Abstract
Tight regulation of surface antigenic expression is crucial for the pathogenic strategy of the Lyme disease spirochete, Borrelia burgdorferi. Here, we report the influence of increasing expression of decorin-binding protein A (DbpA), one of the most investigated spirochetal surface adhesins, on the 50% infectious dose (ID50), dissemination, tissue colonization, pathogenicity, and persistence of B. burgdorferi in the murine host. Our in vitro assays showed that increasing DbpA expression dramatically increased the interaction of B. burgdorferi with decorin and sensitivity to growth inhibition/killing by anti-DbpA antibodies; however, this increased interaction did not affect spirochetal growth and replication in the presence of decorin. Increasing DbpA expression significantly reduced ID50 values and severely impaired dissemination in severe combined immunodeficiency (SCID) and immunocompetent mice. During infection of SCID mice, B. burgdorferi with increased DbpA expression was able to effectively colonize heart and skin tissues, but not joint tissues, completely abrogating arthritis virulence. Although increasing DbpA expression did not affect spirochetal persistence in the skin, it diminished the ability of B. burgdorferi to persist in the heart and joint tissues during chronic infection of immunocompetent mice. Taken together, the study highlights the importance of controlling surface antigen expression in the infectivity, dissemination, tissue colonization, pathogenicity, and persistence of B. burgdorferi during mammalian infection.
Lyme disease caused by the spirochete Borrelia burgdorferi is a multisystem disorder that can result in arthritis, neurological abnormalities, carditis, and cutaneous lesions, such as erythema migrans and acrodermatitis chronica atrophicans (41). As a slow-growing extracellular bacterium with a doubling time of approximately 8 h under the best in vitro conditions, B. burgdorferi has a 50% infectious dose (ID50) of less than 100 organisms in the murine host (1, 34, 39) and can also cause persistent infection, despite the development of vigorous immune responses against the pathogen (38), making it one of the most invasive microbial pathogens in humans and animals.
Tight regulation of surface antigenic expression is crucial for the pathogenic strategy of B. burgdorferi. The pathogen abundantly expresses outer surface proteins A/B (OspA/B) in the unfed tick (11, 28, 36, 37), consistent with an essential role of these lipoproteins in spirochetal persistence in the vector (27, 50). A fresh blood meal down-regulates OspA/B and up-regulates OspC and other proteins, a process that prepares B. burgdorferi for infection of mammals (12, 18, 29, 42). Repression of OspA/B expression during mammalian infection is critical for the maintenance of the enzootic cycle because their expression would ultimately induce strong humoral responses to effectively block acquisition by the vector (10, 45, 46), regardless of whether OspA/B can be effectively targeted by borreliacidal antibodies in mammalian tissues (43). B. burgdorferi abundantly expresses OspC during early infection, when the antigen is required (26, 44). However, OspC is not only a strong immunogen, but also an effective target of protective immunity; its expression induces a robust humoral response that imposes tremendous pressure on the pathogen (15, 26). To cause persistent infection, B. burgdorferi must down-regulate OspC as the specific humoral immune response is developing (7, 24-26). If B. burgdorferi failed to repress OspC expression or to undergo escape mutations on the ospC gene, the infection would be cleared (49). It is also crucial for B. burgdorferi to keep the ospC gene off after it is acquired by the tick vector, as OspC antibodies in the blood meal may kill spirochetes that express the antigen in the vector (17), leading to discontinuation of the enzootic cycle.
During the course of mammalian infection, B. burgdorferi vigorously modifies its surface antigenic expression in response to tissue microenvironmental changes, including specific immune selection pressure. In the absence of humoral immune responses, phenotypes without active BBF01 and VlsE expression dominate in heart and skin tissues, but not in joint tissues, where B. burgdorferi abundantly expresses both antigens (8, 26). The specific immune response down-regulates OspC and many other surface antigens, dramatically up-regulating BBF01 and VlsE (7, 8, 16, 25, 26), a process that allows B. burgdorferi to evade the immune system and proceed to persistent infection.
Decorin-binding protein A (DbpA) is one of the most investigated borrelial surface adhesins and is able to bind both decorin and glycosaminoglycans (3, 6, 14, 19). Mice deficient for decorin, a ligand of DbpA, become less susceptible to murine Lyme disease and harbor few spirochetes during chronic infection, suggesting that the lipoprotein plays an important role during mammalian infection (4, 23). Inactivation of the dbpBA locus does not completely abolish infectivity, indicating that DbpA is not essential for infection of mammals (40). DbpA is not expressed in the tick, indicating that there is no role for this lipoprotein in the vector (21). The antigen is persistently expressed in all tissues at moderate levels during mammalian infection (26). In this study, the influence of increasing DbpA expression on the ID50 value, dissemination, tissue colonization, pathogenicity, and persistence of B. burgdorferi was investigated in the murine model.
MATERIALS AND METHODS
Construction of recombinant plasmid pBBE22-dbpA′.
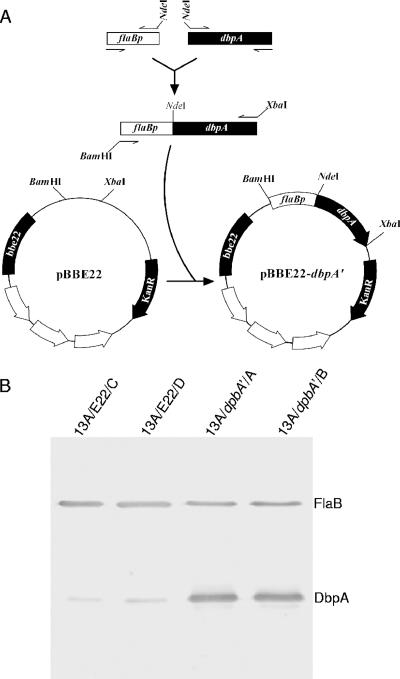
As illustrated in Fig. 1A, a 251-bp fragment of the flaB promoter region was amplified with the use of a primer pair (forward, 5′-AGAAGTACGAAGATAGAGAGAGAAA-3; reverse, 5′-AACACATATGTCATTCCTCCATGATAAA-3). A 748-bp fragment extending from the ATG translational start codon to the 172-bp sequence downstream of the stop codon of the dbpA gene was amplified with the use of a primer pair (forward, 5′-ACCCATATGATTAAATGTAATAATAAAACT-3′; reverse, 5′-GTCTTTTAGGTGAATTGTTGTAACT-3′). (The underlined sequences are NdeI sites.) The two PCR products were pooled, purified by using the QIAquick PCR purification kit (QIAGEN Inc., Valencia, CA), digested with NdeI, repurified, and ligated. The resultant product was used as a template and amplified by nested PCR with the use of a primer pair (forward, 5′-ATAGGATCCAAGATAGAGAGAGAAAAGT-3′; reverse, 5′-TCATCTAGATTATCGGGCGAAGAGTT-3′). (The underlined sequences are either a BamHI (forward) or an XbaI (reverse) site.) The PCR product was purified, digested with BamHI and XbaI, and cloned into the recombinant plasmid pBBE22 (a gift from S. Norris) (34). The insert and flanking regions within the recombinant plasmid were sequenced to ensure the construct was as designed.
