Abstract
Helicobacter pylori infection is associated with an inflammatory response in the gastric mucosa, leading to chronic gastritis, peptic ulcers, and gastric cancer. There is increased T-cell infiltration at the site of infection with H. pylori. CCR6, a specific β-chemokine receptor for CCL20 (MIP-3α/LARC/exodus), has recently been reported to mediate lymphocyte homeostasis and immune responses in mucosal tissue, and it may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation. In this study, we investigated the role of CCR6 and its ligand, CCL20, in inducing an inflammatory response in the gastric mucosa during H. pylori infection. Gastric infiltrating T lymphocytes were isolated from endoscopic biopsy specimens of H. pylori gastritis patients and analyzed for the expression of the CCR6 chemokine receptor. Our results demonstrated that there was significantly increased CCR6 expression in CD3+ T cells infiltrating the gastric mucosa, and the CCR6 ligand, the CCL20 chemokine, was selectively expressed in inflamed gastric tissues. The production of CCL20 was upregulated in response to H. pylori in gastric epithelial cells when there was stimulation by the proinflammatory cytokines interleukin-1β and tumor necrosis factor alpha. Furthermore, recombinant CCL20 induced lymphocyte chemotaxis migration in fresh gastric T cells ex vivo, indicating that the gastric T cells could migrate toward inflammatory sites via CCR6/CCL20 interaction. Our results suggest that the interaction between CCL20 and CCR6 may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation in Helicobacter infection.
Helicobacter pylori, a common human pathogen which infects about 50% of the world's population, is associated with duodenal and peptic ulcer diseases. The clinical consequences range from asymptomatic gastritis to peptic ulceration and gastric malignancy (28, 29). The outcome of the infection is determined by interactions among H. pylori virulence factors, host gastric mucosal factors, and the environment. However, the mechanisms by which host factors cause disease remain unclear. H. pylori colonization induces systemic and mucosal immune responses. There is increased T-cell infiltration at the site of infection with H. pylori (5). Recent studies have suggested that the number of T helper type 1 (Th1) cells is selectively increased during infection (5, 11, 22, 33). Th1 cytokines, such as gamma interferon and tumor necrosis factor alpha (TNF-α), can increase the release of proinflammatory cytokines, augmenting apoptosis induced by H. pylori (35). In addition, H. pylori infection may induce gastric mucosal damage by increasing gastric epithelial cell apoptosis through Fas/FasL or TRAIL/TRAIL-R interactions with infiltrating T cells (19, 36, 38). Recent studies have indicated that T lymphocytes play an important role in the pathogenesis of Helicobacter gastritis (5, 24). The mechanism by which effector T lymphocytes are recruited into the gastric mucosa during the inflammatory response in Helicobacter gastritis is not well understood, and chemokines may play an important role in this process (4, 7, 8, 14, 40).
Chemokines are small, 6- to ∼14-kDa heparin binding proteins which play a role in a variety of biological processes, most notably leukocyte chemotaxis (3, 23). Chemokines are involved in acute and chronic inflammatory processes by attracting neutrophils, monocytes, and T cells to the site of inflammation via their corresponding chemokine receptors (3, 23). Recent reports have shown that there are specific chemokines that mediate the homing of lymphocytes in the intestines (14, 27, 32), suggesting that some chemokines may be involved in lymphocyte trafficking in the gut. It has been demonstrated that distinct sets of chemokines and their receptors are responsible for directing lymphocytes to inflammatory sites (1, 7-9, 27). The following set of proinflammatory chemokines has been shown to be involved in H. pylori gastritis: CXCL1 (Gro-α,), CXCL8 (interleukin-8 [IL-8]), CCL5 (RANTES), CXCL10 (IP-10), CXCL11 (MIG), and CCL20 (MIP-3α/LARC/exodus) (15, 37, 39). Although the activity of chemokines as chemotactic factors for lymphocytes has been recognized for some time, there is now increased interest in this area with the continuing identification of novel lymphocyte-active chemokines and their receptors.
CCR6, a specific β-chemokine receptor for CCL20, is selectively expressed on dendritic cells and some memory T cells (2, 17, 21, 30) and may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation. Recently, it was reported that CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue (10). CCL20 has also been shown to preferentially attract memory T cells (12, 21), and it may play a role in gastric inflammation. In this study, we investigated the role of CCR6 and its ligand, CCL20, in inducing an inflammatory response in gastric mucosa during H. pylori infection. Our results demonstrated that the expression of the chemokine CCL20 is upregulated in inflamed gastritis tissues and that its receptor, CCR6, is selectively expressed on CD3+ T cells infiltrating the gastric mucosa. This implies that the interaction between CCL20 and CCR6 may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation in Helicobacter infection.
MATERIALS AND METHODS
Subjects and patients.
