Abstract
Vibrio vulnificus is an estuarine bacterium that can cause primary septicemia as well as serious wound infections. Generally, clinical isolates have a high lethal effect compared with environmental isolates. However, little is known about the mechanisms by which V. vulnificus causes disease. In this study, we compared the pathogenicity of a clinical isolate, strain M2799, with that of an environmental isolate, strain JCM3731. The clinical isolate showed 100 times higher lethality in mice than the environmental isolate. In strain M2799-inoculated mice, the number of macrophages decreased significantly, whereas there was no appreciable change in the number of macrophages in strain JCM3731-inoculated mice. The clinical isolate showed high cytotoxic activity, especially to macrophages, compared with the environmental isolate in vitro. The growth of the clinical isolate was almost completely inhibited in the presence of macrophages. Moreover, the survival rate of the clinical isolate-inoculated mice increased by recruitment of macrophages. These results indicate that V. vulnificus infection progresses by damage to macrophages during the early phase of infection.
Vibrio vulnificus is a gram-negative halophilic marine bacterium that is found in estuarine waters and contaminates oysters and other seafood. Consumption of raw contaminated seafood or contamination of wounds with V. vulnificus can lead to septicemia or wound infection, respectively. Infection with V. vulnificus is associated with predisposing conditions such as hepatitis, cirrhosis, diabetes, hemochromatosis, and immune compromise (1, 2, 15, 22). V. vulnificus causes a rapid and severe disease process resulting in extensive tissue damage. Mortality was up to 50% in septic patients, with most of them dying within 48 h with a fulminate course after infection (8). In addition, clinical isolates have generally high lethality in mice and high cytotoxicity to various cultured cells compared with environmental isolates (11, 26, 27).
Several putative virulence factors of V. vulnificus, such as metalloprotease (14, 19), hemolysin-cytolysin (7, 23), capsular polysaccharide (CPS) (25, 32), and means of iron acquisition (e.g., siderophore) (18), have been reported in vivo and in vitro. Lethality in animal models is clearly related to CPS expression. Both CPS expression and virulence are associated with an opaque colony morphology (25, 33). V. vulnificus strains with encapsulated phenotypes (opaque colonies) have a much higher lethality in mice than those with unencapsulated phenotypes (translucent colonies). This is considered to be due to CPS providing a protective barrier against phagocytosis by neutrophils and macrophages and against bacteriolysis by complement (24, 29, 31, 32). Many reports have focused on the interaction between phagocytes and CPS (17, 25, 28, 33); however, there is little in the literature on host immune responses to V. vulnificus.
Despite considerable published research on the virulence factors of V. vulnificus, very little definitive information has been obtained (26). Recently, Kashimoto et al. reported that lymphocytes but not neutrophils were depleted, via apoptosis, by V. vulnificus (12). Extensive loss of lymphocytes was observed in patients due to polymicrobial sepsis, and in a mouse model of sepsis, polycaspase and caspase 3 inhibitor prevented lymphocyte apoptosis and improved survival (9, 10). Lymphocyte apoptosis is thus considered to be involved in lethality in polymicrobial sepsis. A clinical isolate of V. vulnificus induced apoptosis in macrophages, but an environmental isolate could not induce apoptosis in vivo or in vitro (11). Much remains to be elucidated about the roles of lymphocytes and macrophages in the host defense against V. vulnificus. Here, we report that V. vulnificus infection progresses by damaging macrophages during the early phase of infection.
MATERIALS AND METHODS
Bacterial strains and media.
The clinical isolate of V. vulnificus, strain M2799, was generously provided by S. Miyoshi (Okayama University, Okayama, Japan), and the environmental isolate, strain JCM3731, was obtained from the Laboratory for Culture Collection, Research Institute for Microbial Diseases, Osaka University. These strains were precultured in heart infusion broth (Eiken Chemical, Tokyo, Japan) containing 2% NaCl (HI medium) at 37°C for 3 h. The culture was diluted 1:100 into fresh broth and then shaken at 37°C until the late logarithmic growth phase. For agar medium, HI medium was solidified with 1.5% (wt/vol) agar (Nacalai Tesque, Kyoto, Japan).