FIG. 1.
Generation of B. burgdorferi with increased DbpA expression. (A) Construction of pBBE22-dbpA′ from the recombinant plasmid pBBE22. The flaB promoter region (flaBp) and the promoterless dbpA gene were PCR amplified, fused, and cloned into pBBE22. (B) Confirmation of increased DbpA expression. The 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B spirochetes were grown to late log phase and subjected to an immunoblot analysis probed with a mixture of FlaB monoclonal antibody and mouse anti-DbpA sera.
Generation of transformants.
B. burgdorferi B31 13A was grown to late logarithmic phase in Barbour-Stoenner-Kelly H (BSK-H) complete medium (Sigma Chemical Co., St. Louis, MO). This highly transformable clone was used in our previous study (47). The 13A spirochetes were harvested from 3.0 ml of culture and transformed with either the recombinant plasmid pBBE22 or pBBE22-dbpA′, as described previously (48). Transformants were identified by PCR using a primer pair specific for the kanamycin cassette, and their plasmid contents were analyzed as described previously (48). Increased DbpA expression resulting from the introduction of pBBE22-dbpA′ was confirmed by immunoblotting, as described below.
Preparation of recombinant DbpA and generation of mouse antisera.
The coding region, excluding the signal peptide-coding sequence of the dbpA gene, was PCR amplified and cloned into the expression vector pET16b and transformed into Escherichia coli strain BL21(DE3) (Novagen, La Jolla, CA). Recombinant proteins were purified using the Hi-Trap affinity column (Amersham-Pharmacia Biotech, Piscataway, NJ). The protein purity and concentration were determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA), respectively. Approximately 70 μg of recombinant protein was dissolved in 100 μl of phosphate-buffered saline (pH 7.3) and emulsified with 30 μl of Freund's complete (first injection) or incomplete (remaining injections) adjuvant and then subcutaneously administered to each BALB/c mouse (age, 5 to 6 weeks; provided by the Louisiana State University [LSU] Division of Laboratory Animal Medicine) at 3-week intervals. The mice were euthanized 3 weeks after the last immunization for antiserum preparation.
Immunoblot analysis.
Transformants were grown in BSK-H complete medium to late log phase at 33°C and harvested by centrifugation. Spirochetes were dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, separated by electrophoresis, and electrotransferred onto nitrocellulose membranes. Blots were probed with a mixture of FlaB monoclonal antibody and mouse antisera raised against recombinant DbpA, as described previously (49).
In vitro assay to examine spirochete-decorin interaction.
B. burgdorferi was grown to densities of 107 cells per ml in BSK-H complete medium at 33°C. Aliquots of 30 μl were transferred to 200-μl PCR tubes, supplemented with decorin at a concentration of either 0 or 500 μg per ml (Sigma), and incubated at 33°C for 48 h. Approximately 10 μl of the preparation was applied to a microscope slide and covered with a coverslip; spirochete movement was recorded using an Olympus IX81 Live-Cell-Imaging System (Hunt Optics and Imaging Inc., Pittsburgh, PA).
In vitro assay to determine the influence of decorin on spirochete growth.
To prepare a spirochete stock, B. burgdorferi was grown to late log phase and diluted with BSK-H complete medium to a cell density of 20 organisms per ×400 field under a dark-field microscope. Approximately 11 μl of 5-mg/ml decorin was added to one of the 200-μl PCR tubes, each of which already contained 90 μl of BSK-H complete medium; then, a 10-fold serial dilution was conducted. To each tube, 10 μl of the spirochete stock was added to reach a final volume of 100 μl, and the tubes were incubated at 33°C. To stop the assay, samples were fourfold diluted with double-distilled H2O 72 h after the initial setup; spirochetes were counted in at least five dark-field microscope fields. The experiment was performed in triplicate.
In vitro killing/inhibition assay.
To prepare a spirochete stock, B. burgdorferi was grown to late log phase and diluted with BSK-H complete medium to a cell density of 100 organisms per ×400 field under a dark-field microscope. Pooled SCID mouse sera (used as a complement source) and BSK-H complete medium were mixed at a ratio of 1:3 and then transferred into 200-μl PCR tubes at 180 or 100 μl per tube. In the PCR tubes containing 180 μl of complement-supplemented BSK-H, 20 μl of pooled mouse anti-DbpA sera was added; a twofold serial dilution was conducted in the tubes containing 100 μl of the same preparation. In the tubes containing 100 μl of complement-supplemented BSK-H, 10 μl mouse sera was added as a negative control. To each tube, 10 μl of the spirochete stock was added to reach a final volume of 110 μl, and the tubes were incubated at 33°C. Viable (motile) spirochetes were counted in at least five dark-field microscope fields at 48 h after the initial setup. The experiment was performed in triplicate.
Infectivity and pathogenicity study in SCID mice.
Severe combined immunodeficiency (SCID) mice on a BALB/c background (age, 4 to 8 weeks; provided by the LSU Division of Laboratory Animal Medicine) were given a single intradermal/subcutaneous injection of 104 spirochetes. The animals were examined for the development of arthritis at 2-day intervals, starting at 10 days; the animals were sacrificed 6 weeks postinoculation. Tibiotarsal joints were randomly chosen for histopathological study as previously described (48). The tibiotarsal joint, heart, and a piece of skin (not from the inoculation site) were used for spirochete isolation and DNA and RNA preparation. DNA was quantified for the copy numbers of flaB and murine actin genes by quantitative PCR (qPCR) as previously described (48). The tissue spirochete burden was expressed as flaB DNA copies per 106 host cells (2 × 106 actin DNA copies). RNA was quantified for the mRNA copy numbers of flaB and dbpA by reverse transcription-qPCR (RT-qPCR) as described previously (23).