Biopsy specimens of the gastric antrum were obtained from patients undergoing gastric endoscopy at National Taiwan University Hospital for dyspepsia; informed consent was obtained from all patients. The study was approved by the Medical Ethics Committee of National Taiwan University Medical Center (Taipei, Taiwan). There were 36 H. pylori-infected and 14 uninfected subjects enrolled in this study. The presence of H. pylori infection was determined by visualization through histology (including Giemsa staining) and/or by a positive rapid urease test performed on at least one additional biopsy sample. The degree of inflammation present in the histological specimens was classified according to the updated Sydney system (13). A grade from absent to marked was assigned for four histological variables: chronic inflammation (mononuclear cell infiltration), activity (polymorphonuclear neutrophil infiltration), glandular atrophy, and intestinal metaplasia.
Isolation of gastric infiltrating lymphocytes.
Gastric infiltrating lymphocytes were isolated using a modification of a previously described technique (16). Briefly, biopsy specimens were collected into sterile collection medium (calcium- and magnesium-free Hanks' balanced salt solution [HBSS] with 5% fetal calf serum and penicillin plus streptomycin). The collected tissues were immediately placed in ice-cold RPMI 1640 complete medium (GIBCO BRL, Gaithersburg, MD) which contained 10% fetal calf serum supplemented with penicillin (50 IU/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), and sodium pyruvate (1 mM). The tissues were washed, diced into 1-mm3 pieces (through a 380-μm tissue sieve), and treated with collagenase (type I; 5 μg/ml; Sigma Chemical, St. Louis, MO) and heparin (5 U/ml) in RPMI 1640 complete medium at 37°C for 60 min. The resulting cell suspensions were washed, and the viability of the mononuclear cells was determined by trypan blue exclusion.
Flow cytometric analysis of surface antigen expression.
Isolated gastric mononuclear cells were labeled with antibodies to CD3, CD4, and CD8 (Becton Dickinson, San Jose, CA) and were immediately postfixed with 1% paraformaldehyde in phosphate-buffered saline using standard techniques. Based on the forward and side scatter, cells were gated on the area corresponding to lymphocytes and analyzed with a FACScan (Becton Dickinson). The gastric infiltrating lymphocytes were stained with fluorescein isothiocyanate-labeled monoclonal antibodies (MAbs) which were used to detect the expression of CD45RO, CD45RA, and chemokine receptors. Cells were incubated with primary antibodies (anti-CCR6; R&D Systems, Minneapolis, MN), anti-CD45RO,CD45RA (BioLegend, San Diego, CA), or appropriate immunoglobulin G isotype controls (Sigma) for 30 min and washed with staining buffer for 5 min. Normal isotype-matched irrelevant anti-mouse antibodies were used as the control antibodies. Chemokine receptor expression on lymphocytes was determined by first gating cells using their side scatter properties. Receptor expression on CD3+ cells was determined by a second histogram of the gated CD3+ lymphocytes (phycoerythrin-Cy5) versus chemokine receptor (fluorescein isothiocyanate) fluorescence.
Culture of primary human gastric epithelial cells.
For culture of human gastric epithelial cells, a previously described method was used (38). In brief, gastric biopsy specimens were collected in Leibowitz's L-15 medium (Life Technologies, Grand Island, NY). Gastric cells were isolated enzymatically after the tissue was mechanically minced into ≤1-mm pieces. The tissue was then pelleted by centrifugation at 1,500 rpm for 5 min at 4°C, the collagenase/dispase was discarded, and then the tissue was washed once in 10 ml of phosphate-buffered saline and pelleted again by centrifugation. Cells were resuspended in the cell culture medium. Gastric epithelial cells obtained as described above were suspended in 2 ml of Ham's F-12 cell culture medium (Life Technologies) with 10% fetal bovine serum and placed into a six-well tissue culture plate.
Chemokine production analysis by real-time RT-PCR and ELISA.
The production of the chemokine CCL20 in gastric tissue was analyzed by real-time quantitative reverse transcription-PCR (RT-PCR) and an enzyme-linked immunosorbent assay (ELISA). For detection of chemokine production by real-time RT-PCR, total RNA was purified using the Trizol reagent (Invitrogen Life Technologies) by following the instructions in the manufacturer's manual. Total RNA (5 μg) was reverse transcribed with Superscript II RNase H reverse transcriptase (Invitrogen Life Technologies) and an oligo(dT) primer. Synthesized cDNA was stored at −20°C until it was used. Two microliters of cDNA was subjected to real-time quantitative RT-PCR using the Opticon system with SYBR green I (Molecular Probes, Eugene, OR) as a fluorescent reporter. Chemokine and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were amplified in separate reactions. The primers used for CCL20 were 5′-GCGCAAATCCAAAACAG-3′ (sense) and 5′-GTCCAGTGAGGCACAA-3′ (antisense). The threshold cycle number of duplicate reactions was determined using the Opticon software, and levels of chemokine mRNA expression were normalized to GAPDH levels using the formula 2(Rt − Et), where Rt is the mean threshold cycle for the reference gene (GAPDH) and Et is the mean threshold cycle for the experimental gene. Data were expressed in arbitrary units. Chemokines produced by gastric epithelial cells were also measured by ELISA, using sandwich ELISA kits (Quantikine; R&D Systems). They were detected by incubation with 125 ng/ml streptavidin-horseradish peroxidase (ICN Biochemical, Aurora, OH) for 20 min, followed by peroxidase substrate (TMB Microwell; KPL, Gaithersburg, MD).