Survival rate.
Specific-pathogen-free BALB/c female mice were purchased from Japan SLC (Shizuoka, Japan). All experimental mice were between the ages of 8 and 10 weeks. V. vulnificus was injected intraperitoneally into mice to investigate the survival rate. All experiments were performed in accordance with the guidelines for the care and use of laboratory animals of Osaka University of Pharmaceutical Sciences.
Distribution of V. vulnificus.
V. vulnificus-inoculated mice were sacrificed, and various tissues were removed. Each tissue was homogenized with phosphate-buffered saline in a loose glass homogenizer. Cell suspensions were plated on sodium dodecyl sulfate-polymyxin B-sucrose agar plates (13) and cultured at 37°C for 12 h.
Flow cytometry analysis.
Fluorescein isothiocyanate-conjugated rat anti-mouse Ly-6G and Ly-6C monoclonal antibodies (BD Pharmingen, Mountain View, CA) and R-phycoerythrin-conjugated rat anti-mouse CD11b monoclonal antibody (BD Pharmingen) were used as primary antibodies. Flow cytometric profiles were analyzed with a FACScan analyzer and CellQuest software (Becton Dickinson, Mountain View, CA).
In vitro cytotoxicity assay.
Neutrophil-rich peritoneal exudate cells (PECs) and macrophage-rich PECs were obtained from the peritoneal cavity by intraperitoneally injecting 12% casein or Brewer modified thioglycolate medium (Becton Dickinson), respectively, into normal BALB/c mice. The cells were found to comprise >80% neutrophils and >85% macrophages, respectively, using a differential cell-staining kit (Diff-Quik kit; Kokusai Shiyaku, Osaka, Japan) or flow cytometry analysis. The mouse macrophage-like cell line J774A (RIKEN BioResource Center, Ibaraki, Japan), thioglycolate-induced macrophages, casein-induced neutrophils, splenocytes, and thymocytes were cultured in RPMI 1640 (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum and 100 μM 2-mercaptoethanol. Serially diluted bacterial suspensions were then inoculated onto several cells at a dose of 7.5 × 105 cells/well in 24-well culture plates. Cytotoxicity was measured after 5 h with a nonradioactive cytotoxicity assay kit (Promega, Madison, WI) according to the manufacturer's instructions. Triton X-100 was used as a positive control, and cytotoxicity was expressed as the ratio of the subject to a positive control.
Effect of leukocytes on bacterial growth.
Thioglycolate-induced macrophages or casein-induced neutrophils (1 × 106 cells/well) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 μM 2-mercaptoethanol, and 10% guinea pig complement (Cedarlane Labs, Ontario, Canada) in 24-well culture plates. The clinical isolate of V. vulnificus (5 × 103 CFU) was cultured on the macrophages or neutrophils for 3 h. After cultivation, bacterial suspensions were plated on sodium dodecyl sulfate-polymyxin B-sucrose agar plates and cultured at 37°C for 12 h.
Effect of leukocytes on the survival of V. vulnificus-inoculated mice.
Macrophages or neutrophils were intraperitoneally injected into BALB/c mice at a dose of 1 × 107 cells/mouse, and then the clinical isolate was intraperitoneally injected at a dose of 2 × 106 CFU/mouse. The survival of the mice was recorded every 1 h for 48 h.
Statistical analysis.
The significance of differences was calculated by one-way analysis of variance. Differences were accepted as statistically significant if the P value was <0.05.
RESULTS
Colony morphology of Vibrio vulnificus.
We first observed the morphology of colonies of V. vulnificus. The clinical isolate, strain M2799, and the environmental isolate, strain JCM3731, produced opaque colonies and translucent colonies, respectively (Fig. 1). The strain M2799 colonies that developed in culture at 37°C were relatively larger than those of strain JCM3731.
FIG. 1.
Morphology of V. vulnificus colonies. Strain M2799 (A) and strain JCM3731 (B) were seeded onto an HI agar plate and cultured for 12 h at 37°C.
