Abstract
The extracytoplasmic-stress-responsive CpxRA two-component signal transduction pathway allows bacteria to adapt to growth in extreme environments. It controls the production of periplasmic protein folding and degradation factors, which aids in the biogenesis of multicomponent virulence determinants that span the bacterial envelope. This is true of the Yersinia pseudotuberculosis Ysc-Yop type III secretion system. However, despite using a second-site suppressor mutation to restore Yop effector secretion by yersiniae defective in the CpxA sensor kinase, these bacteria poorly translocated Yops into target eukaryotic cells. Investigation of this phenotype herein revealed that the expression of genes which encode several surface-located adhesins is also influenced by the Cpx pathway. In particular, the expression and surface localization of invasin, an adhesin that engages β1-integrins on the eukaryotic cell surface, are severely restricted by the removal of CpxA. This reduces bacterial association with eukaryotic cells, which could be suppressed by the ectopic production of CpxA, invasin, or RovA, a positive activator of inv expression. In turn, these infected eukaryotic cells then became susceptible to intoxication by translocated Yop effectors. In contrast, bacteria harboring an in-frame deletion of cpxR, which encodes the cognate response regulator, displayed an enhanced ability to interact with cell monolayers, as well as elevated inv and rovA transcription. This phenotype could be drastically suppressed by providing a wild-type copy of cpxR in trans. We propose a mechanism of inv regulation influenced by the direct negative effects of phosphorylated CpxR on inv and rovA transcription. In this fashion, sensing of extracytoplasmic stress by CpxAR contributes to productive Yersinia sp.-eukaryotic cell interactions.
The CpxRA two-component signal transduction pathway responds to extracytoplasmic stress (ECS) in the bacterial periplasm (for recent reviews, see references 19, 20, 67, and 74). CpxA is the sensor kinase, and CpxR is the response regulator. When phosphorylated by CpxA, CpxR-P acts as a transcriptional factor to activate or repress >100 gene targets in Escherichia coli (17). Several inducing cues are known, including elevated pH (13, 53); adenylate cyclase mutations (79); depletion of phosphatidylethanolamine (48) or outer membrane phospholipase A (PldA) (42); and the accumulation of enterobacterial common antigen lipid II biosynthetic intermediate (14), assembly intermediates of pili (41), type IV secretion systems (90), and the outer membrane lipoprotein NlpE (78). All of these characteristics were seen with the Cpx system of E. coli, and they probably cause inappropriate protein folding in the periplasm. Several downstream targets of CpxR-P therefore encode protein folding and/or degradation factors that exert their function in the periplasm. These include the disulfide bond catalyst DsbA (12, 64), peptidyl-prolyl cis/trans isomerases PpiA and PpiD (15, 64), and the DegP serine protease (11). CpxR-P also activates its own operon (16, 66) and modulates the expression of a second ECS-responsive pathway—the alternative sigma factor σE (17). The Cpx pathway is therefore a central sensory component of periplasmic quality control mechanisms important for bacterial adaptation to stress.
Human-pathogenic Yersinia spp. comprise the two enteropathogens Yersinia pseudotuberculosis and Y. enterocolitica, which are responsible for self-limiting food-borne infections (54), and the infamous species Y. pestis, the causative agent of the often fatal diseases bubonic plague and pneumonic plague (59). Although the routes of infection and disease outcomes are dramatically different, all three species resist antiphagocytic host defense mechanisms, allowing extracellular replication within lymphoid tissue (26, 71, 76). This process is mediated by the Ysc-Yop type III secretion system (T3SS) encoded on a common ∼70-kb virulence plasmid (10). Secreted effector Yops (Yersinia outer proteins) are then localized to the eukaryotic cell interior, where their action disrupts cellular signaling pathways, enabling the bacteria to resist phagocytosis and proliferate extracellularly (82).
With the exception of YmoA, a nucleoid-associated protein responsible for thermoregulation of Yops (9, 40), Ysc-Yop biosynthesis is principally orchestrated via built-in regulatory components located on the virulence plasmid (46). However, we have reported that Cpx pathway activation affects the efficiency of Ysc-Yop T3S (6). Therefore, production of the T3SS may require some factor or factors repressed by CpxR-P. This supports the recently expressed view that envelope stress-responsive pathways are important for bacterial virulence (67, 74). Interestingly, CpxA-defective yersiniae engineered to overproduce and secrete from the Ysc-Yop system, thereby masking the existing CpxA defect, still inefficiently intoxicated target eukaryotic cells (6). This unexpected finding implies that the Yersinia Cpx pathway might also sense target cell contact, an important elicitor of T3S (60, 73).
