3-21 increases with different kinetics, while the abundance of pcf8-13 RNA remains constant during this time interval. The increase in abundance of the three flagellar RNAs indicates a degree of specificity and coordinate gene regulation in the response to Li+ stimulation. In contrast, Wilson and Lefebvre previously reported a decreased abundance of two other flagellar transcripts, RSP3 and PF20, at 60 min of Li+ treatment (50). The single time point used by Wilson and Lefebvre may not truly reflect the timing and kinetics of the abundance change occurring in these genes. An interesting alternative is that Li+ may coordinate the expression of a set of genes that includes only a subset of the flagellar genes. Global analysis of Li+-induced gene expression will distinguish between these possibilities.
Because the effect of Li+ on α1- and β2-tubulin and pcf3-21 RNA abundances was not blocked by cycloheximide, new protein synthesis is not required for these changes in abundance. Li+'s signaling and gene-inducing effects probably depend on modifying the activity of proteins already present in the cell.
Tubulin and pcf3-21 RNA abundances increased to higher levels and decreased more slowly in cells treated with Li+ plus cycloheximide. In acid-shocked cells, cycloheximide treatment does not block the induction of flagellar genes but instead stabilizes flagellar RNAs, resulting in somewhat higher RNA abundance levels and markedly slower degradation of accumulated RNAs (2). Cycloheximide may therefore have a similar stabilizing effect on flagellar RNA abundances after Li+ treatment.
Transcription and Li+-induced RNA abundance increase.
The expression patterns of the tagged α1-tubulin gene constructs reveals that transcription plays a role, and indeed a major one, in the Li+-induced changes in RNA abundance. Two regions of the α1-tubulin promoter were found to influence its expression in the presence of Li+. Because cell lines transformed with the −176 α1-tubΔ/ARG7 construct and the −276Δ α1-tubΔ/ARG7 plasmid construct, which had deleted sequences between bp −156 and −122, expressed the tagged gene at levels of the resident gene, the region between bp −176 and −156 is important for enhancing the Li+-induced response. Deletion of the sequences downstream of bp −122 of the α1-tubulin promoter region led to the complete loss of Li+-stimulated induction but not the loss of basal expression levels of tagged α1-tubulin. Therefore, sequences necessary for Li+ induction are located downstream of bp −122, probably between bp −122 and −85.
Although these studies have delineated sequences necessary for Li+ induction of the α1-tubulin gene, no construct tested was sufficient to drive the expression of the cyc6/ARS reporter gene in response to Li+ treatment (constructs 8 to 10) (Table 1). Additional sequences downstream of bp +75 may also be required for Li+ induction, as in the critical regulatory element within the 5′ untranslated region of the IC70 gene described previously by Kang and Mitchell (16). Alternatively, Li+ induction could require an intact α1-tubulin basal promoter that the cyc6 basal promoter cannot functionally replace. The α1-tubulin promoter differs from the cyc6 promoter at least in that the level of uninduced α1-tubulin basal expression is high relative to the barely detectable expression levels of the uninduced cyc6 gene (7, 35, 37).
Potential elements regulating Li+-induced transcription.
Because the region between bp −122 and −85 was necessary for changes in Li+-induced RNA abundance, we examined this region for similarities to known transcription factor binding sites. The region between bp −118 and −110 of the α1-tubulin promoter has potential for binding C. reinhardtii homologs of the vertebrate transcription factors CREB, AP-1, and ATF/Jun, related subfamilies of transcription factors that form heterodimers among themselves and bind to closely related CRE and AP-1 binding sites (10, 17). In vertebrates, Li+ modulates activities of multiple signal transduction molecules such as GSK3β, protein kinase C, and cyclic AMP (4, 13, 14, 15, 26, 27). For example, GSK3β negatively regulates AP-1 and CREB transcription factors, and Li+ inhibition of GSK3β leads to transcriptional activation by the increased binding of AP-1 and CREB transcription factors (27, 31). It is possible that similar mechanisms of action of Li+ operate in C. reinhardtii and vertebrates, despite their great evolutionary distance. The C. reinhardtii GSK3β homolog is involved in the control of flagellar length, and its activity is inhibited by Li+ (50), suggesting the conservation of Li+ action across divergent species. Interestingly, CRE and AP-1 binding sites were found upstream of the C. reinhardtii α- and β-tubulin genes as well as the pcf3-21 gene, and we demonstrate here that these genes are induced by Li+. CRE and AP-1 binding sites were also found in sequences that control the expression of the C. reinhardtii glutathione peroxidase gene in response to singlet oxygen, implicating the involvement of these factors in other C. reinhardtii gene regulatory circuits (24). It is not yet known whether a transcription factor(s) homologous to CREB, AP-1, or ATF/Jun or coactivators of these factors binds to the α1-tubulin promoter sequence at bp −118 to −110, although database searches have shown the presence of C. reinhardtii homologs of Fos, Jun, and coactivators of CREB. Determining whether these C. reinhardtii homologs bind DNA at these sites is of key importance in future studies.