Survival of V. vulnificus-inoculated mice.
We investigated the survival of strain M2799- and strain JCM3731-inoculated mice. As shown in Fig. 2, inoculation with more than 4 × 106 CFU of strain M2799 killed all of the mice within 12 h, and the survival rate with 2 × 106 CFU was 20% after 24 h. In contrast, with strain JCM3731, all of the mice inoculated with 4 × 107 CFU or less survived for 24 h. The survival of mice inoculated with 2 × 108 CFU began to decrease at 17 h postinoculation, and the survival rate after 24 h was 40%. These results suggest that the clinical isolate has 100 times higher lethality to mice than the environmental isolate.
FIG. 2.
Survival of V. vulnificus-inoculated mice. BALB/c female mice (n = 5 for each dilution of bacterial suspension) were intraperitoneally inoculated with strain M2799 (A) or JCM3731(B). The survival of the mice was recorded every hour for 24 h.
Distribution of V. vulnificus in mice.
We studied the distribution of V. vulnificus in various mouse tissues after intraperitoneal injection. At 1 h postinoculation, strain M2799 was detected not only in the tissues of the peritoneal cavity (e.g., liver, spleen, and kidney) but also in peripheral blood and thymus (Fig. 3A). At 3 h postinoculation, no noticeable change in the count of viable strain M2799 was observed the tissues, though a 100-fold increase was noted in peripheral blood (Fig. 3B). In contrast, Fig. 3B shows that JCM3731 was detected at low levels in the spleen and was not detected in any other tissues. These results suggest that the clinical isolate, strain M2799, proliferated in the peritoneal cavity and then diffused hematogeneously throughout the peritoneal cavity.
FIG. 3.
Detection of V. vulnificus in various tissues. BALB/c mice were intraperitoneally inoculated with strain M2799 (open bars) or strain JCM3731 (closed bars) at a dose of 1 × 107 CFU/mouse. At 1 h (A) and 3 h (B) postinoculation, the viable count was determined in several tissues (n = 3). PB, peripheral blood.
Exudation of leukocytes to the peritoneal cavity.
The above results indicate that strain JCM3731 could not proliferate in the peritoneal cavity as a result of exclusion by host immune responses. We next examined the profile of PECs in intraperitoneally inoculated mice (Fig. 4). In strain M2799-inoculated mice, the number of PECs was significantly decreased at 1 h postinoculation, and after 3 h the number was significantly increased compared with that in sham-inoculated mice. In strain JCM3731-inoculated mice, the number of PECs was increased at 1 h postinoculation, but after 3 h the number had almost recovered to the level in sham-inoculated mice (Fig. 4A).
FIG. 4.

Analysis of exudate leukocytes in the peritoneal cavity. BALB/c mice were intraperitoneally inoculated with M2799 (open bars) or JCM3731 (closed bars) at a dose of 1 × 107 CFU/mouse or were sham inoculated (hatched bars). (A) At 1 h and 3 h postinoculation, the number of PECs was counted by the trypan blue exclusion method. (B) At 1 h and 3 h postinoculation, PECs were collected and analyzed by flow cytometry. Two-color flow cytometry was performed by staining with fluorescein isothiocyanate-conjugated anti-Gr1 and R-phycoerythrin-conjugated anti-Mac1. Mac1+/Gr1high cells and Mac1+/Gr1low/− cells were defined as neutrophils and macrophages, respectively. *, P < 0.05. Error bars indicate standard deviations.
Flow cytometry analysis of PECs in strain M2799- and strain JCM3731-inoculated mice was then performed (Fig. 4B). Mac1+/Gr1high cells and Mac1+/Gr1low/− cells were defined as neutrophils and macrophages, respectively. At 1 h postinoculation, the number of neutrophils in the PECs was significantly increased in both M2799- and JCM3731-inoculated mice. In contrast, the number of macrophages was significantly decreased in strain M2799-inoculated mice, while there was almost no recognizable difference between strain JCM3731-inoculated mice and sham-inoculated mice. At 3 h postinoculation, the number of neutrophils was markedly increased in strain M2799-inoculated mice, whereas the number of macrophages was still decreased. In strain JCM3731-inoculated mice, the numbers of neutrophils and macrophages at 3 h postinoculation were almost the same as those at 1 h postinoculation.
