Abstract
Clostridium perfringens type D isolates cause enterotoxemia in sheep, goats, and probably cattle. While the major disease signs and lesions of type D animal disease are usually attributed to epsilon toxin, a class B select agent, these bacteria typically produce several lethal toxins. Understanding of disease pathogenesis and development of improved vaccines are hindered by the lack of a small-animal model mimicking natural disease caused by type D isolates. Addressing this need, we developed an oral challenge mouse model of C. perfringens type D enterotoxemia. When BALB/c mice with a sealed anus were inoculated by intragastric gavage with type D isolates, 7 of 10 type D isolates were lethal, as defined by spontaneous death or severe clinical signs necessitating euthanasia. The lethalities of the seven type D isolates varied between 14 and 100%. Clinical signs in the lethally challenged mice included seizures, convulsions, hyperexcitability, and/or depression. Mild intestinal gas distention and brain edema were observed at necropsy in a few mice, while histology showed multifocal acute tubular necrosis of the kidney and edema in the lungs of most challenged mice that developed a clinical response. When the lethality of type D isolates in this model was compared with in vitro toxin production, only a limited correlation was observed. However, mice could be protected against lethality by intravenous passive immunization with an epsilon toxin antibody prior to oral challenge. This study provides an economical new model for studying the pathogenesis of C. perfringens type D infections.
Clostridium perfringens type D isolates produce enterotoxemia in sheep, goats, and other animal species (1, 9, 21). The infection in sheep, also known as pulpy-kidney or overeating disease, is characterized by brain lesions and lung edema with usually minor and inconsistent intestinal changes (1). In contrast, the disease in goats is usually characterized by enterocolitis, although systemic changes similar to those seen in sheep can occasionally be observed in the acute and subacute forms of the disease (15, 18). In cattle, although a condition with lesions similar to those observed in sheep enterotoxemia has been described (3, 4), a causal relationship with C. perfringens type D has not been established and the role of this microorganism remains controversial.
C. perfringens type D isolates are found in the normal gastrointestinal flora of many animals, including sheep, goats, and cattle (22). In clinically healthy animals, both the number of type D isolates and their toxin production remain low because of peristalsis and normal gut homeostasis. Clinical disease occurs only when the microbial balance of the gastrointestinal flora is disrupted. It has been proposed that if the intestine is altered by sudden changes in diet or other poorly defined factors, C. perfringens type D cells proliferate rapidly and generate large amounts of toxins that then produce disease. These toxins can act locally, as in the case of chronic enterotoxemia of goats (18), or gain access to the systemic circulation by a yet unknown mechanism, as happens in all forms of sheep enterotoxemia and sometimes with the acute and subacute forms of the disease in goats (18, 22).
C. perfringens type D enterotoxemia has been reproduced experimentally in sheep and goats, with a range of clinical signs and postmortem changes similar to those observed in the natural disease (2, 19, 20). However, neither the factors that predispose animals to develop enterotoxemia nor the pathogenic roles of the various toxins produced by type D isolates are currently well understood.
By definition, type D isolates must produce both alpha (CPA) and epsilon (ETX) toxins. Clinical signs and postmortem changes of natural type D enterotoxemia in sheep or goats may be reproduced by intravenous (i.v.) injection of purified ETX, which is the third most potent clostridial toxin (after botulinum and tetanus toxins) and is listed as a U.S. Department of Agriculture/CDC overlap class B select agent. ETX is produced as an inactive prototoxin in the gastrointestinal tract and is then activated by proteolytic removal of the C-terminal 14 amino acids (10). Activated ETX is then absorbed through the intestinal tract and transported to several target organs, including the brain, lungs, and kidneys. In the brain, ETX affects endothelial cells, producing perivascular edema with the consequent degeneration and necrosis of the cerebral parenchyma (3). It has also been suggested that ETX has direct action on neurons (5).
However, these ETX clinical observations certainly do not preclude the possible involvement of other toxins in natural type D enterotoxemias. For example, a recent survey (13) revealed that during vegetative growth, most genotype D isolates produce various levels of at least three different lethal toxins, including ETX, CPA, and perfringolysin O (PFO). Some type D isolates even produce a fourth lethal toxin, the beta 2 toxin, during vegetative growth. Some or all of these other potent toxins may additively or synergistically supplement ETX effects during type D enterotoxemias. If so, it could be important for type D vaccines to induce protective immunity against several type D toxins.