Determination of ID50 values.
Spirochetes were grown at 33°C to late log phase (108 cells per ml) and 10-fold serially diluted with BSK-H complete medium. BALB/c or BALB/c SCID mice (age, 4 to 5 weeks; provided by the LSU Division of Laboratory Animal Medicine) each received a single intradermal/subcutaneous injection of 100 μl of spirochetal suspension. Ear biopsies were performed up to 6 weeks postinoculation, starting at week 2, as described previously (49). The mice were euthanized 6 weeks postinoculation; heart, tibiotarsal joint, and skin (not from the inoculation site) specimens were harvested for bacterial culture as described previously (48). The ID50 value was calculated as described by Reed and Muench (35).
Chronic-infectivity study.
Subgroups of five BALB/c mice were given a single intradermal/subcutaneous injection of 104 spirochetes. Retro-orbital blood was drawn to monitor the anti-DbpA response at intervals of 2 to 4 weeks, starting at week 2. Anti-DbpA end point titers were determined by an enzyme-linked immunosorbent assay (ELISA) as described below. The mice were euthanized 5 months postinoculation; heart, tibiotarsal joint, and skin specimens were aseptically collected for spirochete culture as previously described (48).
Measurement of anti-DbpA humoral immune response.
Specific DbpA antibody end point titers were determined by an ELISA. Ninety-six-well plates (Fisher Scientific, Pittsburgh, PA) were coated with 100 μl of 2.0-μg/ml recombinant DbpA per well. Sera were twofold serially diluted, starting at 1/200. Five samples drawn from naive BALB/c mice were used as controls. The ELISA was performed as previously described (49).
Mutation analysis.
Spirochetes were recovered from selected mice that had been inoculated with B. burgdorferi with increased DbpA expression and grown to late log phase in BSK-H complete medium; total DNA was extracted by using the DNeasy Tissue Kit (QIAGEN). E. coli DH5α competent cells (Invitrogen Life Technologies, Carlsbad, CA) were transformed with spirochetal DNA by heat shock. Three to five kanamycin-resistant colonies were randomly selected from each transformation experiment and sequenced for the introduced dbpA copy and fused flaB promoter.
Statistical analysis.
A one-way analysis of variance was used to analyze in vitro assay data, followed by a two-tailed Student t test to calculate a P value for every two treatments. The t test was also used to analyze ID50 and qPCR data. A P value of ≤0.05 was considered to be significant.
RESULTS
Generation of transformants with increased DbpA expression.
B. burgdorferi clone 13A is highly transformable due to a lack of lp25 and lp56 (47), the two plasmids that may carry restriction enzymes (22). The plasmid lp25, not lp56, is required for mammalian infection, as it carries bbe22, a gene that codes for a nicotinamidase essential for the survival of B. burgdorferi in the mammalian environment (34). Both pBBE22 and pBBE22-dbpA′ carry a copy of bbe22 and thus should be able to restore infectivity of clone 13A. Transformation of the 13A spirochetes with the recombinant plasmids pBBE22 and pBBE22-dbpA′ produced 14 and 19 transformants, respectively. Plasmid content analyses identified two clones that received each construct, namely, 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B, for further analysis. These four clones had identical plasmid contents; all lacked cp9, lp21, and lp5, in addition to lp25 and lp56.
Increased DbpA expression resulting from the introduction of pBBE22-dbpA′ was confirmed by immunoblotting. Clones 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B and the parental clone, 13A, were grown to late log phase and analyzed for DbpA expression by immunoblotting. Both clones 13A/dbpA′/A and 13A/dbpA′/B expressed significantly more DbpA than the 13A/E22/C or 13A/E22/D spirochetes (Fig. 1B), indicating that increased DbpA expression had been achieved. Clones 13A, 13A/E22/C, and 13A/E22/D expressed DbpA at similar levels (data not shown), indicating that introduction of the recombinant plasmid pBBE22 does not alter the antigen's expression.
Increasing DbpA expression increases the interaction of B. burgdorferi with decorin without affecting spirochetal growth.
The influence of increasing DbpA expression on the interaction of B. burgdorferi with decorin was investigated in vitro. As shown in Video S1A and B in the supplemental material, both 13A/E22/C and 13A/dbpA′/A spirochetes swam very actively and freely in the absence of decorin. The addition of decorin did not significantly affect the motility of the transformant 13A/E22/C (see Video S1C in the supplemental material). In sharp contrast, the presence of decorin severely restrained the movement of 13A/dbpA′/A spirochetes (see Video S1D in the supplemental material). Under these conditions, spirochetes with increased DbpA expression were essentially aggregated. This impaired motility apparently resulted from the increased interaction of B. burgdorferi with decorin due to increasing DbpA expression.
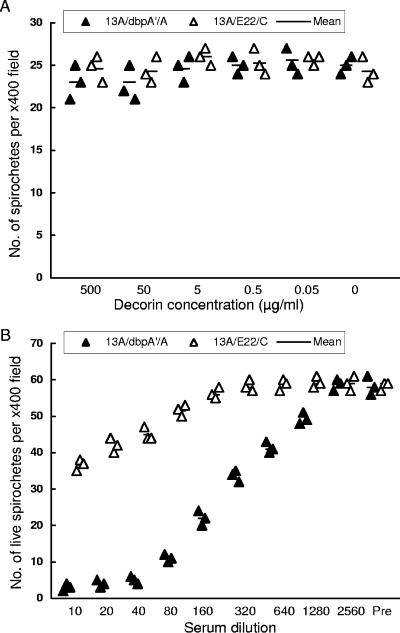
Next, we investigated whether the enhanced interaction of B. burgdorferi with decorin affected in vitro spirochetal growth. B. burgdorferi was grown in the presence of decorin at various concentrations. Surprisingly, the 13A/dbpA′/A spirochetes replicated as well as the 13A/E22/C cells (P > 0.05) (Fig. 2A), despite that the presence of decorin aggregated bacteria and severely interfered with their motility (see video S1 in the supplemental material). Thus, the study indicates that the increased interaction of B. burgdorferi with decorin resulting from increased DbpA expression does not affect spirochetal growth.