In this study, we therefore focused on screening for defects in the expression of the known Yersinia adhesins—invasin (reviewed in reference 29), Ail (reviewed in reference 38), and pH 6 antigen (87). Our results implicate the Cpx pathway in their control. The repercussion was that a ΔcpxA null mutant displayed a reduced capacity to bind and be internalized by mammalian cells. This could be suppressed by ectopic expression of CpxA, invasin, or RovA, a positive regulator of inv expression (52, 68). In addition, these bacteria regained the ability to intoxicate cells with Yop effectors. Significantly, the transcription of both inv and rovA was impaired in the cpxA mutant. In contrast, the ΔcpxR null mutant more efficiently bound to eukaryotic cells and transcribed more inv and rovA mRNAs. These inverse phenotypes are consistent with a critical role for CpxR-P in the synthesis of the invasin adhesin and subsequent Yersinia sp.-host cell interactions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. Unless stated otherwise, bacteria were routinely cultivated in Luria-Bertani (LB) agar or broth at either 26°C (Y. pseudotuberculosis) or 37°C (E. coli) with aeration. Where required, carbenicillin (100 μg/ml), chloramphenicol (25 μg/ml), or kanamycin (50 μg/ml) was added at the final concentration indicated in parentheses.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5 | F−recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 | Vicky Shingler |
| S17-1λpir | recA thi pro hsdRM+ Smr <RP4-2-Tc-Mu-Ku-Tn7> Tpr | 75 |
| BL21(DE3) plysS | F−dcm lon ompT hsdS (rB− mB−) gal λ(DE3) [pLysS Cmr] | Promega |
| Y. pseudotuberculosisa strains | ||
| YPIII/pIB102 | yadA::Tn5 Kmr (parent) | 4 |
| YPIII07/pIB102 | cpxA in-frame deletion of codons 41-449 Kmr | 6 |
| YPIII07/pIB75 | yscU in-frame deletion of codons 25-329 Kmr | This study |
| YPIII08/pIB102 | cpxR in-frame deletion of codons 11-193 Kmr | 6 |
| SF104/pYH7 | ΔyadA (in frame) inv::kan Kmr | 30 |
| Plasmids | ||
| pLS13 | 669-bp PCR fragment of the allele encoding YscUΔ25-329 on pDM4; Cmr | 43 |
| pCR4-TOPO | TA cloning vector; Kmr Cbr | Invitrogen |
| pMMB208 | Expression vector; Cmr | 50 |
| pKEC021 | ∼700-bp XbaI/KpnI PCR fragment of cpxR in pMMB208; Cmr | 6 |
| pJF015 | ∼720-bp XbaI/KpnI PCR fragment of cpxR encoding D51A mutation in pMMB208; Cmr | 6 |
| pET22b(+) | Expression vector; Cbr | Novagen |
| pMF581 | 1,381-bp NdeI/EagI PCR fragment of cpxA in pET22b(+); Cbr | 6 |
| pKEC017 | ∼700-bp NdeI/XhoI PCR fragment of cpxR in pET22b(+) that creates a C-terminal His6 fusion; Cbr | This study |
| pGN37 | pBAD18 rovA+; Cbr | 31 |
| pIRR1 | ∼4.4-kb BamHI fragment of inv in pACYC184; Cmr | 70 |
RNA isolation and reverse transcriptase PCR (RT-PCR).
Yersiniae were grown overnight at 26°C and 37°C. These cultures where either used directly (stationary phase), or 0.1-volumes were subcultured into 5 ml of fresh medium and the bacteria were regrown at the same temperature for a further 90 min (logarithmic phase). One volume of bacterial culture was added to 2 volumes of RNAprotect bacteria reagent (QIAGEN Nordic, West Sussex, United Kingdom) and then extracted by the NucleoSpin RNA II method (Macherey Nagel, Düren, Germany) that included an on-column DNase treatment. Reverse transcription of mRNA and subsequent PCR with the gene-specific primer pair combinations listed in Table 2 are described in detail elsewhere (6). Briefly, cDNA was generated by the ImProm-II reverse transcription system (Promega, Madison, WI). Specific gene transcripts were detected by PCR in 20-μl reaction mixture volumes that included the Dynazyme II DNA polymerase (Finnzymes, Espoo, Finland) or Easy-A high-fidelity PCR cloning enzyme (Stratagene). Cycling conditions were an initial denaturation at 94°C for 3 min and then denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s (5 cycles) and then denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s (25 cycles) before a final extension at 72°C for 5 min in a GeneAmp PCR system.
TABLE 2.