A major factor currently limiting type D pathogenesis and vaccine development studies is the lack of an economical small-animal model that reproduces some or all aspects of natural disease. Mice have been used to study the effects of intravenously administered type D vegetative-culture supernatants or pure ETX (5, 13), and the nature and distribution of brain lesions produced by i.v. injections of ETX or type D vegetative-culture supernatants are similar in mice and sheep (3). While the mouse i.v. injection model is useful for studying the systemic effects of ETX and indicates the sensitivity of the species to type D toxins, it obviously differs significantly from natural type D enterotoxemias, in which toxins are produced in the gastrointestinal tract and then absorbed into the circulation. In response to renewed interest in ETX and type D disease due to biodefense concerns, we now report the development of a new intragastric oral challenge mouse model for studying type D disease.
MATERIALS AND METHODS
Animals.
BALB/C mice (20 to 25 g), purchased from Charles River Laboratories (Hollister, CA), were housed in a temperature- and light cycle-controlled room. All procedures involving animals were reviewed and approved by the University of California, Davis, Committee for Animal Care and Use (permit 04-11593).
Bacterial strains.
The 10 C. perfringens type D isolates examined in this study came from our laboratory collections and originated from animal infections (Table 1). Strain toxin genotypes were previously determined by multiplex PCR (13).
TABLE 1.
Correlation between production of ETX, CPA, and PFO in vitro and lethality in the oral mouse model with 10 isolates of C. perfringens type D
| Straina | Origin | Lethality (%) | ETX (μg/ml) | CPA (103 U/ml) | PFO (103 U/ml)b |
|---|---|---|---|---|---|
| 1 | Reference strain | 100 | 25 | 1.85 | ND |
| 2 | Ovine | 0 | 2.0 | 4.59 | 3.49 |
| 3 | Unknown | 38 | 5.0 | 0.75 | 0.9 |
| 4 | Ovine | 14 | 6.0 | 2.46 | 2.47 |
| 5 | Unknown | 0 | 6.0 | 0.6 | 1.8 |
| 6 | Caprine | 50 | 8.0 | 1.25 | 1.1 |
| 7 | Guinea pig | 38 | 10 | 1.52 | ND |
| 8 | Unknown | 25 | 53 | 4.17 | 5.47 |
| 9 | Ovine | 29 | 17 | 1.57 | 0.7 |
| 10 | Caprine | 0 | 1.7 | 1.37 | ND |
Type D isolates are arbitrarily designated 1 to 10 to avoid possible misuse from identifying high ETX producers.
ND, not detected.
Quantification of toxin levels in type D vegetative-culture supernatants.
To evaluate whether in vitro toxin production differences among type D isolates correlate with their lethality in the oral challenge mouse model (described below), type D isolates were grown for 12 h in TGY, as described previously (13). ETX levels present in the vegetative-culture supernatants were then assessed by Western blotting, as previously described (6, 13), using an ETX-specific monoclonal antibody (MAb) (5B7; kindly provided by Paul Hauer, Center for Veterinary Biologics, Ames, IA) as the primary antibody. CPA activity levels in the vegetative-culture supernatants were measured using egg yolk agar plates, as previously described (13, 14). C. perfringens PLC (Sigma) was used as a standard for quantifying CPA activity. Finally, hemolytic activity against horse erythrocytes was measured to determine PFO activity levels in these type D vegetative-culture supernatants, as described previously (17).
Intraduodenal inoculation of concentrated culture supernatant.
To determine if type D toxins are absorbed into the circulation from the intestines of mice, and to establish the amount of toxin needed in the duodenum to produce disease, initial studies were conducted in which concentrated type D filtered culture supernatants were inoculated directly into the mouse duodenum. In these experiments, concentrated C. perfringens type D filtered culture supernatant was prepared from an overnight cooked-meat medium (CMM) culture of type D isolate no. 1 (Table 1). That supernatant was then filtered through a 0.22-μm filter. The sterile concentrated type D culture supernatant was then obtained by precipitating the filtered supernatant with 45% ammonium sulfate, followed by centrifugation and dialysis for 24 h at 4°C in phosphate-buffered saline. Before inoculation into mice, the concentrated culture supernatant was incubated with 0.05% trypsin for 30 min at 37°C to activate ETX (16). The 50% mouse lethal dose (MLD50) and the presence of ETX were then determined for a concentrated supernatant aliquot, as previously described (13).