FIG. 2.
Influence of increasing DbpA expression on in vitro spirochetal growth in the presence of decorin or anti-DbpA antibodies. (A) Enhanced interaction of B. burgdorferi with decorin does not affect growth. The 13A/dbpA′/A or 13A/E22/C spirochetes were grown in BSK medium supplemented with decorin at concentrations of 0 to 500 μg/ml for 72 h. Samples were fourfold diluted with double-distilled H2O; spirochetes were counted in at least five dark-field microscope fields; calculated means are presented. The experiment was performed in triplicate. (B) B. burgdorferi with increased DbpA expression becomes more sensitive to in vitro killing/inhibition by anti-DbpA antibodies. Clones 13A/dbpA′/A and 13A/E22/C were grown in BSK-H medium containing different dilutions (1:10 to 1:2,560) of anti-DbpA sera or control mouse sera (Pre) in the presence of complement for 48 h. Viable spirochetes were counted in at least five dark-field microscope fields, and calculated means are presented. The experiment was performed in triplicate.
Increasing DbpA expression increases sensitivity to in vitro growth inhibition by anti-DbpA antibodies.
An in vitro killing/inhibition assay was used to determine whether higher DbpA expression increased sensitivity to specific antibody. At 1:10 dilution, DbpA antisera reduced 13A/E22/C and 13A/dbpA′/A growth by 1.6- and 19.3-fold, respectively, in comparison with preimmune sera (Fig. 2B). The antisera showed significant inhibition activity against the control spirochetes at a dilution of 1:80 (P = 0.007), in contrast to clone 13A/dbpA′/A at dilutions as high as 1:1,280 (P = 0.006). These data clearly indicate that increasing DbpA expression dramatically increases the sensitivity of B. burgdorferi to growth inhibition by specific antibody.
Increasing DbpA expression diminishes arthritis virulence and reduces the tissue spirochetal burden in the joints of SCID mice.
The influence of increasing DbpA expression on infectivity and pathogenicity was first assessed in immunodeficient mice. Subgroups of five SCID mice were challenged with clone 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B. Joint swelling evolved in each of the 10 mice that had received either 13A/E22/C or 13A/E22/D after approximately 10 days and quickly developed into severe arthritis (Fig. 3A). In contrast, none of the 10 mice that were inoculated with clone 13A/dbpA′/A or 13A/dbpA′/B ever presented with joint swelling during the 6-week period. Histopathological examination confirmed that intensive lesions appeared in the tissues in or around the tibial bones of mice that had received the 13A/E22/C or 13A/E22/D spirochetes, but not in those that were inoculated with clone 13A/dbpA′/A or 13A/dbpA′/B (Fig. 3B). The inability of clones 13A/dbpA′/A and 13A/dbpA′/B to induce arthritis could be due to loss of infectivity. To rule out this possibility, the heart, joint, and skin samples were cultured for spirochetes. B. burgdorferi was readily recovered from all 30 samples of the 10 mice inoculated with either clone 13A/dbpA′/A or 13A/dbpA′/B (data not shown), indicating that increasing DbpA expression does not abrogate infectivity but diminishes arthritis virulence.
FIG. 3.
Increasing DbpA expression diminishes arthritis virulence in SCID mice. (A) SCID mice were inoculated with either transformant 13A/E22/C or 13A/dbpA′/A, and sacrificed 6 weeks later. Severe joint swelling was noted only in mice challenged with 13A/E22/C. (B) Intensive tissue lesions caused by a severe inflammatory response adjacent to the tibial bone was noted in mice infected with 13A/E22/C, but not those infected with 13A/dbpA′/A. Tissue sections were stained with hematoxylin and eosin. tb, tibial bone; pe, periostium; at, adipose tissue; te, tendon; lct, loose connective tissue; de, dermis.
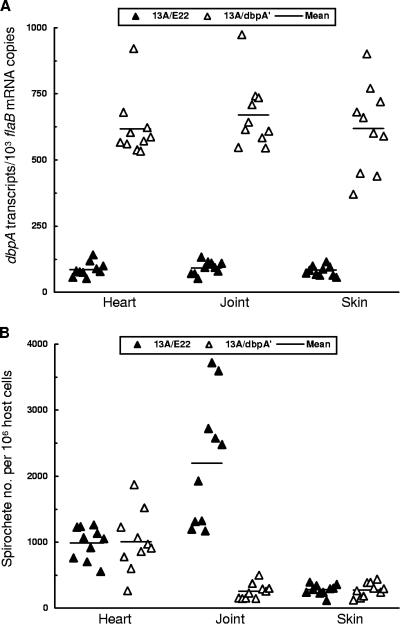
To confirm in vivo increased dbpA expression driven by the fused flaB promoter, RNA was prepared from all heart, joint, and skin specimens of the 20 mice and assessed for the relative copy numbers of dbpA and flaB mRNAs by RT-qPCR. The presence of the construct pBBE22-dbpA′ increased the dbpA mRNA copy numbers by 7.3-, 7.3-, and 7.5-fold in the heart (P = 3.0 × 10−11), joint (P = 4.2 × 10−11), and skin (P = 5.7 × 10−9) tissues, respectively (Fig. 4A), indicating that increased dbpA expression indeed occurred during murine infection.
FIG. 4.
Increasing DbpA expression dramatically reduces the spirochetal load in the joint tissue, but not in the heart or skin, of SCID mice. Subgroups of five BALB/c SCID mice were infected with clone 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B and euthanized 6 weeks later. The four subgroups were combined into two groups (13A/E22/C and 13A/E22/D; 13A/dbpA′/A and 13A/dbpA′/B) when the data were analyzed. (A) RNA samples were prepared from the heart, joint, and skin tissues, and flaB and dbpA expression was quantified by RT-qPCR. The data are presented as dbpA mRNA copy numbers per 1,000 flaB transcripts. (B) DNA samples were prepared from the heart, joint, and skin specimens and analyzed for spirochetal flaB and murine actin DNA copies by qPCR. The data are expressed as spirochete numbers per 106 host cells.