Oligonucleotides used in this study
| Application and specific genea | Name | Sequenceb |
|---|---|---|
| RT-PCR | ||
| rpoA (330 bp) | prpoAa | 5′-GTT CGA CGC ACG CCA AGG TGA-3′ |
| prpoAb | 5′-ACG TCC TGC GGC TTG ACG AT-3′ | |
| cpxR (390 bp) | pcpxRfor | 5′-GTG AAC TGA CGT CGC TGT TGA-3′ |
| pcpxRrev | 5′-TTG CAG GCA ATC AAC TTC CAG-3′ | |
| cpxA (295 bp) | pcpxAkpn | 5′-ACG TAC GGT ACC ATC AGT GCA CCG CTG-3′ |
| pcpxAsac | 5′-ACG TAC GAG CTC TTC ACC ATG ACG GCG-3′ | |
| cpxP (220 bp) | pcpxPfor | 5′-GTT ATG GCG TCA ATG TTC GTT C-3′ |
| pcpxPrev | 5′-CAA GAT CAA GGC GCG GCT GAC-3′ | |
| ppiA (210 bp) | pppiAfor | 5′-CCG GGA ATA TTG AGC TGG AG-3′ |
| pppiArev | 5′-CGC AGG CCA TTA TCT GCT TC-3′ | |
| degP (410 bp) | pdegPkpn | 5′-ACG TAC GGT ACC ATC TGA CTG CGA TTA-3′ |
| pdegPsac | 5′-ACG TAC GAG CTC CAA CCT TCA TGG CTT-3′ | |
| ail (240 bp) | pail2867for | 5′-ATT GCA TGT TTA TCA ATT GCG-3′ |
| pail2867rev | 5′-CCA TCA ATA AGT TTG AAT CCG-3′ | |
| ail-like (280 bp) | pail2113for | 5′-CAC GGT ATC TTT CGG TTA CGC-3′ |
| pail2113rev | 5′-TGT ACT CTT ACC TTG AGT TGC-3′ | |
| ail2 (200 bp) | pail1731for | 5′-CAG GGA GAT GTA AGA CTC GGT-3′ |
| pail1731rev | 5′-GCG AAT AAT AAT CAA AGG ATG-3′ | |
| ompX (235 bp) | pompXfor | 5′-TTG CAT GTC TTT CAG CGG TAG-3′ |
| pompXrev | 5′-TTG TAA ACC GCT TCG TCA CCG-3′ | |
| psaA (265 bp) | ppsaAfor | 5′-ATC GCT GCT TGT GGT ATG GC-3′ |
| ppsaArev | 5′-ACC AAC ATA GTC ACC ATC GG-3′ | |
| inv (235 bp) | pinvfor | 5′-CCA GCC TTA TTC TGT CTC TTC-3′ |
| pinvrev | 5′-CCG CAT CGC CCA CCA TTG AG-3′ | |
| rovA (230 bp) | provAfor | 5′-GGC GCG CAT TAA TTG ACC ATC-3′ |
| provArev | 5′-AAT TCT CTT CGC ACG ACG ATC-3′ | |
| hns (345 bp) | phnskpn | 5′-ACG TAC GGT ACC TTG CTC TTC AAT GGC-3′ |
| phnssac | 5′-ACG TAC GAG CTC TTA ACA ACA TCC GTA-3′ | |
| Protein expression | ||
| cpxR::his(6) (700 bp) | pcpxR-Nde(ET) | 5′-CAT ATG CAT AAA ATC CTA TTA GTT GAT G-3′ |
| pcpxR-Xho(HisET) | 5′-CTC GAG TGT TTC TGA TAC CAT CA-3′ | |
| Mobility shift assay | ||
| cpxR/cpxP (245 bp) | pcpxRb | 5′-GTC ATC ATC AAC TAA TAG GA-3′ |
| pcpxPb | 5′-AAC GAA CAT TGA CGC CAT AAC-3′ | |
| cpxR (internal, 390 bp) | pcpxRfor | 5′-GTG AAC TGA CGT CGC TGT TGA-3′ |
| pcpxRrev | 5′-TTG CAG GCA ATC AAC TTC CAG-3′ | |
| degP (245 bp) | pdegPfulla | 5′-ACG CTC GAG CTG CGG AAT AGT ATG CA-3′ |
| pdegPfullb | 5′-CAA TGC CAA TGC ACT TAA TAC-3′ | |
| rovA (715 bp) | provAfor1 | 5′-CCG ACG CTA AGT GTC AAT AAC-3′ |
| provArev1 | 5′-GAA CTA ATC GTG CTA GAT CAG-3′ | |
| inv (505 bp) | pinvfor1 | 5′-TCA TCA AGG CAA CCA TCA GGA-3′ |
| pinvrev1 | 5′-AGA AAC TCA CTG ATT GGC TGG-3′ |
The number of base pairs in parentheses is the approximated size of the amplified PCR fragment.