The mice were fasted for 24 h before the experiment but were allowed access to water up to 1 h before the start of the experiment. The abdomen was shaved and disinfected with iodine solution (Betadine; Purdue Pharma LP, CT) immediately before surgery. The animals were anesthetized by intraperitoneal administration of Avertine (Winthrop Laboratories, New York, NY). An abdominal laparotomy was performed, and concentrated culture supernatant, diluted in 1% peptone water containing 200, 400, 800, 1,600 or 3,200 MLD50, was injected into the mouse duodenum (10 mice for each MLD50 concentration) immediately distal to the stomach. Sterile, nontoxic CMM was injected into the duodenums of another 10 mice as a negative control. The injections were performed by insertion of a 0.5-in., 27-gauge needle oblique to the intestinal lumen in a direction away from the stomach. After inoculation, the incision in the peritoneum, abdominal muscles, and skin was sutured in one plane using 3-0 Prolene. The surgical procedure lasted approximately 10 min per animal, and all mice were awake within 30 min after surgery. The animals were then monitored until the assay endpoint (see below).
Oral challenge model optimization.
Initially, several attempts were made to develop an intragastric oral challenge model using unsealed mice (as opposed to mice with a sealed anus; see below). Briefly, these initial attempts were all made using type D isolate no. 1 (Table 1) and included intragastric gavage with trypsinized or nontrypsinized whole cultures or washed cells. For these initial attempts, we also assayed a number of different pretreatments, which included fasting for 1 to 24 h, water deprivation for up to 18 h, broad-spectrum antibiotic treatment for 1 to 7 days, and diet change, increasing the lipid and/or carbohydrate level for 1 to 15 days before inoculation.
Preparation of inocula for the final oral challenge mouse model.
Washed cells were prepared from each C. perfringens type D isolate tested in this study (Table 1) and were used as the inocula for the oral challenge mouse studies. To prepare the washed-cell inocula, freeze-dried cells of each of the 10 tested C. perfringens type D isolates were reconstituted in CMM and subcultured onto blood agar plates, from which a single colony was then subcultured into CMM. A 10% inoculum of this culture was added to fresh CMM before further incubation overnight at 37°C under anaerobic conditions. The overnight cultures were centrifuged at 10,000 × g for 25 min at 4°C. The resultant pellet was then resuspended in 20 ml of phosphate-buffered saline, pH 7.2, and centrifuged again as described above. That pellet was finally resuspended in fresh CMM, the bacterial concentration was adjusted to the desired concentration using the McFarland scale, and the suspension was used as the final washed-cell inoculum. Sterile, nontoxic CMM was used as an inoculum in negative control animals.
Description of the final oral challenge mouse model.
The mice were allowed unlimited access to food and water up to 1 h before the start of the experiments. The mice were then inoculated by intragastric gavage with 1 ml of CMM. Forty-five minutes later, the perianal skin and ∼0.5 cm of the rectal canal were sealed with a gel of cyanoacrylamide ester glue (producing “sealed mice”). Approximately 1 h after the first inoculation, 0.5 ml of washed cells (see below) of one of the 10 type D challenge strains (Table 1) was administered intragastrically to each mouse in a group of 10. Three hours after the experiment start time, 0.5 ml of CMM-0.1% trypsin was administered intragastrically to all mice.
Characterization of the final oral challenge mouse model.
To determine if pathological effects in orally challenged mice were bacterial-dose dependent, groups of 10 mice were inoculated with 2 × 109, 4 × 109, or 8 × 109 CFU of washed cells from type D isolate no. 1. Controls included in this assay consisted of (i) replacement of the bacterial inocula by sterile, nontoxic CMM; (ii) inoculation of washed cells of a C. perfringens type A strain, obtained from a healthy sheep, in place of type D isolates; (iii) inoculation of CMM alone; and (iv) use of the type D model without trypsin dosing. The mice were periodically observed until the assay endpoint (see below).
Antibody protection experiments.
Ten sealed mice received an i.v. injection containing 50 IU/ml of anti-ETX MAb in 0.5 ml of normal saline solution 1 hour before they were given an intragastric inoculation containing washed cells of C. perfringens type D isolate no. 1. The mice were periodically observed until the assay endpoint (see below).