In summary, when strain M2799, which showed high lethality, was inoculated into mice, the number of neutrophils increased dramatically, whereas the number of macrophages decreased compared with that in sham-inoculated mice. In contrast, when strain JCM3731, which showed low lethality, was inoculated into mice, the number of neutrophils increased but the number of macrophages showed no change compared with that in sham-inoculated mice. These results indicate that the clinical isolate has strong cytotoxicity to macrophages but not to neutrophils. We then examined the cytotoxic activities of strains M2799 and JCM3731 in various cells.
Cytotoxic activity of V. vulnificus in various cells.
The mouse macrophage-like cell line J774A was used as a positive control of cytotoxicity of V. vulnificus. Strain M2799 showed high cytotoxic activity in macrophages and in splenocytes containing a number of macrophages. Cytotoxic activity was also observed in neutrophils and thymocytes, the constituent cells of which were mostly T cells, but this activity was only approximately 1/50 of that in macrophages. Strain JCM3731 showed much less cytotoxic activity in both cell types and no specificity to macrophages (Table 1). These results suggest that V. vulnificus has cytotoxic activity against macrophages, and as a consequence, the number of macrophages decreased in strain M2799-inoculated mice.
TABLE 1.
Cytotoxic activity of V. vulnificus in various cellsa
| Strain | Multiplicity of infection at 50% cytotoxic activity in:
|
||||
|---|---|---|---|---|---|
| Cell line J774A | Primary culture cells
|
||||
| Macrophages | Splenocytes | Neutrophils | Thymocytes | ||
| M2799 | 0.041b | 0.080b | 0.073b | 4.1b | 11.3b |
| JCM3731 | 26.7 | 90.5 | 34.7 | 35.2 | 183.2 |
Target cells at a dose of 1.5 × 105 cells/well (J774A) or 7.5 × 105 cells/well (primary culture cells) and bacteria were cocultured for 5 h. The supernatants were assayed for released lactate dehydrogenase.
The difference between M2799 and JCM3731 was statistically significant (P < 0.01).
Effect of leukocytes on bacterial growth.
The number of macrophages decreased with time in strain M2799-inoculated mice, and eventually these mice died, whereas the number of macrophages in strain JCM3731-inoculated mice was similar to that in sham-inoculated mice, and all of these mice survived. These results may indicate that the growth of V. vulnificus can be inhibited by macrophages. We then examined the effect of macrophages on bacterial growth. Strain M2799 was cultured with or without macrophages or neutrophils. In medium without macrophages or neutrophils, the count of viable strain M2799 had increased approximately 16-fold after 3 h (Fig. 5). In contrast, the count of viable strain M2799 cultured with macrophages or neutrophils had increased 1.7-fold or 8.2-fold, respectively, after 3 h. These results indicate that the growth of V. vulnificus was almost completely inhibited in the presence of macrophages. Neutrophils showed a relatively weak inhibitory effect on bacterial growth compared with macrophages.
FIG. 5.
Effect of leukocytes on bacterial growth. Thioglycolate-induced macrophages or casein-induced neutrophils were prepared as described in Materials and Methods. Strain M2799 was cultured with macrophages (open bars), without macrophages or neutrophils (hatched bars), or with neutrophils (closed bars) for 3 h and counted (n = 3 for each condition). *, P < 0.05. Error bars indicate standard deviations.
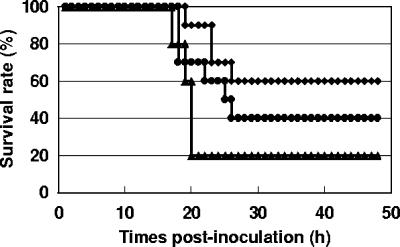
Effect of leukocytes on the survival of V. vulnificus-inoculated mice.