The NdeI and XhoI restriction endonuclease sites are in italics.
Preparation of cell extracts and Western blotting.
Overnight Yersinia cultures were grown at 26°C and 37°C. After standardization of the optical density at a wavelength of 600 nm, pelleted fractions were lysed in sample buffer, heat inactivated prior to fractionation by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to Schleicher & Schuell nitrocellulose membrane (Whatman, Middlesex, United Kingdom). Invasin and RovA were identified with rabbit polyclonal anti-invasin and anti-RovA antibodies, respectively. Identification of H-NS was performed with rabbit polyclonal antibodies raised against H-NS of E. coli. Proteins were detected with a combination of horseradish peroxidase-conjugated anti-rabbit antibody (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and chemiluminescent detection with homemade solutions.
HeLa cell infection assays.
Cultivation and infection of HeLa cells for cytotoxicity assays were performed by standard methods (28, 72). At hourly intervals, the extent of morphological change was visualized by light microscopy (72). Cytotoxicity resulting from infection with the parental Y. pseudotuberculosis strain (YPIII/pIB102) defined the upper limit, while the lower limit was defined by the ΔyadA inv::kan double mutant (SF104/pYH7) (30).
To analyze the extent of bacterial association with eukaryotic cells, HeLa cells were grown to near confluence in six-well tissue culture trays. Washed cells were infected with standardized bacterial cultures pregrown in tissue culture medium, at a multiplicity of infection of ∼10. Infected cells were synchronized by a brief mild centrifugation and incubated at either 26°C or 37°C in a humidified 5% CO2 environment. At 90 min postinfection, cells were gently washed multiple times to remove unattached and loosely attached bacteria. Those bacteria tightly associated with eukaryotic cells were recovered in a solution of 0.1% (vol/vol) Triton X-100 and quantitated by viable-cell counting. Data are representative of multiple independent experiments expressed as the mean percentage of the total number of infecting bacteria.
The invasion assay is essentially the same as the attachment assay, except that the bacteria which remained tightly associated with cells after initial washes were overlaid with fresh cell culture medium containing gentamicin (20 μg/ml) and incubated for another 90 min. The extracellular bacteria were removed by gentle washing. Internalized bacteria protected from gentamicin exposure were then recovered via a solution of 0.1% (vol/vol) Triton X-100 and quantitated by viable-cell counting. Their number was expressed as a percentage of those bacteria tightly associated with cells, derived from at least four independent experiments.
CpxR-His6 purification and in vitro phosphorylation.
The cpxR allele from parental Y. pseudotuberculosis was amplified with gene-specific primers (Table 2) and cloned in front of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter of pET22b(+). This expression plasmid, pKECO17, maintained in E. coli BL21(DE3) plysS, gave rise to CpxR fused to a C-terminal His6 tag. An overnight culture of this strain grown in LB broth at 26°C was subcultured into 100 ml of the same medium and grown for 2 h at 26°C. IPTG at a final concentration of 0.4 mM was then added, and the culture was grown for a further 4 h. After chilling on ice, bacteria were collected by centrifugation at 6,000 rpm for 20 min. They were then washed in an equal volume of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 1 mM imidazole, pH 8) and concentrated 10-fold in the same buffer before being disrupted by ultrasonication with 5-s pulses followed by 5-s pauses for 2 min in the presence of Complete protease inhibitor (EDTA free; Roche Diagnostics). The soluble lysate was clarified by low-speed centrifugation prior to the addition of 0.1 volume of a 50% Ni-nitrilotriacetic acid slurry (QIAGEN) for 1 h at 4°C. Protein was then purified on a Poly-Prep chromatography column (Bio-Rad) as previously described (21). Samples of each flowthrough were collected for analysis by SDS-PAGE and Western blotting with mouse monoclonal antisera recognizing His6 (QIAGEN) fused to the C terminus of CpxR. The concentration of purified CpxR::His6 was approximately 3.35 mg/ml. CpxR was phosphorylated by incubating 104 μg/ml purified protein for 1 h at 30°C with a mixture of 100 mM Tris-HCl (pH 7.0), 10 mM MgCl2, 125 mM KCl, and 50 mM acetyl phosphate (lithium, potassium salt; Sigma-Aldrich).
Electrophoretic mobility shift assay.