Effects of live H. pylori on chemokine production in primary human gastric epithelial cells.
Confluent monolayers of primary human gastric epithelial cells (1 × 106 cells/ml) were stimulated with TNF-α or IL-1β (50 ng/ml each) alone or with both TNF-α and IL-1β for 12 h. Supernatants were harvested, centrifuged, and collected for chemokine measurement by ELISA. For bacterial infection, the confluent primary gastric epithelial monolayers were stimulated with IL-1β or TNF-α (50 ng/ml each) alone or with both TNF-α and IL-1β for 12 h and then infected with H. pylori strain ATCC 43504 (1 × 108 cells/ml) at a multiplicity of infection of 100. After 12 h of incubation, supernatants were harvested, centrifuged, and collected for chemokine production analysis.
Lymphocyte chemotaxis assay.
Lymphocyte chemotaxis experiments were performed using 6.5-mm polycarbonate transwell tissue culture inserts with a 5-μm pore size (Corning, Corning, NY). The lower compartment was filled with 600 μl of epithelial conditioned medium or HBSS containing the recombinant chemokine CCL20 (0.1 to ∼100 ng/ml). Gastric infiltrating lymphocytes (5 × 105 cells) in 100 μl of HBSS were placed on the filters and incubated for 4 h at 37°C. Lymphocytes that migrated through the filter into the lower compartment were counted. Triplicate wells were prepared for each condition. The results are expressed as percentages of input cells corrected for the percentage of cells migrating in the absence of chemokines.
Statistical analyses.
The results for expression of chemokine receptors and production of chemokines for gastric infiltrating lymphocytes and peripheral blood T cells were evaluated by the nonparametric Mann-Whitney rank sum test. A P value of <0.05 was regarded as indicating statistical significance.
RESULTS
Most of the gastric infiltrating T lymphocytes are activated memory T cells and express Th1-type chemokine receptors CXCR3, CCR5, and CXCR6.
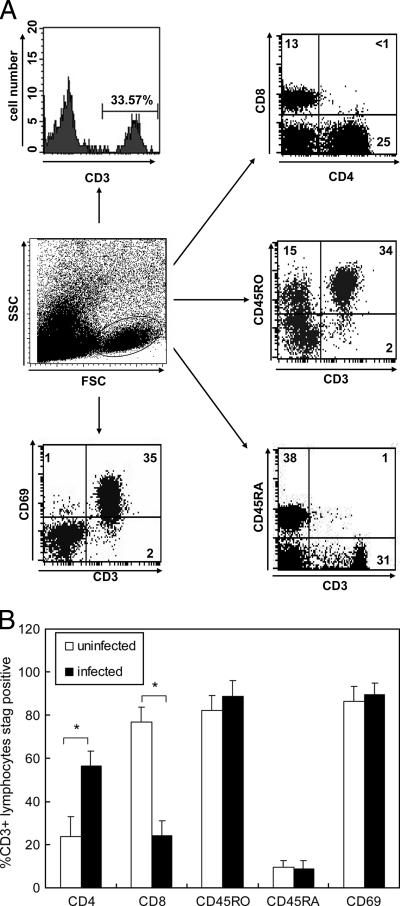
Gastric T cells were isolated from biopsy specimens obtained from H. pylori-infected and uninfected subjects. The yields of viable lymphocytes ranged from 0.5 × 106 to 5 × 106 cells for the gastric mucosa of infected subjects. The yields for uninfected subjects, however, were less than one-fifth of these yields. Flow cytometric analysis of isolated lymphocytes revealed that most of the gastric infiltrating lymphocytes expressed CD45RO, a memory T-cell marker, but not CD45RA, a marker of naïve T cells. In addition, over 90% of gastric T cells were CD69+, indicating that most of the gastric infiltrating T cells were activated memory T cells (Fig. 1). There was an increased CD4/CD8 T cell ratio in H. pylori-infected subjects (Fig. 1). The number of CD4+ T cells in H. pylori-infected subjects was 18.2 × 104 ± 2.4 × 104 cells per sample, and the number of CD8+ T cells was 6.6 × 104 ± 0.68 × 104 cells per sample. In contrast, the number of CD4+ T cells in uninfected subjects was 0.94 × 104 ± 0.12 × 104 cells per sample, and the number of CD8+ T cells was 2.96 × 104 ± 0.32 × 104 cells per sample. These results indicate that the number of CD8+ T cell appears not to be reduced, and the increase in the CD4+/CD8+ T cell ratio in H. pylori-infected subjects seems to reflect the accumulation of CD3+ CD4+ memory lymphocytes.
FIG. 1.