The above results indicate that macrophages markedly inhibit the growth of strain M2799. Conversely, strain M2799 showed cytotoxic activity against macrophages in the peritoneal cavity; thus, strain M2799 spread to the entire body, and the infected mice died. We investigated whether the survival of strain M2799-inoculated mice increases with recruitment of macrophages. The survival rate of sham-inoculated mice was 20%, whereas those of macrophage- and neutrophil-recruited mice increased to 60% and 40%, respectively (Fig. 6). Our results indicate that the clinical isolate of V. vulnificus has high cytotoxic activity, especially against macrophages, and that the growth of the strain was inhibited by macrophages. Moreover, the survival of V. vulnificus-inoculated mice increased with macrophage recruitment. Thus, the clinical isolate of V. vulnificus has strong pathogenicity in mice by protecting cells from macrophage attack and simultaneously by cytotoxic activity against macrophages.
FIG. 6.
Effect of leukocytes on survival of strain M2799-inoculated mice. BALB/c mice were intraperitoneally injected with a combination of strain M2799 and macrophages (diamonds) (n = 10), strain M2799 and neutrophils (circles) (n = 10), or strain M2799 and phosphate-buffered saline (triangles) (n = 5). Strain M2799 was injected at a dose of 2 × 106 CFU/mouse, and macrophages and neutrophils were injected at a dose of 1 × 107 cell/mouse. The survival of the mice was recorded every hour for 48 h.
DISCUSSION
An assay system using the most sensitive animal model, the iron-overloaded mouse, demonstrated that V. vulnificus could be classified into virulent and avirulent strains (27, 30). Most virulent and avirulent strains were clinical and environmental isolates, respectively (26). The clinical isolate strain M2799 showed a higher lethal effect in BALB/c mice than the environmental isolate strain JCM3731 (Fig. 2). The lethal effect of strain M2799 in C57BL/6 mice was almost as great as that in BALB/c mice (data not shown). It is well known that C57BL/6 and BALB/c mice exhibit dominant Th1-type and Th2-type immune responses, respectively. Mice inoculated with strain M2799 died within a few hours, and the lethal effects of the strain on BALB/c mice and C57BL/6 mice were similar, indicating that leukocytes but not lymphocytes play a central role in the clearance of V. vulnificus.
When strain M2799 was intraperitoneally injected into mice, it was detected not only in the tissues of the peritoneal cavity but also in peripheral blood and thymus (Fig. 3). This finding indicates that strain M2799 proliferated in the peritoneal cavity and then disseminated hematogeneously throughout the peritoneal cavity. In contrast, strain JCM3731 was scarcely detected in the various tissues tested, indicating that this strain could not proliferate in the peritoneal cavity because of exclusion by host immune responses. This difference in the proliferation of strains M2799 and JCM3731 in mice might be explained by the morphology of their colonies. Strain M2799 and strain JCM3731 produced opaque and translucent colonies, respectively (Fig. 1). Electron microscopy has shown that the colonies with the opaque phenotype contain capsular materials, while those with the translucent phenotype have no observable or incomplete capsular materials (3, 25, 33). CPSs are recognized virulence determinants and act by increasing adherence to host tissues and conferring resistance to phagocytosis. Strain M2799 was highly resistant to human serum, but strain JCM3731 could not survive in human serum (data not shown). Therefore, the environmental isolate strain JCM3731 appears to be excluded by host immune responses such as complement and phagocytosis by leukocytes because it produces little if any CPS.