Comparison of Li+ and acid shock responses.
C. reinhardtii responses to Li+ and acid shock stimuli differ in several ways. Li+ signals sustained flagellar elongation over ∼3 to 6 h, while acid shock signals immediate loss followed by the regrowth of flagella over ∼2 h. Li+ signals sustained elevated expression of at least three flagellar genes over 3 to 6 h, while acid shock signals a rapid induction/deinduction of flagellar genes, which returns to preshock levels within ∼2 h. While sequences that enhance the transcription of α1-tubulin in both Li+ and acid shock lie upstream of bp −122, other sequence elements responsive to these stimuli downstream of bp −122 differ in their sequence and location in the α1-tubulin gene promoter (Table 1). Also, the promoter region between bp −85 and −16 was necessary and sufficient to confer inducibility after acid shock upon the reporter gene cyc6/ars, while none of the α1-tubulin promoter fragments containing sequences were sufficient for Li+-induced changes in RNA abundance. Thus, the kinetics of flagellum length change, the kinetics of flagellar gene expression, and the promoter elements governing Li+- and acid shock-activated transcription differ significantly. A global analysis of Li+-induced gene expression similar to that undertaken previously for acid shock (46) will aid in defining the Li+-induced gene set and identifying the signaling pathway(s) and sensors involved in the cellular response to Li+.
The effect of Li+ on both flagellar morphology and gene expression in C. reinhardtii makes this organism a useful tool for investigating the relationship between the regulation of flagellar size and gene expression. Moreover, the diverse and dramatic effects of Li+ on C. reinhardtii as well as the possible conservation of mechanisms by which Li+ affects gene expression in mammals and C. reinhardtii make this organism a suitable model for determining the still elusive molecular mechanisms of Li+ action.
Acknowledgments
We thank J. Quinn for CYC6/ARS plasmids, J. P. Davies for plasmid pJD54 and ARS restriction enzyme maps, C. Silflow for the 8(1)A lambda genomic clone, and the Chlamydomonas Genetics Center for the pARG7.8 plasmid and strains used in this work. This work represents partial fulfillment of the requirements for the dissertation of G.P.
This work was supported by NSF grant 9316552 and FSU COFRS grants to L.R.K.
Footnotes
Published ahead of print on 15 June 2007.
REFERENCES
- 1.Ambion Inc. 1995. Ambion TechNotes, vol. 2. Ambion Inc., Austin, TX.