Assay endpoint and interpretation of results.
The assay endpoint for all oral challenge experiments was defined as spontaneous death, development of severe neurological or respiratory signs necessitating euthanasia, or survival without clinical alterations after 48 h.
Postmortem examinations.
Postmortem examinations were performed immediately after death on mice inoculated intragastrically. The whole gastrointestinal tract, brain, kidneys, lungs, spleen, and liver were collected and fixed by immersion in 10% formalin, pH 7.2, for a minimum of 24 h before being processed routinely to obtain 4-μm-thick sections, which were stained with hematoxylin and eosin.
RESULTS
Intraduodenal inoculations.
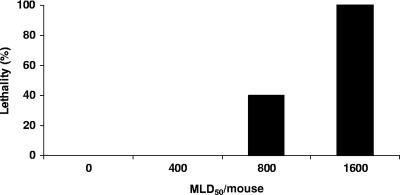
To assess whether mice are responsive to type D toxins present in their gastrointestinal tracts, an initial study administered different doses of concentrated culture supernatant (prepared from type D isolate no. 1) directly into the mouse duodenum. This experiment showed that, via intraduodenal administration, a dose containing at least 1,600 MLD50 (as measured by i.v. injection) of concentrated type D culture supernatant was necessary to induce neurological signs or death in 100% of mice (Fig. 1). In these animals, neurological signs were observed between 1.5 and 4 h after inoculation; the signs consisted of convulsions, opistothonus, circling, and depression. Most of these animals were euthanized as soon as they showed severe clinical signs, but death occurred spontaneously in a few animals before they could be euthanized.
FIG. 1.
Percentages of lethality in groups (10 mice per group) of mice after intraduodenal inoculation with different doses of concentrated filtered culture supernatant of C. perfringens type D (isolate no. 1) (Table 1).
Initial attempts to establish a model using unsealed mice.
Having confirmed that the presence of concentrated type D culture supernatants in the intestines can be lethal for mice, we next explored development of a oral challenge mouse model of infection with viable type D vegetative cells. Despite variations in a number of parameters (as described in Materials and Methods), no consistent lethal effects were obtained using mice without a sealed anus.
Development of an oral challenge model using sealed mice.
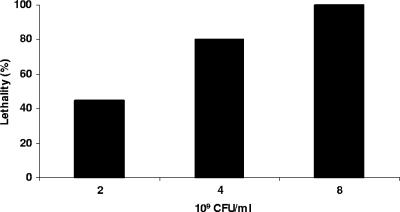
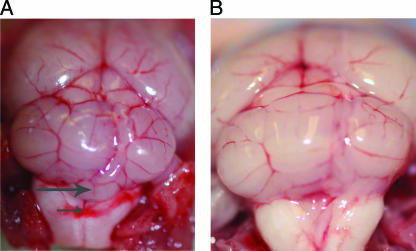
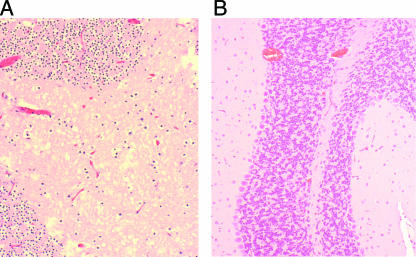
Using mice with a sealed anus, an intragastric inoculation of 8 × 109 CFU of type D isolate no. 1 produced 100% lethality (Fig. 2), i.e., within 4 h postinoculation, these mice showed either significant neurological signs necessitating euthanasia or died. Those animals showing neurological signs at 4 h lived for an additional 1 to 8 h after the onset of clinical signs. Grossly, mild brain edema, manifested as cerebellar coning, was observed in a few animals in this longest-survival group (Fig. 3A). Dilation of the small intestines of a few mice by gas was the only gross abnormality observed in the gastrointestinal tract. Histologically, the same animals showed interstitial and perivascular proteinaceous edema of the brain (Fig. 4A). This change was most marked at the level of basal ganglia, cerebellar white matter, and cerebellar peduncles. Multifocal acute tubular necrosis of the proximal convoluted tubules of the kidney was also observed in most cases (Fig. 5A). These lesions were usually small, comprising only a few tubules per focus, and were characterized by swollen sloughed off epithelial cells with vacuolated acidophilic cytoplasm and pyknotic and/or karyorrhectic nuclei. The lungs showed diffuse proteinaceous alveolar edema. The only histological abnormality observed in the gastrointestinal tract was dilation of the small intestine with blunting of the villi.