Characterization of gastric infiltrating lymphocytes isolated from gastric biopsy specimens from H. pylori-infected subjects. (A) Suspensions of mononuclear cells were isolated from gastric biopsy specimens as described in Materials and Methods. Immediately after isolation, cells were stained for CD3, CD4, CD8, CD69, CD45 RA, and CD45RO and analyzed by flow cytometry. The numbers indicate the percentages of cells detected in each quadrant in the flow cytometric scatter plots. The data are representative of the phenotype observed in gastric infiltrating lymphocytes isolated from 36 infected subjects. (B) Percentages of CD4, CD8, CD45RO, CD45RA, and CD69 expression in CD3+ gastric T cells from the 36 H. pylori-infected subjects mentioned above and 14 uninfected subjects. The data are the means and standard errors of the means. Statistical analysis by the Mann-Whitney rank sum test revealed significant differences in the expression of CD4 and CD8 in gastric T cells between infected and uninfected samples (asterisk, P < 0.01).
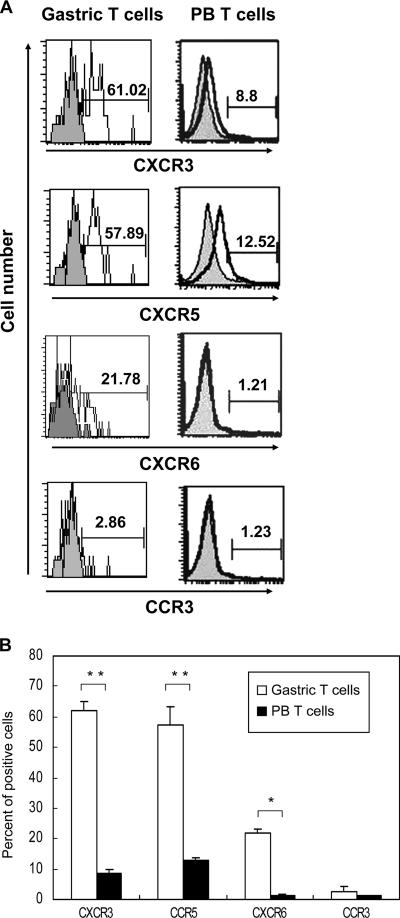
To characterize the expression of chemokine receptors in gastric infiltrating lymphocytes from H. pylori-associated chronic gastritis tissues, freshly isolated gastric T cells from gastric specimens were stained for the expression of T-cell surface antigens and chemokine receptors by flow cytometry. The results in Fig. 2 demonstrate that the gastric infiltrating lymphocytes expressed much higher levels of Th1-type chemokine receptor CCR5, CXCR3, and CXCR6 receptors than peripheral blood T cells expressed, which is compatible with a Th1-type response pattern. In contrast, the Th2-type chemokine receptor CCR3 was rarely detected in gastric T cells (Fig. 2), indicating that in human H. pylori gastritis, a Th1 response predominates. This finding is compatible with previous reports (5) indicating that the Th1 response is induced during infection with H. pylori.
FIG. 2.
Gastric infiltrating T cells express Th1-type chemokine receptors CXCR3, CCR5, and CXCR6 but not Th2-type chemokine receptor CCR3. (A) Gastric infiltrating T cells (Gastric T cells) and peripheral blood T cells (PB T cells) isolated from H. pylori-infected subjects were incubated with the anti-CXCR3, anti-CCR5, anti-CXCR6, and anti-CCR3 MAbs and analyzed by flow cytometry (shaded histograms, isotype control). The data represent the results of 16 independent experiments performed with different donors. (B) Percentages of CD3+ lymphocytes expressing CXCR3,CCR5, CXCR6, and CCR3 in gastric infiltrating lymphocytes and peripheral blood T cells isolated from the subjects described above. The data are the means and standard deviations. Statistical analysis by the Mann-Whitney rank sum test revealed significant differences between gastric T-cell and peripheral blood T-cell samples in expression of Th1-type chemokine receptors CXCR3, CCR5, and CXCR6 (one asterisk, P < 0.05; two asterisks, P < 0.005).
Gastric infiltrating T lymphocytes express the CCR6 chemokine receptor.
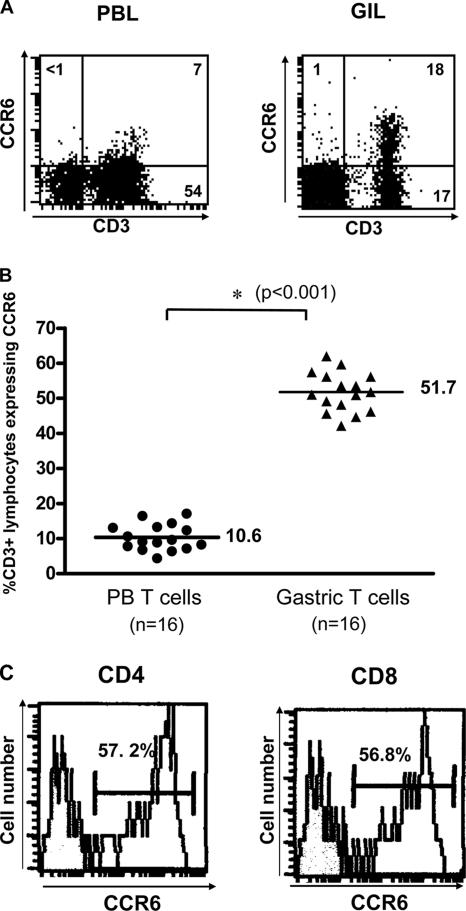
CCR6, a specific β-chemokine receptor for CCL20, is selectively expressed on some memory T cells and may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation. To determine the roles of CCR6 and CCL20 in H. pylori gastritis, we examined the expression of the CCR6 chemokine receptors in gastric infiltrating lymphocytes from gastric biopsy specimens. The results in Fig. 3 show that the percentage of CD3+ lymphocytes expressing CCR6 was significantly elevated in gastric infiltrating lymphocytes compared with that in peripheral blood from the same patients (P < 0.001). Both CD4+ and CD8+ gastric T cells expressed similar levels of CCR6 on the cell surface. These results indicate that the CCR6 chemokine receptor is selectively expressed in gastric infiltrating T lymphocytes during gastric inflammation.