Target DNA fragments containing the control regions to the inv, rovA, degP, and cpxR/cpxP genes were amplified by PCR with the specific primers described in Table 2. These fragments were gel extracted via Genelute minus ethidium bromide spin columns (Sigma-Aldrich) and then phenol-chloroform purified before being concentrated by isopropanol precipitation. DNA-protein binding assays were perform at 25°C for 30 min after combining 40 to 80 μg/ml DNA (depending on the template) with 26 μg/ml CpxR::His6 in a solution of 100 mM Tris-HCl (pH 7.4), 100 mM KCl, 10 mM MgCl2, 10% glycerol, 1.5 mM dithiothreitol, and 1 mM EDTA. To the completed reaction mixtures, 3 μl of 75% (vol/vol) glycerol was added before the samples were analyzed on a 5% nondenaturing polyacrylamide gel in TAE buffer (0.08 M Tris-acetate, 1 mM EDTA).
RESULTS
CpxA is required for Y. pseudotuberculosis to interact with mammalian cells.
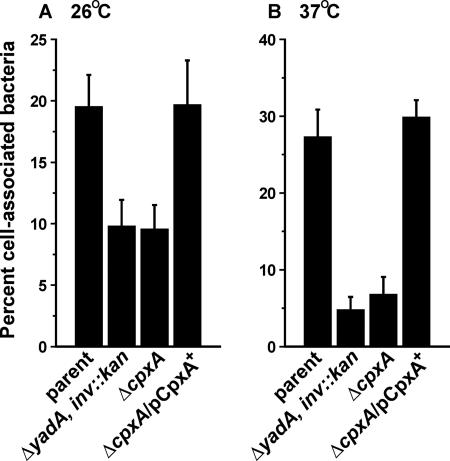
Y. pseudotuberculosis lacking the CpxA sensor kinase but engineered to secrete Yops extensively still poorly translocated these Yops into mammalian cells (data not shown) (6). Since Yop translocation requires intimate target cell contact (73), perhaps these bacteria only weakly interact with eukaryotic cells. To investigate this hypothesis, we infected HeLa cell monolayers with the parent strain and ΔcpxA null mutant bacteria to determine the ability of each strain to associate with target cells. At 26°C, the ΔcpxA null mutant displayed an ∼50% reduction in cell-associated bacteria, while the reduction was ∼75% when the bacteria were cultured at 37°C (Fig. 1). These results are comparable to those obtained with isogenic control bacteria defective for two principal adhesins, invasin and YadA (25, 29, 39). Poor cell association by our mutant was due entirely to the loss of CpxA because mutant bacteria bound to cells with normal efficiency when producing CpxA from an expression plasmid. Thus, the inability of the ΔcpxA null mutant to engage mammalian cells could explain why these cells were not susceptible to the T3S-dependent injection of effector toxins (6). Furthermore, it suggests that the Cpx pathway is required for the expression and/or function of one or more Yersinia adhesins.
FIG. 1.
HeLa cell association by Y. pseudotuberculosis. Strains were allowed to infect a monolayer of growing HeLa cells at either 26°C (A) or 37°C (B). Unattached and loosely attached bacteria were removed, and viable-cell counts were performed on the remaining tightly cell-associated bacteria. Shown is the mean percentage ± the standard error of the mean from at least four independent experiments of infecting bacteria that remained tightly associated with cells. Strains: parent, YPIII/pIB102; ΔyadA inv::kan double mutant, SF104/pYH7; ΔcpxA null mutant, YPIII07/pIB102; ΔcpxA mutant complemented with pcpxA+, YPIII07/pIB102/pMF581. Expression of cpxA from pMF581 was induced by IPTG.
The Cpx pathway influences the transcription of adhesin-encoding genes in Y. pseudotuberculosis.
To engage mammalian cells, yersiniae use at least four prominent surface-located adhesins, invasin (inv), pH 6 antigen (psa), YadA (yadA), and Ail (ail/YPTB2867) (25, 29, 38). Furthermore, yersiniae also possess up to three ail paralogues (8) designated ail-like (YPTB2113), ail2 (YPTB1731), and ompX (YPTB2542) according to the Pfam server (http://www.sanger.ac.uk/cgi-bin/Pfam/). Could CpxA influence the function of one or more of these adhesins? To explore this notion, we used RT-PCR to analyze gene transcription in the exponentially grown Y. pseudotuberculosis parental strain and ΔcpxA null mutant bacteria. Isolated mRNA was reversed transcribed, and this cDNA served as the template in PCRs with gene-specific primer pairs recognizing inv, psaA (which encodes the pH 6 antigen tip adhesin), and the four ail paralogues. Analysis of yadA was not included because this gene is disrupted in our strain (4). As a control to visualize the effect that loss of CpxA has on gene expression within the CpxR-P regulon, we analyzed the transcription of three genes, ppiA, degP, and cpxP, which in E. coli are positively regulated by an activated Cpx pathway (11, 13, 17, 64, 78). Indeed, the expression of all three genes is elevated in response to loss of CpxA (Fig. 2). Since temperature is an important cue for the control of adhesin gene expression (25, 29, 38), we opted to grow bacteria to logarithmic phase at both 26°C and 37°C. Significantly, the transcription of inv, psaA, and ail was notably reduced in the ΔcpxA null mutant (Fig. 2). This reduction could be trans complemented with a wild-type copy of cpxA. On the other hand, the transcription of the ail-like allele that encodes YPTB2113 was elevated in the absence of CpxA (Fig. 2). Thus, the Cpx pathway can apparently impart both positive and negative transcriptional control on genes that encode a number of important Yersinia adhesins.