FIG. 2.
Percentages of lethality in groups (10 mice per group) of sealed mice after intragastric inoculation with different doses of washed cells of C. perfringens type D (isolate no. 1) (Table 1).
FIG. 3.
(A) Brain of a sealed mouse inoculated intragastrically with a C. perfringens type D isolate. Note the cerebellar coning (the vermis of the cerebellum protrudes slightly backwards) (large arrow) and hemorrhage on the dorsal part of the medulla (small arrow). (B) Brain of a healthy control mouse shown for comparison.
FIG. 4.
(A) Interstitial edema in the brain of a sealed mouse inoculated intragastrically with a C. perfringens type D isolate. Observe the presence of diffuse acidophilic fluid and vacuolation of the interstitium. (B) Brain of a healthy control mouse shown for comparison. The tissues were stained with hematoxylin and eosin. Magnification, ×200.
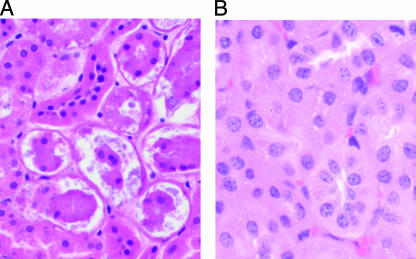
FIG. 5.
(A) Tubular necrosis in the kidney of a sealed mouse inoculated intragastrically with a C. perfringens type D isolate. (B) Kidney of a healthy control mouse shown for comparison. The tissues were stained with hematoxylin and eosin. Magnification, ×200.
No clinical disease or mortality was observed when C. perfringens type D washed cells were replaced by washed cells of C. perfringens type A or by sterile, nontoxic CMM or when CMM alone was inoculated. Neither control produced clinical, gross (Fig. 3B), or histological (Fig. 4B and 5B) abnormalities in the sealed-mouse model. Inoculation of mice without the use of trypsin produced occasional and inconsistent mortality. The anal occlusion with cyanoacrylamide glue did not by itself induce any macroscopic lesions in the treated animals and was completely reversible, i.e., all animals that did not die from experimental challenge were spontaneously freed of the occlusion (evidenced by normal passage of feces) 10 to 14 h after glue application and showed complete clinical recovery.
Evaluation of additional type D isolates in the oral challenge mouse model.
Because a minimum dose of 8 × 109 CFU produced 100% lethality in mice inoculated intragastrically with isolate no. 1, the same dose was used for intragastric inoculation of sealed mice with nine additional type D isolates. The lethality results for these 10 tested type D strains are shown in Table 1. When produced, neurological signs or death occurred 4 h after bacterial delivery, and all affected mice showed neurological signs or died between 4 and 8 h postinoculation. No consistent gross abnormalities were observed in the sealed mice inoculated intragastrically with any of the strains tested, although moderate gas distension of the small intestine and cerebellar coning were observed in a few mice that developed clinical disease. Histological lesions in all sealed mice that became clinically ill were similar to those described in mice inoculated intragastrically with isolate no. 1.
Comparison between in vivo lethality and type D isolate in vitro toxin production levels.
To demonstrate the usefulness of the sealed-mouse model for exploring type D pathogenesis, we compared the lethalities of the 10 surveyed type D isolates to their in vitro toxin-producing abilities (Fig. 6 and Table 1). Interestingly, no strong correlation was apparent between the amount of ETX, PFO, or CPA produced by a type D isolate and the lethality of that isolate in the oral challenge model using sealed mice. However, if isolate no. 8 is excluded from this analysis, a moderate correlation (r2 = 0.66) was noted between isolate lethality and in vitro ETX production levels.
FIG. 6.
Correlation between ETX (A), CPA (B), and PFO (C) production in vitro and lethality after oral inoculation of 10 isolates of C. perfringens type D (10 mice were inoculated per isolate).
Antibody protection experiments.