FIG. 3.
Gastric infiltrating T lymphocytes express the CCR6 chemokine receptor. (A) Gastric infiltrating lymphocytes (GIL) and peripheral blood lymphocytes (PBL) isolated from H. pylori-infected subjects were incubated with the anti-CCR6 MAb and analyzed by flow cytometry. The data represent the results of 16 independent experiments performed with different donors. (B) Percentages of CD3+ lymphocytes expressing CCR6 in gastric infiltrating lymphocytes (Gastric T cells) and peripheral blood lymphocytes (PB T cells) isolated from the subjects described above. The percentage of CD3+ lymphocytes expressing CCR6 was significantly elevated in gastric infiltrating lymphocytes. The symbols are results for individual samples, and the horizontal lines indicate the means. The P value was determined by the Mann-Whitney rank sum test. (C) Expression of CCR6 in CD4+ and CD8+ gastric T cells isolated from H. pylori-infected subjects.
Upregulation of the CCL20 chemokine in inflamed Helicobacter gastritis tissues.
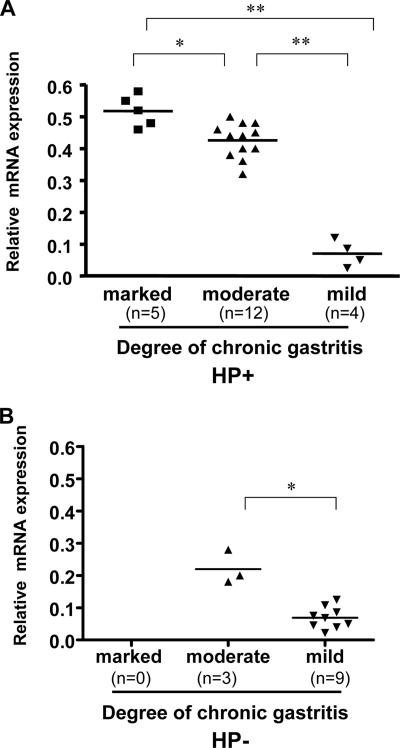
We then examined the expression of CCL20/MIP-3α, the chemokine which interacts with CCR6, which may attract effector and memory T cells in H. pylori-associated inflamed gastric tissues. The expression of CCL20 in gastric tissues was analyzed by a real-time quantitative RT-PCR. To determine whether the expression of CCL20 is related to the degree of chronic inflammation in H. pylori-infected gastric samples, we divided the H. pylori-positive gastric biopsy samples into three groups based on their histology scores for chronic inflammation (marked, moderate, or mild) using the criteria described in Materials and Methods. The results in Fig. 4 demonstrate that expression of CCL20 was upregulated in inflamed gastric tissues and was correlated with the degree of gastric inflammation. Compared to the H. pylori-infected group, the gastric biopsy specimens from uninfected subjects had much lower histology scores for the degree of inflammation, and they also had significantly lower levels of expression of CCL20 compared to the H. pylori-infected gastric tissues. The results indicate that CCL20 may play a role in inducing gastric inflammation in H. pylori infection.
FIG. 4.
Upregulation of the chemokine CCL20 in inflamed Helicobacter gastritis tissues. Gastric biopsy specimens from chronic gastritis tissues in H. pylori-infected (HP+) (A) and uninfected (HP−) (B) subjects were collected, and the RNAs were isolated. The production of CCL20 in gastric tissues was analyzed by real-time quantitative RT-PCR. The degree of inflammation present in the histological specimens was classified according to the updated Sydney system (13). The CCL20 mRNA expression was calculated by determining the ratio of the concentrations of CCL20 and GAPDH expressed in gastric tissues. Expression of CCL20 is related to the degree of chronic inflammation in gastric samples (one asterisk, P < 0.05; two asterisks, P < 0.001; determined by the Mann-Whitney rank sum test).
Production of CCL20 is upregulated in response to H. pylori when there is stimulation by the proinflammatory cytokines IL-1β and TNF-α.