Next, the tissue spirochete burden was determined as an indication of tissue colonization. DNA was prepared from the heart, joint, and skin specimens of the 20 mice and quantified by qPCR (Fig. 4B). Increasing DbpA expression did not affect the spirochete load, either in the heart (P = 0.92) or skin tissue (P = 0.94), but caused an 8.7-fold decrease in the joint (P = 6.1 × 10−6).
Increasing DbpA expression significantly reduces ID50 and severely impairs spirochetal dissemination in both SCID and immunocompetent mice.
The influence of increasing DbpA expression on the ID50 value and spirochetal dissemination was first investigated in immunodeficient mice. Groups of three animals each received a single inoculation of 101 to 105 spirochetes of clone 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B. Ear biopsy specimens were taken for bacterial culture at 2, 3, 4, 5, and 6 weeks postinoculation. The animals were euthanized immediately after the last biopsy; then, heart, joint and skin samples were cultured for spirochetes and for ID50 determination. At 2 weeks postinoculation, 21 out of the 24 mice inoculated with clone 13A/E22/C or 13A/E22/D at doses of 102 or higher had a positive biopsy (Table 1). In contrast, none of the mice inoculated with clone 13A/dbpA′/A or 13A/dbpA′/B at these doses produced a positive biopsy in the same time frame. Seven mice did not show a positive biopsy until 5 weeks after inoculation with the two clones. The study allows us to conclude that increasing DbpA expression severely impairs the dissemination of B. burgdorferi in immunodeficient mice.
TABLE 1.
Increasing DbpA expression severely impairs dissemination and significantly reduces ID50 of B. burgdorferi in SCID micea
| Inoculum and dose | No. of biopsies positive/total no. of ear biopsies conducted at postinoculation weekb:
|
No. of cultures positive/total no. of specimens examined
|
No. of mice infected/total no. of mice inoculated | ID50 (no. of organisms) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | Heart | Joint | Skin | All sites | |||
| Expt I | |||||||||||
| 13A/E22/C | 18 | ||||||||||
| 105 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 2/3 | 3/3 | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| 13A/E22/D | 32 | ||||||||||
| 105 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 1/3 | 3/3 | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 13A/dbpA′/A | 3 | ||||||||||
| 105 | 0/3 | 2/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 0/3 | 3/3 | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 1/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 2/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 1/3 | 3/3 | ND | 3/3 | 2/3 | 3/3 | 8/9 | 3/3 | |
| 13A/dbpA′/B | 6 | ||||||||||
| 105 | 0/3 | 2/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 0/3 | 1/3 | 2/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 2/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 2/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 8/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 2/3 | 2/3 | 2/3 | 3/3 | 2/3 | 6/9 | 2/3 | |
| Expt II | |||||||||||
| 13A/E22/C | 32 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 13A/E22/D | 18 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| 13A/dbpA′/A | 3 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 3/3 | 2/3 | 3/3 | 8/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 13A/dbpA′/B | 6 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 2/3 | 2/3 | 2/3 | 6/9 | 2/3 | |
The 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B spirochetes were grown to late log phase (108 cells per ml) and 10-fold serially diluted with BSK-H medium. Groups of three BALB/c SCID mice each received a single intradermal/subcutaneous dose of 100 μl of bacterial suspension; ear biopsies were performed up to 6 weeks postinoculation, starting at week 2. Once all three animals of a dose group became positive, biopsies were no longer performed on the group. All animals were sacrificed immediately after the last biopsy; heart, tibiotarsal joint, and skin specimens were harvested for bacterial isolation. Experiment II was designed to assess ID50 values only, so a biopsy was not conducted. The ID50 values were calculated by the method of Reed and Muench (35).
ND, not determined.
An arthritis assessment found that all 25 mice infected with clone 13A/E22/C or 13A/E22/D developed severe arthritis and none of the mice inoculated with 13A/dbpA′/A or 13A/dbpA′/B showed joint swelling (data not shown). Again, the study confirmed that increasing DbpA expression abrogates arthritis virulence in SCID mice.
The ID50 values of clones 13A/E22/C and 13A/E22/D were 18 and 32 organisms, respectively, in comparison to 13A/dbpA′/A and 13A/dbpA′/B, with values of 3 and 6 in experiment I (Table 1). Consistently, the values measured for 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B were 32, 18, 3, and 6 organisms, respectively, in experiment II (Table 1). The combination of these two experiments indicates a 5.6-fold decrease in the ID50 value resulting from increasing DbpA expression in SCID mice (P = 0.003).
Next, the influence of increasing DbpA expression on the ID50 value and spirochetal dissemination was investigated in immunocompetent mice. At 2 weeks postinoculation, all 18 mice that received 103 or more organisms of clone 13A/E22/C or 13A/E22/D had a positive biopsy; all 6 mice given 102 bacteria became positive a week later (Table 2). In contrast, none of the mice inoculated with transformant 13A/dbpA′/A or 13A/dbpA′/B produced a positive biopsy at 2 weeks; only two of the 30 inoculated mice produced a positive biopsy at 3 weeks, and seven more became positive a week later. Most inoculated mice did not develop a positive ear biopsy until week 5 or 6. Two inoculated mice did not produce a single positive ear biopsy during the period of 6 weeks but were found to be infected only during necropsy. Again, increasing DbpA expression even more severely delayed the dissemination of B. burgdorferi in immunocompetent mice.
TABLE 2.