In strain M2799-inoculated mice, the number of neutrophils increased markedly and that of macrophages decreased by approximately one-half compared with sham-inoculated mice (Fig. 4). In addition, strain M2799 showed high cytotoxic activity against macrophages and splenocytes containing macrophages (Table 1). In strain JCM3731-inoculated mice, neutrophils were also increased compared with those in sham-inoculated mice, though the number was lower than that in strain M2799-inoculated mice. Strain JCM3731 did not specifically affect macrophages, and the cytotoxic activity against macrophages was approximately 1,000-fold lower than that of strain M2799 (Fig. 4; Table 1). These results suggest that macrophages in the peritoneal cavity were damaged by virulence factors such as secretory proteins from strain M2799, and as a consequence their numbers decreased. Kashimoto et al. reported that a clinical isolate of V. vulnificus induced apoptosis of macrophages, and the ratio of apoptotic macrophages was up to 10% in vivo (11). Strain M2799 also induced a slight degree of DNA fragmentation (data not shown); however, a 50% decrease of macrophages in vivo could not be explained by apoptosis alone. Recent studies have shown that caspase 1 is activated by the flagellin of Legionella and Salmonella via nucleotide-binding oligomerization domain leucine-rich repeat proteins such as Naip5 and Ipaf, and proinflammatory programmed death was induced in macrophages (5, 6, 20, 21). Caspase 1 is not involved in apoptotic cell death, and an important function is to process the proforms of the inflammatory cytokines such as IL-1β and IL-18 to their active forms (4, 16). Expression of IL-1β and IL-18 in peritoneal macrophages and splenocytes in strain M2799-inoculated mice was increased (data not shown). Therefore, these cells might undergo proinflammatory programmed death via the same pathway. Detailed analysis of the cytotoxic mechanisms of strain M2799, especially against macrophages, is needed.
In mice inoculated with strain M2799, the number of macrophages decreased and the strain was detected in several tissues tested. In contrast, in mice inoculated with strain JCM3731, the number of macrophages did not change and the strain was only slightly detected in the tissues. The number of neutrophils increased in both M2799- and JCM3731-inoculated mice (Fig. 3 and 4). We then examined the effect of macrophages and neutrophils on the growth of V. vulnificus (Fig. 5). The growth of strain M2799 was completely inhibited by macrophages, and the effect of neutrophils was about one-half of that of macrophages. Thus, the survival of strain M2799-inoculated mice was increased by recruitment of macrophages (Fig. 6). The effect of neutrophils on both proliferation and survival was less marked that that of macrophages. Although the number of neutrophils in the peritoneal cavity increased significantly in strain M2799-inoculated mice, these mice died (Fig. 2 and 4). These results indicate that neutrophils may assist the function of macrophages, but neutrophils alone are insufficient to eliminate V. vulnificus. Therefore, macrophages plays a critical role in preventing V. vulnificus infection. Further research is now required to determine the virulence factor that specifically attacks macrophages.
Acknowledgments
This study was supported in part by a Grant-in-Aid for High Technology Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
We are grateful to Shin-ichi Miyoshi for supplying Vibrio vulnificus clinical isolate strain M2799.
Editor: A. Camilli
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Brennt, C., A. C. Wright, S. K. Dutta, and J. G. Morris, Jr. 1991. Growth of Vibrio vulnificus in serum from alcholics: association with high transferring iron saturation. J. Infect. Dis. 164:1030-1032. [DOI] [PubMed] [Google Scholar]
- 2.Bullen, J. J., P. B. Spalding, C. G. Ward, and J. M. Gutteridge. 1991. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch. Intern. Med. 151:1606-1609. [PubMed] [Google Scholar]
- 3.Chatzidaki-Livanis, M., M. K. Jones, and A. C. Wright. 2006. Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. J. Bacteriol. 188:1987-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Fink, S. L., and B. T. Cookson. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8:1812-1825. [DOI] [PubMed] [Google Scholar]
- 6.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7:576-582. [DOI] [PubMed] [Google Scholar]