- 2.Baker, E. J., L. R. Keller, J. A. Schloss, and J. L. Rosenbaum. 1986. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardtii. Mol. Cell. Biol. 6:54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman, S. A., N. F. Wilson, N. A. Haas, and P. A. Lefebvre. 2003. A novel protein kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 13:1145-1149. [DOI] [PubMed] [Google Scholar]
- 4.Bitran, J. A., H. K. Manji, W. Z. Potter, and F. Gusovsky. 1995. Down-regulation of PKC α by lithium in vitro. Psychopharmacol. Bull. 31:449-452. [PubMed] [Google Scholar]
- 5.Cheshire, J. L., J. H. Evans, and L. R. Keller. 1994. Ca2+ signaling in the Chlamydomonas flagellar regeneration system: cellular and molecular responses. J. Cell Sci. 107:2491-2498. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 7.Davies, J. P., and A. R. Grossman. 1994. Sequences controlling transcription of the Chlamydomonas reinhardtii β2-tubulin gene after deflagellation and during the cell cycle. Mol. Cell. Biol. 14:5165-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debuchy, R., S. Purton, and J. D. Rochaix. 1989. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 8:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dentler, W. 2005. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 17:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulkes, N. S., and P. Sassone-Corsi. 1996. Transcription factors coupled to the cAMP-signaling pathway. Biochim. Biophys. Acta 1288:F101-F121. [DOI] [PubMed] [Google Scholar]
- 11.Gillham, N. W. 1965. Induction of chromosomal and nonchromosomal mutations in Chlamydomonas reinhardi with N-methyl-N′-nitro-N-nitrosoguanidine. Genetics 52:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris, E. H. 1989. The Chlamydomonas sourcebook. Academic Press, San Diego, CA. [DOI] [PubMed]
- 13.Jope, R. S. 2003. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24:441-443. [DOI] [PubMed] [Google Scholar]
- 14.Jope, R. S., and M. S. Roh. 2006. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets 7:1421-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jope, R. S., and M. B. Williams. 1994. Lithium and brain signal transduction systems. Biochem. Pharmacol. 47:429-441. [DOI] [PubMed] [Google Scholar]
- 16.Kang, Y., and D. R. Mitchell. 1998. An intronic enhancer is required for deflagellation-induced transcriptional regulation of a Chlamydomonas reinhardtii dynein gene. Mol. Biol. Cell 9:3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karin, M., Z. G. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 18.Keller, L. R., J. A. Schloss, C. D. Silflow, and J. L. Rosenbaum. 1984. Transcription of α and β tubulin genes in vitro in isolated Chlamydomonas reinhardtii nuclei. J. Cell Biol. 98:1138-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindle, K. L. 1990. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozminski, K. G., P. L. Beech, and J. L. Rosenbaum. 1995. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131:1517-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le, N. H., P. vanderBent, G. Huls, M. van de Wetering, M. Loghman-Adham, A. C. M. Ongi, J. P. Calvert, H. Clevers, M. H. Breuning, H. van Dam, and D. J. M. Peters. 2004. Aberrant polycystin-1 expression results in modification of activator protein-1 activity, whereas Wnt signaling remains unaffected. J. Biol. Chem. 279:27472-27481. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre, P. A., S. A. Nordstrom, J. E. Moulder, and J. L. Rosenbaum. 1978. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J. Cell Biol. 78:8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefebvre, P. A., C. D. Silflow, E. D. Wieben, and J. L. Rosenbaum. 1980. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell 20:469-477. [DOI] [PubMed] [Google Scholar]
- 24.Leisinger, U., K. Rufenacht, B. Fischer, M. Pesaro, A. Spengler, A. J. B. Zehnder, and R. I. L. Eggen. 2001. The glutathione peroxidase homologous gene from Chlamydomonas reinhardtii is transcriptionally up-regulated by singlet oxygen. Plant Mol. Biol. 46:395-408. [DOI] [PubMed] [Google Scholar]
- 25.Manji, H. K., Y. Bersudsky, G. Chen, R. H. Belmaker, and W. Z. Potter. 1996. Modulation of protein kinase C isozymes and substrates by lithium: the role of myoinositol. Neuropsychopharmacology 15:370-381. [DOI] [PubMed] [Google Scholar]