Increasing DbpA expression severely impairs dissemination and significantly reduces ID50 of B. burgdorferi in immunocompetent micea
| Inoculum and dose | No. of biopsies positive/total no. of ear biopsies conducted at postinoculation week:
|
No. of cultures positive/total no. of specimens examined
|
No. of mice infected/total no. of mice inoculated | ID50 (no. of organisms) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | Heart | Joint | Skin | All sites | |||
| Expt I | |||||||||||
| 13A/E22/C | 32 | ||||||||||
| 105 | 3/3 | NDb | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | ND | 2/3 | 3/3 | 3/3 | 8/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 13A/E22/D | 32 | ||||||||||
| 105 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 13A/dbpA′/A | 18 | ||||||||||
| 105 | 0/3 | 0/3 | 0/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 0/3 | 0/3 | 0/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 1/3 | 3/3 | ND | ND | 3/3 | 2/3 | 3/3 | 8/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 2/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 1/3 | 1/9 | 1/3 | |
| 13A/dbpA′/B | 18 | ||||||||||
| 105 | 0/3 | 0/3 | 0/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 104 | 0/3 | 1/3 | 1/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 0/3 | 1/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 2/3 | 0/3 | 2/3 | 3/3 | 2/3 | 3/3 | 8/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| Expt II | |||||||||||
| 13A/E22/C | 32 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 13A/E22/D | 18 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 2/3 | 3/3 | 3/3 | 8/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 0/3 | 1/3 | 1/3 | 2/9 | 0/3 | |
| 13A/dbpA′/A | 18 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 2/3 | 2/3 | 3/3 | 7/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 1/3 | 0/3 | 1/3 | 2/9 | 1/3 | |
| 13A/dbpA′/B | 6 | ||||||||||
| 102 | ND | ND | ND | ND | ND | 2/3 | 1/3 | 3/3 | 6/9 | 3/3 | |
| 101 | ND | ND | ND | ND | ND | 1/3 | 1/3 | 2/3 | 4/9 | 2/3 | |
The 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B spirochetes were grown to late log phase (108 cells per ml) and 10-fold serially diluted with BSK-H medium. Groups of three BALB/c mice each received a single intradermal/subcutaneous dose of 100 μl of bacterial suspension; ear biopsies were performed up to 6 weeks post-inoculation, starting at week 2. Once all three animals of a dose group became positive, biopsies were no longer performed on the group. All animals were sacrificed immediately after the last biopsy; heart, tibiotarsal joint, and skin specimens were harvested for bacterial isolation. Experiment II was designed to assess ID50 values only, so a biopsy was not conducted. The ID50 values were calculated by the method of Reed and Muench (35).
ND, not determined.
The ID50 values of clones 13A/E22/C and 13A/E22/D were 32 and 32 organisms, respectively, in comparison to 13A/dbpA′/A and 13A/dbpA′/B, with values of 18 and 18 in experiment I (Table 2). The values were 32, 18, 18, and 6 for clones 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, and 13A/dbpA′/B, respectively, in a repeat experiment. Again, the study indicates that increasing DbpA expression results in a 2.0-fold increase in infectivity, as measured by the ID50 values in immunocompetent mice (P = 0.03).
Increasing DbpA expression diminishes the ability of B. burgdorferi to persist in the heart and joint tissues during chronic infection of immunocompetent mice.
Subgroups of five BALB/c mice each received a single intradermal/subcutaneous injection of 104 spirochetes of clone 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B. Retro-orbital blood was selectively drawn for assessing the immune response at intervals of 2 to 4 weeks, starting at week 2. Animals were euthanized 5 months postinoculation; B. burgdorferi was grown from each skin specimen of all 20 mice regardless of whether they received 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B bacteria (Table 3). The control spirochetes were successfully grown from all 10 hearts and 9 out of the 10 joint specimens; however, B. burgdorferi with increased DbpA expression was cleared from 7 of the 10 hearts and 7 of the 10 joint specimens. The study indicates that increasing DbpA expression diminishes the ability of B. burgdorferi to persist in the heart and joint tissues during chronic infection of immunocompetent mice.
TABLE 3.
Increasing DbpA expression diminishes the ability of B. burgdorferi to persist in the heart and joint tissues during chronic infection of immunocompetent micea
| Inoculum | No. of cultures positive/total no. of specimens examined
|
No. of mice infected/total no. of mice inoculated | |||
|---|---|---|---|---|---|
| Heart | Joint | Skin | All sites | ||
| 13A/E22/C | 5/5 | 4/5 | 5/5 | 14/15 | 5/5 |
| 13A/E22/D | 5/5 | 5/5 | 5/5 | 15/15 | 5/5 |
| 13A/dbpA′/A | 2/5 | 1/5 | 5/5 | 8/15 | 5/5 |
| 13A/dbpA′/B | 1/5 | 2/5 | 5/5 | 8/15 | 5/5 |
Groups of five BALB/c mice were inoculated with clone 13A/E22/C, 13A/E22/D, 13A/dbpA′/A, or 13A/dbpA′/B and sacrificed 5 months later. Heart, tibiotarsal joint, and skin specimens were harvested and cultured for spirochetes in BSK-H medium.
Retro-orbital blood was used to monitor the specific humoral response by a DbpA ELISA. Infection with clone 13A/dbpA′/A elicited a 17-, 10-, and 8.4-fold-stronger anti-DbpA humoral response than 13A/E22/C at 2 (P = 0.02), 4 (P = 0.005), and 6 weeks postinoculation (P = 0.04) (Fig. 5). The anti-DbpA response reached a plateau within 4 weeks after inoculation with clone 13A/dbpA′/A (Fig. 5) but did not reach the level in mice infected with 13A/E22/C until 12 weeks (data not shown). This stronger humoral response suggests increased DbpA expression resulting from the introduction of an extra dbpA copy driven by a flaB promoter.
FIG. 5.
B. burgdorferi with increased DbpA expression stimulates a faster and stronger anti-DbpA humoral response. Five BALB/c mice were inoculated with either transformant 13A/E22/C or 13A/dbpA′/A. Retro-orbital sinus blood was drawn every 2 to 4 weeks for 5 months, starting at week 2 postinoculation, and monitored for anti-DbpA titers by an end point ELISA. Data for samples collected at 2, 4, 6, and 26 weeks are selectively presented.
B. burgdorferi recovered from all 16 positive heart, joint, and skin specimens from the 10 mice infected with clone 13A/dbpA′/A or 13/A/dbpA′/B was analyzed for possible mutations. Isolates were grown to mid-log phase and examined for DbpA expression using immunoblotting. All 16 isolates abundantly expressed DbpA like the original inocula (data not shown), suggesting that it was unlikely that mutations had occurred on the introduced dbpA copy. DNA was extracted from 5 of the 16 isolates and sequenced after replication in E. coli; no mutation was noticed (data not shown). All the analyses indicated that no escape mutations on the introduced copy were selected for during chronic infection of immunocompetent mice.