FIG. 2.
RT-PCR of mRNA isolated from Y. pseudotuberculosis. RNA was isolated from logarithmic-phase bacterial cultures grown at 26°C or 37°C in LB medium. Samples were subjected to RT-PCR with primers specific for ail, ail-like, ail2, ompX, psaA, and inv. Amplification of rpoA was used as an internal standard. The ppiA, degP, and cpxP alleles are representative members of the Cpx regulon that served as controls to monitor the regulatory influence of Cpx pathway activation. Where indicated, IPTG was added to induce ectopic expression of cpxA. Lanes: parent, YPIII/pIB102; ΔcpxA null mutant, YPIII07/pIB102; complemented ΔcpxA/pcpxA+ mutant, YPIII07/pIB102/pMF581. All images where first acquired with a Fluor-S MultiImager (Bio-Rad). After image inversion, the intensity of each band was quantified with the Quantity One quantitation software version 4.2.3 (Bio-Rad) and is given below each image as a value relative to the gene expression in parental bacteria grown at the lower temperature.
To our knowledge, this is the first report indicating that all ail paralogues are actively transcribed in Yersinia spp. While temperature does not seem to affect the transcription of the ail-like allele, a lower growth temperature may slightly favor transcription of the ail2 and ompX alleles (Fig. 2). However, this temperature dependency is not as great as that of inv transcription. As expected, the transcription of ail and psaA was elevated at higher growth temperatures (44, 62).
Production of invasin depends upon functional CpxA.
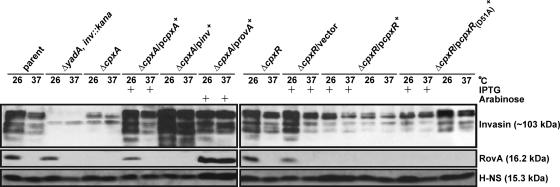
It is intriguing that the ΔcpxA null mutant, which also lacks a functional yadA gene, poorly transcribes inv-specific mRNA and engages mammalian cells at a frequency as low as that of the ΔyadA inv::kan double mutant. This suggests that the ΔcpxA null mutant may exhibit a critical defect in invasin production. We investigated the levels of invasin production in the Yersinia parental strain and ΔcpxA null mutant bacteria grown to stationary phase at either 26°C or 37°C. Consistent with optimal production at low temperature (37, 52, 57, 61), invasin levels were significantly elevated when parent bacteria were grown at 26°C compared to 37°C (Fig. 3). However, invasin was barely detectable in the ΔcpxA null mutant, regardless of the growth temperature (Fig. 3). Complementation with a wild-type copy of cpxA restored normal temperature-dependent invasin production (Fig. 3). Thus, CpxA sensor activity is necessary for controlled production of invasin.
FIG. 3.
Analysis of invasin, RovA, and H-NS production by Y. pseudotuberculosis. Protein was isolated from stationary-phase bacteria grown in LB medium at either 26°C or 37°C, separated by SDS-PAGE, and then identified by immunoblot analysis with polyclonal rabbit antiserum raised against invasin, RovA, or E. coli H-NS. Where indicated, IPTG (final concentration of 0.4 mM) or arabinose (0.02%) was added. Lanes: parent, YPIII/pIB102; ΔyadA, inv::kan double mutant, SF104/pYH7; ΔcpxA null mutant, YPIII07/pIB102; ΔcpxA mutant complemented with pcpxA+, YPIII07/pIB102/pMF581; ΔcpxA mutant suppressed with pinv+, YPIII07/pIB102/pIRR1; ΔcpxA mutant suppressed with provA+, YPIII07/pIB102/pGN37; ΔcpxR null mutant, YPIII08/pIB102; ΔcpxR mutant with vector control, YPIII08/pIB102/pMMB208; ΔcpxR mutant producing wild-type CpxR in trans, YPIII08/pIB102/pKEC021; ΔcpxR mutant producing nonphosphorylatable CpxRD51A in trans, YPIII08/pIB102/pJF015. Molecular masses in parentheses were deduced from the primary sequence.