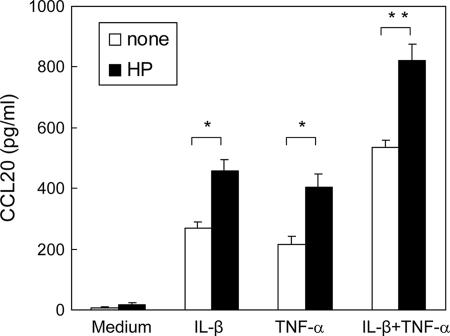
Because recent studies have shown that proinflammatory cytokines or bacterial infection alters chemokine production in the intestinal epithelia, we further investigated whether gastric epithelial cells respond to H. pylori or proinflammatory cytokines to induce CCL20 production. Primary human gastric epithelial cells were isolated from gastric biopsy specimens and cultured in the presence or absence of H. pylori. The results in Fig. 5 demonstrate that the proinflammatory cytokines IL-1β and TNF-α induced human primary gastric epithelial cells to produce CCL20, and the production of CCL20 was upregulated in response to H. pylori when there was stimulation by IL-1β and TNF-α (Fig. 5).
FIG. 5.
Production of the cytokine CCL20 was upregulated in response to H. pylori when there was stimulation by the proinflammatory cytokines IL-1β and TNF-α in primary human gastric epithelial cells. Primary human gastric epithelial cells were isolated from gastric biopsy specimens as described in Materials and Methods and cultured in the presence or absence of H. pylori (HP). Primary human gastric epithelial cells were incubated with IL-1β (50 ng/ml) and/or TNF-α (50 ng/ml) in the presence or absence of H. pylori, and the production of CCL20 was assayed by ELISA. The data are the means and standard deviations. One asterisk, P < 0.05; two asterisks, P < 0.01.
Gastric infiltrating T lymphocytes express CCR6 and migrate toward recombinant CCL20 in the chemotaxis assay.
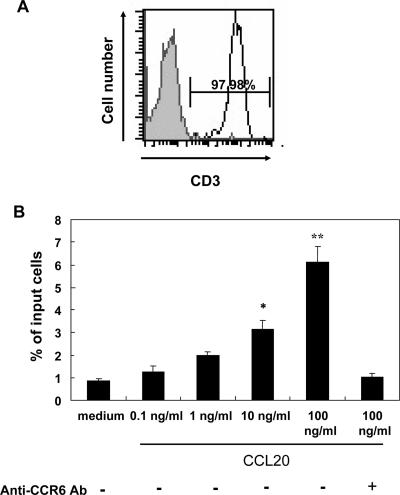
To specifically investigate whether the epithelial chemoattractant CCL20 contributes to the recruitment of gastric infiltrating T cells in humans with H. pylori gastritis, we next measured lymphocyte chemotaxis in response to recombinant CCL20 using a chemotaxis chamber system. Gastric infiltrating T cells were purified from biopsy specimens and assayed for chemotaxis in response to CCL20 in vitro. As shown in Fig. 6, the isolated gastric infiltrating T cells migrated toward recombinant CCL20 in a dose-dependent manner in the chemotaxis assay. Furthermore, the chemotactic migration of gastric T cells was completely inhibited by the addition of anti-CCR6 MAb, indicating that the gastric infiltrating T cells could migrate toward inflammatory sites via the CCR6/CCL20 interaction. This implies that CCR6 has a role in gastric infiltrating T cells in response to gastric inflammation mediated via CCL20 during H. pylori infection.
FIG. 6.
Chemotactic migratory abilities of gastric T cells in response to the chemokine CCL20. (A) Gastric infiltrating lymphocytes isolated from different H. pylori-infected donors and gastric T cells were purified with MAC beads. The purity of T cells isolated was greater than 97% as determined using anti-CD3 MAb by flow cytometry. Purified gastric T cells were subjected to a chemotaxis assay (shaded histogram, isotype control). (B) Gastric T cells migrated toward recombinant CCL20 in the transwell chemotaxis assay. The lymphocyte chemotaxis experiments were performed as described in Materials and Methods. Gastric T cells were placed in the upper compartment and migrated through the filter into the lower compartment containing recombinant CCL20, the concentration of which is indicated. Lymphocytes that migrated through the filter into the lower compartment were counted. The results are representative of the results of three separate experiments. The data are the means and standard deviations (one asterisk indicates that the P is <0.05 and two asterisks indicate that the P value is <0.005 for comparisons to the group with medium alone). Ab, antibody.
DISCUSSION
In this study, we investigated the role of the CCR6 chemokine receptor and its ligand, CCL20, in inducing an inflammatory response in gastric mucosa during H. pylori infection. We demonstrated that the expression of the CCL20 chemokine is upregulated in inflamed gastritis tissues and that its receptor, CCR6, is expressed on CD3+ T cells infiltrating the gastric mucosa. This implies that the interaction between CCL20 and CCR6 may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation in Helicobacter gastritis.