DISCUSSION
Tight regulation of surface antigenic expression is crucial for the pathogenic strategy of B. burgdorferi. To investigate the influence of increasing DbpA expression on overall infectivity and pathogenicity, B. burgdorferi was modified to constitutively express the surface adhesin by introducing a promoterless dbpA copy fused with a flaB promoter. Increasing DbpA expression dramatically increased the interaction of B. burgdorferi with decorin; however, this enhanced interaction did not affect in vitro spirochetal growth in the presence of decorin. Higher DbpA expression also remarkably increased sensitivity to in vitro inhibition/killing by specific antibodies. Increasing DbpA expression significantly reduced ID50 values and severely delayed spirochetal dissemination to distal tissues in both SCID and immunocompetent mice. During infection of SCID mice, increasing DbpA expression did not affect tissue colonization in the heart and skin but dramatically reduced the spirochetal load in the joint and completely diminished arthritis virulence. Finally, B. burgdorferi with increased DbpA expression was able to effectively persist in the skin but was essentially cleared from both heart and joint tissues during chronic infection of immunocompetent mice.
In the study, immunodeficient mice were used to provide a mammalian environment in which adaptive immune responses were completely absent, allowing us to investigate the influence of increasing DbpA expression on the infectivity, dissemination, pathogenicity, and tissue colonization of B. burgdorferi under these special circumstances. In contrast, immunocompetent mice were used to study the influence of increasing DbpA expression on spirochetal infectivity and persistence in the presence of vigorous immune responses against the pathogen.
As a surface-exposed lipoprotein, DbpA binds decorin and glycosaminoglycans (14, 19), two key building blocks of proteoglycans, which are found in the extracellular matrix and connective tissues, as well as on the surfaces of mammalian cells. As an extracellular bacterium, B. burgdorferi primarily resides in niches where these two ligands abundantly exist. After inoculation into the dermis of a murine host, B. burgdorferi first replicates in the local tissues, where the pathogen interacts with host components, and then disseminates to distal tissues. Our in vitro assay showed that increasing DbpA expression dramatically enhanced the interaction of B. burgdorferi with decorin and that this increased interaction severely reduced the motility of spirochetes but did not affect spirochetal growth or replication. The increase in the interaction of B. burgdorferi with decorin attributed to the higher DbpA expression likely facilitates the attachment of spirochetes to the dermis tissue, where decorin expression is extremely high (23). This enhanced attachment may lead to better protection for the pathogen and may help it gain a foothold during the initial infection, thus significantly reducing ID50 values in both SCID and immunocompetent mice. However, this increased interaction may severely restrain the motility of B. burgdorferi, and as a result, it dramatically impairs spirochetal dissemination to distal tissues in SCID, as well as immunocompetent, mice.
Increasing DbpA expression did not affect in vitro spirochetal growth in the presence of decorin. The significant reduction in the ID50 value resulting from the increased DbpA expression in both SCID and immunocompetent mice indicates that increasing DbpA expression does not reduce in vivo spirochetal growth or replication. B. burgdorferi with increased DbpA expression colonized both the heart and skin tissues, as well as the control spirochetes, in the absence of specific immune responses, further suggesting that increasing interactions with host decorin does not reduce the viability of spirochetes in mice. However, increasing DbpA expression indeed significantly reduced the bacterial load in the joint tissues. In addition to DbpA, B. burgdorferi expresses several other surface-adhesive molecules (6), including DbpB (3, 19-21), the fibronectin-binding protein BBK32 (32, 33), Bgp (Borrelia glycosaminoglycan-binding protein) (30, 31), and P66 (5, 9). Bgp binds glycosaminoglycans (30); DbpA, DbpB, and BBK32 bind decorin and fibronectin (3, 19, 32, 33), in addition to the binding of glycosaminoglycans (13, 14). The outer membrane protein P66 binds a cell surface receptor, the integrin αIIbβ3 (5, 9). In addition, there are unidentified borrelial adhesins that interact with a different cell surface receptor, the integrin α3β1 (2). Although DbpA, DbpB, BBK32, and Bgp all bind glycosaminoglycans, they show distinct specificities for members of the large glycosaminoglycan family (13, 14, 30). Different glycosaminoglycans are found in different tissues. Increasing DbpA expression on the spirochete's surface may hinder the interactions of other adhesins with their host ligands, thus changing the binding specificity of B. burgdorferi. This unbalanced surface antigen expression due to increasing DbpA expression may reduce the overall interactions of B. burgdorferi in the joint tissues, and as a consequence, it decreases the bacterial load in this specific tissue.
It should not be surprising that increasing DbpA expression completely diminishes the ability of B. burgdorferi to induce arthritis in SCID mice if the tissue spirochetal burden is the only crucial factor that determines arthritis virulence. It should also be kept in mind that the joint consists of multiple tissues, which are composed of various cell types, extracellular matrices, and connective tissues. As discussed above, increasing DbpA expression may alter the binding specificity of B. burgdorferi and facilitates the colonization of selective tissues, but it reduces colonization of other tissues, so increasing DbpA expression may restrict B. burgdorferi from colonizing the site that is essential for the development of arthritic pathology. Finally, there has been no evidence against the notion that DbpA, when expressed on the spirochete's surface, may reduce the inflammatory response. If this is true, it also remains to be determined whether the reduction in the ID50 value is, in part, due to a reduced inflammatory response resulting from increased DbpA expression.
Our in vitro assay showed that increasing DbpA expression dramatically increases sensitivity to inhibition/killing by anti-DbpA antibodies. However, B. burgdorferi with increased DbpA expression was consistently grown from all heart, joint, and skin specimens from immunocompetent mice that had been inoculated with a higher dose, at least within the first 6 weeks of infection, despite the development of a strong anti-DbpA antibody response. Five months after infection, although spirochetes with increased DbpA expression were cleared from almost all heart and joint samples, all of the skin tissues remained persistently infected. Based on these observations, DbpA appears to be ineffectively targeted by protective antibodies in mammalian tissues, in sharp contrast to other surface antigens, such as OspC and OspA/B. Constitutive expression of either OspC or OspA/B completely diminishes the ability of B. burgdorferi to cause persistent infection in immunocompetent mice (43, 49). A previous study suggested that the interaction of DbpA with decorin may provide B. burgdorferi a protective strategy to evade specific humoral immunity (23). Decorin is abundantly expressed in skin tissue (23); because the pathogen is provided with sufficient decorin to interact with, B. burgdorferi with higher DbpA expression can persist against the strong immune response. The interaction of DbpA with decorin may reduce the effectiveness of anti-DbpA antibodies in targeting B. burgdorferi in this specific tissue. In the joint and heart tissues, lower decorin expression may be unable to provide sufficient ligands to occupy overexpressed DbpA; thus, the antigen is better exposed to specific antibodies and mediates killing/inhibition effects, resulting in clearance of the pathogen in these tissues.