Our results indicated that, in our new oral challenge mouse model, type D toxins produced in the intestine were damaging the brain and other internal organs. Because of that finding, and the previous determination (by i.v. injection) that ETX is the most important toxin of type D supernatants for systemic lethality (13), we used the new model to test whether passive immunization can protect animals against type D toxins produced in the gastrointestinal tract. When mice were passively immunized with an ETX-neutralizing MAb prior to receiving an intragastric inoculation of washed cells of isolate no. 1, they were protected from neurologic clinical signs and no deaths were observed. However, transient diarrhea was observed 18 h after bacterial inoculation in all of these immunized mice. Recovery was complete 36 h after the start of the experiment.
DISCUSSION
Although C. perfringens type D infections have major veterinary disease and biosafety significance, there is currently no economical small-animal oral challenge model of such infections. Our report shows that conventional laboratory mice can be utilized to reproduce C. perfringens type D disease by intragastric administration of washed type D vegetative cells. Based upon our results, this model will prove invaluable for the study of C. perfringens type D pathogenesis and for efficacy trials of improved type D vaccines.
Previous studies using rabbits, guinea pigs, and mice had failed to produce enterotoxemia when C. perfringens type D was administrated orally, for reasons that are not known (7, 8). Our study first evaluated whether lethal quantities of type D toxins can be absorbed from the gastrointestinal tract into the systemic circulation when mice are inoculated intraduodenally with concentrated type D culture supernatants. When this experiment confirmed that the mouse is sensitive to the presence of type D toxins in its gastrointestinal tract, several different approaches were used to develop an oral challenge model using unsealed mice and viable cells of a C. perfringens type D isolate that produces relatively high ETX levels. However, all efforts to reproduce enterotoxemia in unsealed mice were unsuccessful or gave inconsistent results.
It is possible that the anatomical configuration of the mouse intestinal tract was responsible for this inability to reproduce the disease. An important difference between the gastrointestinal tracts of mice and ruminants is the relationship between intestinal length and animal weight, with mice having a smaller intestinal absorptive surface/mass ratio than ruminants. Additionally, intestinal transit is faster in mice than in ruminants, which should diminish bacterial retention and the intestinal accumulation of toxins.
The intestinal level of toxins required to reach toxicity might be difficult to reach when the bowel can evacuate its contents. By sealing the anus in our model, we transformed the gastrointestinal tract into a static in vivo incubator in which toxin could be produced and then absorbed into the systemic circulation. Our sealed type D oral challenge mouse model is a variation of the model previously used for studying Vibrio cholerae (12). With specific modifications for type D isolates (see below), this sealed-mouse model permitted isolate no. 1 to consistently reproduce most of the neurologic clinical signs and some of the lesions observed in natural cases of type D enterotoxemia.
The final model involves the intragastric administration of washed vegetative cells, together with an energy source for bacterial growth (CMM), which mimics carbohydrate feeding under natural conditions in ruminants. Overeating of high-carbohydrate diets is considered the most important predisposing factor for natural enterotoxemia in sheep and goats (1). Also, trypsin administered orally was found (data not shown) to increase lethality in this model compared with mice that did not receive supplemental trypsin. This observation suggests that the amount of trypsin present naturally in the mouse intestine is not sufficient to activate enough epsilon prototoxin to cause disease, at least under the model challenge conditions. In ruminants, epsilon prototoxin can be intestinally activated by trypsin or by lambda toxin, a metalloproteinase produced by some C. perfringens type D strains (10, 13). However, when 9 of the 10 type D isolates used in this study were assayed by PCR for the presence of the lambda toxin gene (data not shown), only one (isolate no. 4) was positive, indicating that in vivo induction of lambda toxin production is not a major factor in the results shown in Table 1. Of course, it is possible that these strains produce other proteases that activate the prototoxin, although that possibility is not supported by the need for trypsin supplementation in our model.