Most of the gastric infiltrating T cells were CD45RO+ CD69+, indicating that there was accumulation of activated memory CD4+ T cells during Helicobacter infection (Fig. 1). Recent reports have indicated that the Th1 response is induced during infection with H. pylori (5, 11, 20, 22). It has been demonstrated that gamma interferon and TNF-α, whose levels are increased in the gastric mucosa during H. pylori infection, augment the apoptosis induced by H. pylori (31, 35, 38), and these results suggest a role for immune-mediated apoptosis of gastric epithelial cells by infiltrating T cells during Helicobacter infection. Therefore, in addition to bacterial virulence factors, the degree of gastric mucosa damage is also determined by the inflammation response induced during H. pylori infection.
It has been demonstrated that the gastrointestinal epithelium senses the invading microorganisms and produces cytokines, i.e., chemokines that attract lymphocytes and dendritic cells to the site of inflammation (14, 27, 32). Nishi et al. (26) reported that H. pylori infection significantly induces the production of CCL20, the thymus- and activation-regulated chemokine, and the B-lymphocyte chemoattractant. Wen et al. also reported that CCL20 is significantly induced in inflammatory gene profiles in gastric mucosa during H. pylori infection in humans (37). Recent studies have also shown that CCL20 is abundantly expressed in mouse and human inflammatory enteric mucosa (18, 34). Here, we demonstrated that CCL20 is expressed in inflamed gastric tissues in Helicobacter infection and that the production of CCL20 is upregulated in response to H. pylori when there is stimulation by the proinflammatory cytokines IL-1β and TNF-α (Fig. 4). Importantly, these studies indicate that in the presence of proinflammatory cytokines, H. pylori can induce an amount of CCL20 sufficient to induce the inflammatory response via attracting lymphocytes infiltrating into gastric mucosa. Thus, induction of CCL20 expression by gastric epithelial cells upon interaction with H. pylori might be mediated through induction of the proinflammatory cytokines IL-1β and TNF-α from H. pylori-activated macrophages or other cells. Our results suggest that synergy between the innate immune response induced by H. pylori and gastric epithelial cells induces a sufficient amount of CCL20 production, resulting in recruitment of infiltrating lymphocytes and gastric inflammation during Helicobacter infection. Taken together, these results suggest a role for CCL20 in induction of the inflammatory response in H. pylori gastritis.
Recent results have demonstrated that CCR6 is selectively expressed on subsets of memory T cells, including α4β7-expressing gut homing cells and cutaneous lymphocyte-associated antigen (CLA)-expressing cells, and on B cells (21). It is of interest that almost all α4β7+ memory cells and many CLA+ cells express CCR6. These data suggest that CCR6 and CCL20 may play roles in recruiting these highly differentiated, resting memory cells and dendritic cells to sites of inflammation in both the skin and intestinal mucosa (6, 25). Cook et al. (10) demonstrated that mice lacking CCR6 have impaired immunity with defective lymphocyte homeostasis in the small intestine mucosa, suggesting that CCR6 is a mucosa-specific regulator of humoral immunity and lymphocyte homeostasis in the intestinal mucosa. However, the role of CCR6 in recruiting gastric inflammatory cells is still not clear.
In this study, we demonstrated that the expression of CCL20 is upregulated in inflamed gastritis tissues and that the CCL20 receptor, CCR6, is selectively expressed on CD45RO+ T cells in the gastric mucosa. Moreover, the CCR6-expressing gastric T cells migrated in response to CCL20 in the ex vivo lymphocyte migration assay. These results imply that the interaction between CCL20 and CCR6 may play a role in recruiting CD45RO+ memory T cells to the sites of inflammation in the gastric mucosa during Helicobacter infection. This improves our understanding of the immune pathogenesis of the disease and should help us develop more effective therapies.
Acknowledgments
We thank M. L. Yen (National Health Research Institute, Taiwan) for critically reviewing the manuscript, S.-B. Wang (Department of Medicine, National Taiwan University Hospital) for supporting endoscopic biopsy specimens, and W.-I. Tsai and W.-H. Wu for assisting with the H. pylori cultures.