During mammalian infection, numerous events occur, including initial tissue colonization, dissemination to and colonization in distal tissues, and persistence. Increasing DbpA expression significantly reduces the ID50 value and severely delays dissemination. Although increased DbpA expression only reduces tissue colonization in the joint in the absence of specific immune responses, the specific immune response more effectively clears spirochetes with high DbpA expression in both joint and heart tissues. DbpA may significantly contribute to overall infectivity and pathogenicity when expressed at an appropriate level; however, increasing its expression profoundly changes the phenotype of B. burgdorferi in a negative way. Taken together, the study highlights the importance of tight regulation of surface antigen expression in the infectivity, dissemination, tissue colonization, pathogenicity, and persistence of B. burgdorferi during mammalian infection.
Supplementary Material
Acknowledgments
We thank S. Norris for providing pBBE22 and M. T. Kearney for assistance with statistical analysis.
This work was supported in part by a career development award and a grant from NIH/NIAMS and an Arthritis Foundation Investigators award.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 11 June 2007.
Supplemental material for this article may be found at http://iai.asm.org.
REFERENCES
- 1.Barthold, S. W. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J. Infect. Dis. 163:419-420. [DOI] [PubMed] [Google Scholar]
- 2.Behera, A. K., E. Hildebrand, S. Uematsu, S. Akira, J. Coburn, and L. T. Hu. 2006. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin α3β1. J. Immunol. 177:657-664. [DOI] [PubMed] [Google Scholar]
- 3.Brown, E. L., B. P. Guo, P. O'Neal, and M. Hook. 1999. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J. Biol. Chem. 274:26272-26278. [DOI] [PubMed] [Google Scholar]
- 4.Brown, E. L., R. M. Wooten, B. J. Johnson, R. V. Iozzo, A. Smith, M. C. Dolan, B. P. Guo, J. J. Weis, and M. Hook. 2001. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Investig. 107:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn, J., W. Chege, L. Magoun, S. C. Bodary, and J. M. Leong. 1999. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926-940. [DOI] [PubMed] [Google Scholar]
- 6.Coburn, J., J. R. Fischer, and J. M. Leong. 2005. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol. Microbiol. 57:1182-1195. [DOI] [PubMed] [Google Scholar]
- 7.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72:5063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defoe, G., and J. Coburn. 2001. Delineation of Borrelia burgdorferi p66 sequences required for integrin αIIbβ3 recognition. Infect. Immun. 69:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva, A. M., D. Fish, T. R. Burkot, Y. Zhang, and E. Fikrig. 1997. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect. Immun. 65:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fingerle, V., G. Goettner, L. Gern, B. Wilske, and U. Schulte-Spechtel. 2007. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int. J. Med. Microbiol. 297:97-107. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, J. R., N. Parveen, L. Magoun, and J. M. Leong. 2003. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 100:7307-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung, B. P., G. L. McHugh, J. M. Leong, and A. C. Steere. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 62:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, A. J. Nowalk, D. R. Clifton, C. Nolder, J. L. Hughes, and J. A. Carroll. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes bba64, bba65, and bba66 during persistent infection in mice. Infect. Immun. 75:2753-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, R. D., Jr., and J. Piesman. 2000. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect. Immun. 68:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 20.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, F. T., E. L. Brown, T. Wang, R. V. Iozzo, and E. Fikrig. 2004. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am. J. Pathol. 165:977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. Deponte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parveen, N., and J. M. Leong. 2000. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:1220-1234. [DOI] [PubMed] [Google Scholar]
- 31.Parveen, N., D. Robbins, and J. M. Leong. 1999. Strain variation in glycosaminoglycan recognition influences cell-type-specific binding by Lyme disease spirochetes. Infect. Immun. 67:1743-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 33.Probert, W. S., J. H. Kim, M. Hook, and B. J. Johnson. 2001. Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect. Immun. 69:4129-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 35.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoint. Am. J. Hygiene 27:493-497. [Google Scholar]
- 36.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seiler, K. P., and J. J. Weis. 1996. Immunity to Lyme disease: protection, pathology and persistence. Curr. Opin. Immunol. 8:503-509. [DOI] [PubMed] [Google Scholar]
- 39.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Hook, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 40.Shi, Y., Q. Xu, S. V. Seemanapalli, K. McShan, and F. T. Liang. 2006. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect. Immun. 74:6509-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 42.Stewart, P. E., X. Wang, D. M. Bueschel, D. R. Clifton, D. Grimm, K. Tilly, J. A. Carroll, J. J. Weis, and P. A. Rosa. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 74:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strother, K. O., E. Hodzic, S. W. Barthold, and A. M. de Silva. 2007. Infection of mice with Lyme disease spirochetes constitutively producing outer surface proteins A and B. Infect. Immun. 75:2786-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. Vanraden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsao, J., A. G. Barbour, C. J. Luke, E. Fikrig, and D. Fish. 2001. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:65-74. [DOI] [PubMed] [Google Scholar]
- 46.Tsao, J. I., J. T. Wootton, J. Bunikis, M. G. Luna, D. Fish, and A. G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA 101:18159-18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu, Q., K. McShan, and F. T. Liang. 2007. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol. Microbiol. 64:220-231. [DOI] [PubMed] [Google Scholar]
- 48.Xu, Q., S. V. Seemanapalli, L. Lomax, K. McShan, X. Li, E. Fikrig, and F. T. Liang. 2005. Association of linear plasmid 28-1 with an arthritic phenotype of Borrelia burgdorferi. Infect. Immun. 73:7208-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, Q., S. V. Seemanapalli, K. McShan, and F. T. Liang. 2006. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 74:5177-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.