The cpxA mutant is defective for invasin-dependent interactions with eukaryotic cells.
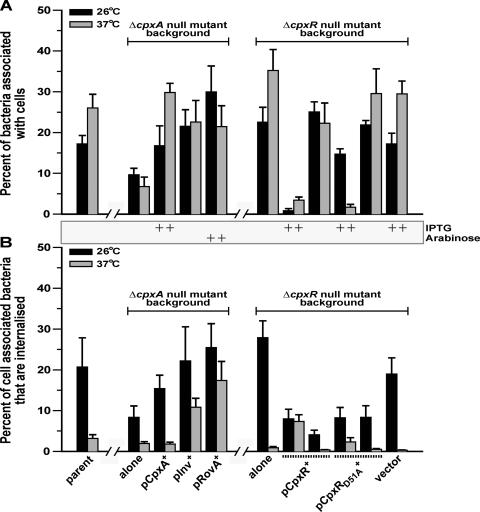
If a defect in invasin production is the principal reason why the ΔcpxA null mutant fails to establish productive eukaryotic cell interactions, then ectopically expressing inv should suppress this mutant phenotype. A low-copy-number vector containing inv under the control of its native promoter was introduced into the ΔcpxA null mutant. Significantly, invasin produced by this strain was abundant even at higher temperatures (Fig. 3). We next wondered whether this invasin could restore bacterial association with and invasion of HeLa cell monolayers. Because close cell contact induces the antiphagocytic properties of the Yersinia T3SS (82), we performed infections at 26°C to restrict the T3SS and at 37°C to induce the T3SS. As expected, the ΔcpxA null mutant poorly associated with HeLa cells at both temperatures (Fig. 4A). Moreover, these cell-associated bacteria did not effectively invade eukaryotic cells (Fig. 4B). Intriguingly, cell association and uptake were restored to this mutant when inv was expressed in trans (Fig. 4). Not surprisingly, uptake was more efficient at 26°C, which is a dual reflection of the down regulation of elevated invasin production at this temperature (37, 52, 57, 61) and the antiphagocytic properties of the T3SS preferentially induced at 37°C (10, 82). This finding implies that additional copies of the inv gene can suppress the cell binding and uptake defects exhibited by the ΔcpxA null mutant. We therefore conclude that these cell contact defects are principally due to a loss of invasin at the bacterial surface.
FIG. 4.
Y. pseudotuberculosis-HeLa cell association and uptake efficiency. Strains were allowed to infect monolayers of growing HeLa cells at 26°C (black bars) and at 37°C (gray bars). (A) The percentage of infecting bacteria that remained tightly associated with cells was determined as outlined in the legend to Fig. 1. (B) The remaining duplicates were subjected to gentamicin to recover internalized bacteria protected from antibiotic exposure. Their number is expressed as a mean percentage ± the standard error of the mean from at least four independent experiments of those bacteria tightly associated with cells. Where indicated, ectopic expression of cpxA and the cpxR allelic variants was induced with IPTG. rovA expression was induced with arabinose. Strains: parent, YPIII/pIB102; ΔcpxA null mutant alone, YPIII07/pIB102; ΔcpxA mutant complemented with pcpxA+, YPIII07/pIB102/pMF581; ΔcpxA mutant suppressed with pinv+, YPIII07/pIB102/pIRR1; ΔcpxA mutant suppressed with provA+, YPIII07/pIB102/pGN37; ΔcpxR null mutant alone, YPIII08/pIB102; ΔcpxR mutant producing wild-type CpxR in trans, YPIII08/pIB102/pKEC021; ΔcpxR mutant producing nonphosphorylatable CpxRD51A in trans, YPIII08/pIB102/pJF015; ΔcpxR mutant with vector control, YPIII08/pIB102/pMMB208.
Production of RovA in CpxA-defective bacteria suppresses the loss of bacterium-eukaryotic cell interactions.
RovA is a chromosomally encoded transcriptional activator of virulence gene expression that belongs to the SlyA/MarR family (24). It is autoregulated and required for the control of inv expression (52, 68). RovA functions as an antirepressor, antagonizing H-NS-mediated repression of inv and rovA expression through competition for similar binding sites within each promoter region (23, 31, 80). Therefore, we examined if increasing the levels of RovA produced in the ΔcpxA null mutant could elevate invasin production and also suppress the cell binding and uptake phenotype. RovA was expressed from an arabinose-inducible promoter, and this was sufficient to increase invasin production independently of temperature (Fig. 3). Consistent with this observation, this strain efficiently bound to (Fig. 4A) and invaded (Fig. 4B) HeLa cell monolayers even at higher temperatures. Thus, elevated RovA levels mask the effect of removing CpxA and the antiphagocytic properties of the T3SS, enabling invasin to function at the bacterial surface.