- 7.Gray, L. D., and A. S. Kreger. 1985. Purification and characterization of an extracellular cytolysin production by Vibrio vulnificus. Infect. Immun. 48:62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hlady, W. G., and K. C. Klontz. 1996. The epidemiology of vibrio infections in Florida, 1981-1993. J. Infect. Dis. 173:1176-1183. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss, R. S., K. C. Chang, P. E. Swanson, K. W. Tinsley, J. J. Hui, P. Klender, S. Xanthoudakis, S. Roy, C. Black, E. Grimm, R. Aspiotis, Y. Han, D. W. Nicholson, and I. E. Karl. 2000. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1:496-501. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, R. E. Schmieg, Jr., J. J. Hui, K. C. Chang, D. F. Osborne, B. D. Freeman, J. P. Cobb, T. G. Buchman, and I. E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166:6952-6963. [DOI] [PubMed] [Google Scholar]
- 11.Kashimoto, T., S. Ueno, M. Hanajima, H. Hayashi, Y. Akeda, S. Miyoshi, T. Hongo, T. Honda, and N. Susa. 2003. Vibrio vulnificus induces macrophage apoptosis in vitro and in vivo. Infect. Immun. 71:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashimoto, T., S. Ueno, H. Hayashi, M. Hanajima, K. Yoshioka, K. Yoshida, K. Mutoh, and N. Susa. 2005. Depletion of lymphocytes, but not neutrophils, via apoptosis in a murine model of Vibrio vulnificus infection. J. Med. Microbiol. 54:15-22. [DOI] [PubMed] [Google Scholar]
- 13.Kitaura, T., S. Doke, I. Azuma, M. Imaida, K. Miyano, K. Harada, and E. Yabuuchi. 1983. Halo production by sulfatase activity of V. vulnificus and V. cholerae O1 on a new selective sodium dodecyl sulfate-containing agar medium: a screening marker in environmental surveillance. FEMS Microbiol. Lett. 17:205-209. [Google Scholar]
- 14.Kothary, M. H., and A. S. Kreger. 1987. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J. Gen. Microbiol. 133:1783-1791. [DOI] [PubMed] [Google Scholar]
- 15.Kraffert, C. A., and D. J. Hogan. 1992. Vibrio vulnificus infection and iron overload. J. Am. Acad. Dermatol. 26:140. [DOI] [PubMed] [Google Scholar]
- 16.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, J. Salfeld, E. Towne, D. Tracey, S. Wardwell, F. Wei, W. Wong, R. Kamen, and T. Seshadri. 1995. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80:401-411. [DOI] [PubMed] [Google Scholar]
- 17.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 18.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 64:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyoshi, N., C. Shimizu, S. Miyoshi, and S. Shinoda. 1987. Purification and characterization of Vibrio vulnificus protease. Microbiol. Immunol. 31:13-25. [DOI] [PubMed] [Google Scholar]
- 20.Molofsky, A. B., L. M. Shetron-Rama, and M. S. Swanson. 2005. Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death. Infect. Immun. 73:5720-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molofsky, A. B., B. G. Byrne, N. N. Whitfield, C. A. Madigan, E. T. Fuse, K. Tateda, and M. S. Swanson. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203:1093-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muench, K. H. 1989. Hemochromatosis and infection: alchol and iron, oysters and sepsis. Am. J. Med. 87:40N-43N. [PubMed] [Google Scholar]
- 23.Shinoda, S., S. Miyoshi, H. Yamanaka, and N. Miyoshi-Nakahara. 1985. Some properties of Vibrio vulnificus hemolysin. Microbiol. Immunol. 29:583-590. [DOI] [PubMed] [Google Scholar]
- 24.Shinoda, S., M. Kobayashi, H. Yamada, S. Yoshida, M. Ogawa, and M. Mizuguchi. 1987. Inhibitory effect of capsular antigen of Vibrio vulnificus on bactericidal activity of human serum. Microbiol. Immunol. 31:393-401. [DOI] [PubMed] [Google Scholar]
- 25.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio culnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stelma, G. N., Jr., A. L. Reyes, J. T. Peeler, C. H. Johnson, and P. L. Spaulding. 1992. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl. Environ. Microbiol. 58:2776-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis in Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 29.Tamplin, M. L., S. Specter, G. E. Rodrick, and H. Friedman. 1985. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect. Immun. 49:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tison, D. L., and M. T. Kelly. 1986. Virulence of Vibrio vulnificus strains from marine environments. Appl. Environ. Microbiol. 51:1004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright, A. C., J. L. Powell, M. K. Tanner, L. A. Ensor, A. B. Karpas, J. G. Morris, Jr., and M. B. Sztein. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida, S., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]