The experimental model used here produces a pathology that is similar to natural enterotoxemia in sheep, regardless of the origin of the isolate. For example, the model produces central nervous system signs at a frequency similar to that in naturally occurring disease in sheep. However, histological brain lesions were observed in only a few animals, which is different from the situation in sheep, a species in which most animals with enterotoxemia show such lesions (22). Renal lesions were observed in most orally challenged mice that developed clinical disease. This is an important finding, since in ruminants, renal lesions (i.e., pulpy-kidney disease) are considered to result from autolysis and have not been observed when sheep with enterotoxemia are necropsied immediately after death (22). Miyamoto et al. (11) showed that ETX accumulates in the kidneys of mice inoculated with this toxin, which is consistent with the results presented here. Also, the absence of significant histological abnormalities in the intestines of the mice in these experiments is consistent with the findings in natural sheep type D enterotoxemia, in which intestinal changes are not considered to be a feature of the disease (22). By contrast, in goats, necrotizing colitis is frequently described in cases of type D enterotoxemia (18). The differences in susceptibility to type D infection of the gastrointestinal tracts of sheep and goats is not understood, but it has been suggested to be related to the absence of receptors for ETX in the gastrointestinal tract of sheep (18). The same hypothesis can be proposed to explain the lack of lesions in the gastrointestinal tract of mice.
In our new oral challenge mouse model, the severity and speed of onset of experimental disease was related to the dose of the orally administered isolate, with clinical signs developing most prominently in animals that received the highest bacterial dose. Our results also clearly identify ETX as a major contributor to the lethality associated with oral challenge of mice, since passive i.v. immunization with an ETX-neutralizing MAb protected the mice against lethality induced by intragastric administration of isolate no. 1. This experiment is very important, as it provides the essential validation for the mouse model. The toxigenic potential of C. perfringens type D varies greatly between isolates. It is therefore not surprising that 7 of 10 isolates induced lethalities that varied between 14% and 100% of inoculated mice using our model.
We used Western blotting to quantitate ETX production in vitro because we have shown before that there is a high correlation between ETX production in vitro as measured by Western blotting and by mouse bioassay (13). When nine additional isolates of C. perfringens type D were tested in the oral challenge mouse model, no strong correlation was noted between lethality and in vitro ETX, PFO, or CPA production. This observation contrasts with the strong correlation noted between in vitro ETX production levels and the lethality of type D culture supernatants administered to mice via i.v. injection. There are several possible explanations for this difference. First, this result could indicate that type D toxin (particularly ETX) regulation in the gastrointestinal tract differs significantly from growth in culture medium. This possibility would be consistent with the strong correlation observed between in vitro ETX production and lethality of i.v.-injected type D culture supernatants (which use ETX produced in vitro) (13). Note that in the oral challenge mouse model, washed cells are used, so in vivo ETX production is required for the disease pathology. A second possibility is that in vivo growth may represent a major component of type D in vivo virulence; if so, some type D isolates may grow faster than others in vivo and thus produce high intestinal toxin levels more quickly. The final alternative explanation is that additional in vivo-expressed virulence factors, possibly including novel toxins, may contribute to the disease caused by type D isolates.
There is currently little or no information about C. perfringens type D growth or toxin production in vivo, in large part due to the absence of economical animal models. The new oral challenge mouse model should now allow investigators to address these important pathogenesis questions. Furthermore, this model should be applicable for elucidating the roles of individual toxins in the pathogenesis of type D infections using toxin knockout mutants, and such studies are already under way in our laboratories. Future studies will also assess whether this model (or variations thereof) could be used to study the pathogeneses of diseases caused by other C. perfringens toxin types.
Acknowledgments
National Institute of Allergy and Infectious Diseases grant AI056177-03 supported this research. Research at Monash University was also supported by a grant to the Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics.
We thank P. Hauer for supplying MAbs against ETX and J. Glenn Songer for supplying some type D isolates used in the study. We thank S. Fitisemanu for excellent secretarial work.