This work was supported by grants from the National Health Research Institute, Taiwan (grant NHRI-EX95-9532SI), the National Science Council (grants NSC95-2320-B-002-026 and NSC95-2321-B-002-025), and National Taiwan University Hospital (grant NTUH 89S2011).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Ansel, K. M., V. N. Ngo, P. L. Hyman, S. A. Luther, R. Forster, J. D. Sedgwick, J. L. Browning, M. Lipp, and J. G. Cyster. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309-314. [DOI] [PubMed] [Google Scholar]
- 2.Baba, M., T. Imai, M. Nishimura, M. Kakizaki, S. Takagi, K. Hieshima, H. Nomiyama, and O. Yoshie. 1997. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 272:14893-14901. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565-568. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini, M., and P. Loetscher. 2000. Chemokines in inflammation and immunity. Immunol. Today 21:418-420. [DOI] [PubMed] [Google Scholar]
- 5.Bamford, K. B., X. J. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, L. M., and S. R. Watson. 1996. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr. Opin. Immunol. 8:312-316. [DOI] [PubMed] [Google Scholar]
- 7.Butcher, E. C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209-253. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336-341. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, J. J., G. Haraldsen, J. Pan, J. Rottman, S. Qin, P. Ponath, D. P. Andrew, R. Warnke, N. Ruffing, N. Kassam, L. Wu, and E. C. Butcher. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400:776-780. [DOI] [PubMed] [Google Scholar]
- 10.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495-503. [DOI] [PubMed] [Google Scholar]
- 11.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 12.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis: the updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 14.Dwinell, M. B., N. Lugering, L. Eckmann, and M. F. Kagnoff. 2001. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 120:49-59. [DOI] [PubMed] [Google Scholar]
- 15.Eck, M., B. Schmausser, K. Scheller, A. Toksoy, M. Kraus, T. Menzel, H. K. Muller-Hermelink, and R. Gillitzer. 2000. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin. Exp. Immunol. 122:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan, X. J., A. Chua, C. N. Shahi, J. McDevitt, P. W. N. Keeling, and D. Kelleher. 1994. Gastric T lymphocyte response to Helicobacter pylori in patients with H. pylori colonisation. Gut 35:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaves, D. R., W. Wang, D. J. Dairaghi, M. C. Dieu, B. D. Saint-Vis, K. Franz-Bacon, D. Rossi, C. Caux, T. McClanahan, S. Gordon, A. Zlotnik, and T. J. Schall. 1997. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3a and is highly expressed in human dendritic cells. J. Exp. Med. 186:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izadpanah, A., M. B. Dwinell, L. Eckmann, N. M. Varki, and M. F. Kagnoff. 2001. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G710-719. [DOI] [PubMed] [Google Scholar]
- 19.Jones, N., A. Day, H. A. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karttunen, R., T. Karttunen, H.-P. T. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao, F., R. L. Rabin, C. S. Smith, G. Sharma, T. B. Nutman, and J. M. Farber. 1999. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J. Immunol. 162:186-194. [PubMed] [Google Scholar]
- 22.Lindholm, C., M. Quiding-Jalrbrink, H. Lonroth, A. Hamlet, and A.-M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luster, A. D. 1998. Chemokines: chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 24.Mattapallil, J. J., S. Dandekar, D. R. Canfield, and J. V. Solnick. 2000. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology 118:307-315. [DOI] [PubMed] [Google Scholar]
- 25.Moser, B., and P. Loetscher. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123-128. [DOI] [PubMed] [Google Scholar]
- 26.Nishi, T., K. Okazaki, K. Kawasaki, T. Fukui, H. Tamaki, M. Matsuura, M. Asada, T. Watanabe, K. Uchida, N. Watanabe, H. Nakase, M. Ohana, H. Hiai, and T. Chiba. 2003. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect. Immun. 71:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadakis, K. A., J. Prehn, V. Nelson, L. Cheng, S. W. Binder, P. D. Ponath, D. P. Andrew, and S. R. Targan. 2000. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165:5069-5076. [DOI] [PubMed] [Google Scholar]
- 28.Parsonnet, J. 1997. Molecular mechanisms for inflammation-promoted pathogenesis of cancer—The Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 57:3620-3624. [PubMed] [Google Scholar]
- 29.Parsonnet, J., G. D. Friedman, D. G. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1994. Helicobacter pylori infection and risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 30.Power, C. A., D. J. Church, A. Meyer, S. Alouani, A. E. I. Proudfoot, I. Clark-Lewis, S. Sozzani, A. Mantovani, and T. N. C. Wells. 1997. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3a from lung dendritic cells. J. Exp. Med. 186:825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudi, J., D. Kuck, S. Strand, A. Von Herbay, S. M. Mariani, P. H. Krammer, P. R. Galle, and W. Stremmel. 1998. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Investig. 102:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibahara, T., J. N. Wilcox, T. Couse, and J. L. Madara. 2001. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology 120:60-70. [DOI] [PubMed] [Google Scholar]
- 33.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka, Y., T. Imai, M. Baba, I. Ishikawa, M. Uehira, H. Nomiyama, and O. Yoshie. 1999. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur. J. Immunol. 29:633-642. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, S., W. Beil, J. Westermann, R. P. H. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 36.Wang, J., X. Fan, C. Lindholm, M. Bennett, J. O'Connoll, F. Shanahan, E. G. Brooks, V. E. Reyes, and P. B. Ernst. 2000. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect. Immun. 68:4303-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen, S., C. P. Felley, H. Bouzourene, M. Reimers, P. Michetti, and Q. Pan-Hammarstrom. 2004. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans1. J. Immunol. 172:2595-2606. [DOI] [PubMed] [Google Scholar]
- 38.Wu, Y. Y., H. F. Tsai, W. C. Lin, A. H. Chou, H. T. Chen, J. C. Yang, P. I. Hsu, and P. N. Hsu. 2004. Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J. Gastroenterol. 10:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaoka, Y., M. Kita, T. Kodama, S. N., T. T., K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, S. K., L. Eckmann, A. Panja, and M. F. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 113:1214-1223. [DOI] [PubMed] [Google Scholar]