- 26.Manji, H. K., and R. H. Lenox. 1994. Long-term action of lithium: a role for transcriptional and posttranscriptional factors regulated by protein kinase C. Synapse 16:11-28. [DOI] [PubMed] [Google Scholar]
- 27.Miller, J. C., P. Jimenez, and A. A. Mathe. 12 October 2006, posting date. Restraint stress influences AP-1 and CREB DNA-binding activity induced by chronic lithium treatment in the rat frontal cortex and hippocampus. Int. J. Neuropsychopharmacol. doi: 10.1017/S1461145706007279. [DOI] [PubMed]
- 28.Nakamura, S., H. Tabino, and M. K. Kojima. 1987. Effect of lithium on flagellar length in Chlamydomonas reinhardtii. Cell Struct. Funct. 12:369-374. [Google Scholar]
- 29.Nakamura, S., M. Watanabe, K. Hatase, and M. K. Kojima. 1990. Light inhibits flagellation in a Chlamydomonas mutant. Plant Cell Physiol. 31:399-401. [Google Scholar]
- 30.Nguyen, R. L., L. W. Tam, and P. A. Lefebvre. 2005. The LF1 gene of Chlamydomonas reinhardtii encodes a novel protein required for flagellar length control. Genetics 169:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozaki, N., and D.-M. Chuang. 1997. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J. Neurochem. 69:2336-2344. [DOI] [PubMed] [Google Scholar]
- 32.Pan, J., and W. J. Snell. 2005. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev. Cell 9:431-438. [DOI] [PubMed] [Google Scholar]
- 33.Pan, J., Q. Wang, and W. J. Snell. 2005. Cilium-generated signaling and cilia related disorders. Lab. Investig. 85:452-463. [DOI] [PubMed] [Google Scholar]
- 34.Pazour, G., and G. B. Witman. 2003. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 15:105-110. [DOI] [PubMed] [Google Scholar]
- 35.Periz, G., and L. R. Keller. 1997. DNA elements regulating α1-tubulin gene induction during regeneration of eukaryotic flagella. Mol. Cell. Biol. 17:3858-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quandt, K., K. Frech, H. Karas, E. Wingender, and T. Werner. 1995. MatInd and MatInspector—new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn, J. M., and S. Merchant. 1995. Two copper-responsive elements associated with the Chlamydomonas cyc6 gene function as targets for transcriptional activators. Plant Cell 7:623-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum, J. L., and G. B. Witman. 2002. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3:813-825. [DOI] [PubMed] [Google Scholar]
- 39.Sager, R., and S. Granick. 1953. Nutritional studies with Chlamydomonas reinhardtii. Ann. N. Y. Acad. Sci. 56:831-838. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 41.Schloss, J. A. 1990. A Chlamydomonas gene encodes a G protein β subunit-like polypeptide. Mol. Gen. Genet. 221:443-452. [DOI] [PubMed] [Google Scholar]
- 42.Schloss, J. A., C. D. Silflow, and J. L. Rosenbaum. 1984. mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol. Cell. Biol. 4:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silflow, C. D., P. A. Lefebvre, T. W. McKeithan, J. A. Schloss, L. R. Keller, and J. L. Rosenbaum. 1982. Expression of flagellar protein genes during flagellar regeneration in Chlamydomonas. Cold Spring Harbor Symp. Quant. Biol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 44.Singla, V., and J. F. Reiter. 2006. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313:629-633. [DOI] [PubMed] [Google Scholar]
- 45.Solter, K. M., and A. Gibor. 1978. The relationship between tonicity and flagellar length. Nature 275:651-652. [DOI] [PubMed] [Google Scholar]
- 46.Stolc, V., M. P. Samanta, W. Tongprasit, and W. F. Marshall. 2005. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl. Acad. Sci. USA 102:3703-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam, L. W., W. L. Dentler, and P. A. Lefebvre. 2003. Defective flagellar assembly and length regulation in LF3 null mutants in Chlamydomonas. J. Cell Biol. 163:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuxhorn, J., T. Daise, and W. L. Dentler. 1998. Regulation of flagellar length in Chlamydomonas. Cell Motil. Cytoskeleton 40:133-146. [DOI] [PubMed] [Google Scholar]
- 49.Wang, Q., J. Pan, and W. J. Snell. 2006. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell 125:549-562. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, N. F., and P. A. Lefebvre. 2004. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot. Cell 3:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wissocq, J. C., J. Attias, and M. Thellier. 1991. Exotic effects of lithium, p. 7-34. In N. J. Birch (ed.), Lithium and the cell. Academic Press, London, United Kingdom.