Editor: D. L. Burns
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Barker, I. K., A. A. Van Dreumel, and N. Palmer. 1993. The alimentary system, p. 1-318. In K. F. Jubb, P. C. Kennedy, and N. Palmer (ed.), Pathology of domestic animals, 4th ed., vol. 2. Academic Press, San Diego, CA. [Google Scholar]
- 2.Blackwell, T. E., D. G. Butler, J. F. Prescott, and B. P. Wilcock. 1991. Differences in signs and lesions in sheep and goats with enterotoxaemia induced by intraduodenal infusion of Clostridium perfringens type D. Am. J. Vet. Res. 52:1147-1152. [PubMed] [Google Scholar]
- 3.Buxton, D., N. S. M. Macleod, and T. B. Nicolson. 1981. Focal symmetrical encephalomalacia in young cattle. Vet. Rec. 108:459. [DOI] [PubMed] [Google Scholar]
- 4.Fairley, R. A. 2005. Lesions in the brains of three cattle resembling the lesions of enterotoxaemia in lambs. NZ Vet. J. 53:356-358. [DOI] [PubMed] [Google Scholar]
- 5.Finnie, J. W. 2003. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: a review. Aust. Vet. J. 81:219-221. [DOI] [PubMed] [Google Scholar]
- 6.Fisher, D. J., M. E. Fernandez-Miyakawa, S. Sayeed, V. Adams, R. Poon, J. I. Rood, F. A. Uzal, and B. A. McClane. 2006. Dissecting the lethality contributions of Clostridium perfringens genotype C toxins in the mouse intravenous injection model. Infect. Immun. 74:5200-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton, R. E., D. L. Madden, and N. B. McCullough. 1970. Pathogenicity of Clostridium perfringens for germ-free guinea pigs after oral ingestion. Appl. Microbiol. 19:314-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudault, S., C. Bridonneau, and P. Raibaud. 1983. Pathogenicity of various toxinogenic types of Clostridium perfringens administered by mouth to axenic and holoxenic mice. Ann. Microbiol. 134B:277-283. [PubMed] [Google Scholar]
- 9.Lyerly, D. M., T. Wilkins, F. A. Uzal, M. Fernandez-Miyakawa, and B. A. McClane. 2004. The enterotoxigenic clostridia, p. 698-752. In M. Dworkin and S. Falkow (ed.), The prokaryotes, 3rd ed. Springer-Verlag, New York, NY.
- 10.Minami, J., S. Katayama, O. Matsushita, C. Matsushita, and A. Okabe. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527-535. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto, O., K. Sumitani, T. Nakamura, S. Yamagami, S. Miyata, T. Itano, T. Negi, and A. Okabe. 2000. Clostridium perfringens epsilon toxin causes excessive release of glutamate in the mouse hippocampus. FEMS Microbiol. Lett. 189:109-113. [DOI] [PubMed] [Google Scholar]
- 12.Richardson, S. H., J. C. Giles, and K. S. Kruger. 1984. Sealed adult mice: new model for enterotoxin evaluation. Infect. Immun. 43:482-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayeed, S., M. E. Fernandez-Miyakawa., D. J. Fisher, V. Adams, R. Poon, J. I. Rood, F. A. Uzal, and B. A. McClane. 2005. Epsilon-toxin is required for most Clostridium perfringens genotype D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect. Immun. 73:7413-7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan, J., T. A. Warner, P. T. Scott, T. L. Bannam, D. I. Berryman, and J. I. Rood. 1992. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27:207-219. [DOI] [PubMed] [Google Scholar]
- 15.Smith, M. C., and D. M. Sherman. 1994. Goat medicine. Lea and Febiger, Pittsburgh, PA.
- 16.Sterne, M., and I. Batty. 1975. Pathogenic clostridia. Butterworths, London, England.
- 17.Stevens, D. L., J. Mitten, and C. Henry. 1987. Effects of alpha and theta toxins from Clostridium perfringens on human polymorphonuclear leukocytes. J. Infect. Dis. 156:324-333. [DOI] [PubMed] [Google Scholar]
- 18.Uzal, F. A., and W. R. Kelly. 1996. Enterotoxaemia in goats: a review. Vet. Res. Comm. 20:481-492. [DOI] [PubMed] [Google Scholar]
- 19.Uzal, F. A., and W. R. Kelly. 1997. The effects of intravenous administration of Clostridium perfringens type D epsilon toxin on young goats and lambs. J. Comp. Pathol. 116:63-71. [DOI] [PubMed] [Google Scholar]
- 20.Uzal, F. A., and W. R. Kelly. 1998. Experimental Clostridium perfringens type D enterotoxaemia in goats. Vet. Pathol. 35:132-140. [DOI] [PubMed] [Google Scholar]
- 21.Uzal, F. A., W. R. Kelly, W. E. Morris, and R. Assis. 2002. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J. Comp. Pathol. 126:71-75. [DOI] [PubMed] [Google Scholar]
- 22.Uzal, F. A., W. R. Kelly, W. E. Morris, J. Bermudez, and M. Biason. 2004. The pathology of experimental Clostridium perfringens type D enterotoxemia in sheep. J. Vet. Diagn. Investig. 16:403-411. [DOI] [PubMed] [Google Scholar]