A cpxA mutant engineered to produce elevated levels of invasin can intoxicate infected eukaryotic cells with Yop effector toxins.
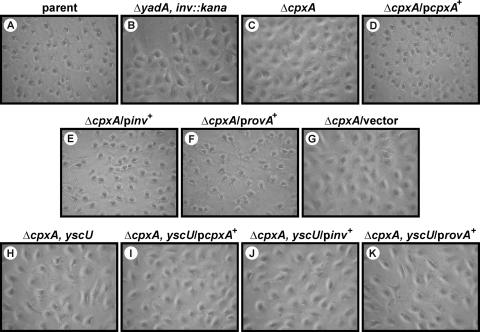
Yersiniae defective for CpxA poorly translocate Yop effectors into eukaryotic cells, an effect that reflects not only low Yop secretion (6) but also reduced bacterium-target cell contact (Fig. 1 and Fig. 4). To investigate if Yop translocation can be restored in the ΔcpxA null mutant by artificially elevating invasin levels, we used a HeLa cell monolayer cytotoxicity assay to measure Yop intoxication (72). Translocation of the YopE cytotoxin induces actin depolymerization, which causes infected cells to become round. This cytotoxicity is easily viewed by phase-contrast light microscopy. As expected, the morphology of cells infected with the ΔcpxA null mutant remained unaltered (Fig. 5). However, the ΔcpxA null mutant producing CpxA, invasin, or RovA in trans induced a dramatic change in cellular morphology akin to an infection with the parent Yersinia strain (Fig. 5). This cytotoxicity is strictly dependent on the Ysc-Yop T3SS and not some other Cpx-induced factor because isogenic strains carrying an additional deletion of yscU, which encodes an essential component of the secretion apparatus (43), no longer supported effector translocation (Fig. 5). Thus, ectopic production of invasin or RovA can induce the ΔcpxA null mutant to form an intimate association with target cells that, in turn, promotes T3SS-dependent translocation of Yop effectors.
FIG. 5.
Infection of HeLa cells by Y. pseudotuberculosis. Strains were allowed to infect a monolayer of growing HeLa cells. At ∼1 h postinfection, the effect of the bacteria on the HeLa cells was recorded by phase-contrast microscopy. Infection with bacteria capable of translocating the YopE cytotoxin caused extensive rounding of the HeLa cells. In the absence of translocated YopE, infected cells maintained a typically elongated morphology. Ectopic expression of cpxA and rovA was induced with IPTG and arabinose, respectively. Shown are phase-contrast images of parental strain YPIII/pIB102 (A); ΔyadA inv::kan double-mutant strain SF104/pYH7 (B); ΔcpxA null mutant strain YPIII07/pIB102 (C); a ΔcpxA mutant strain complemented with pcpxA+, YPIII07/pIB102/pMF581 (D); a ΔcpxA mutant strain suppressed with pinv+, YPIII07/pIB102/pIRR1 (E); a ΔcpxA mutant strain suppressed with provA+, YPIII07/pIB102/pGN37 (F); a ΔcpxA mutant strain with vector control, YPIII07/pIB102/pMF200 (G); ΔcpxA yscU double-mutant strain YPIII07/pIB75 (H); a ΔcpxA yscU mutant strain producing CpxA in trans, YPIII07/pIB75/pMF581 (I); a ΔcpxA yscU mutant strain producing invasin in trans, YPIII07/pIB75/pIRR1 (J); and a ΔcpxA yscU mutant strain producing RovA in trans, YPIII07/pIB75/pGN37 (K).
Loss of the cognate CpxR response regulator enhances Yersinia sp.-target cell interactions.
Activated CpxA acts as a kinase transferring a phosphate molecule to CpxR (65). Phosphorylated CpxR (CpxR-P) can then bind to DNA to activate or repress target gene promoters. When homeostasis has been reached, CpxR-P is silenced by the intrinsic phosphatase activity of CpxA (65). One consequence of generating a ΔcpxA null mutant is to raise the levels of CpxR-P inside bacteria. Presumably, this would chiefly occur under non-Cpx-inducing conditions because under inducing conditions, CpxA would normally act as a kinase rather than a phosphatase. CpxR-P levels are further augmented by the nonspecific phosphorylation via small phosphodonors such as acetyl phosphate (11, 13). Therefore, it is possible that hyperphosphorylated CpxR represses invasin production in the ΔcpxA null mutant. If this is true, a ΔcpxR null mutant would be expected to produce extra invasin and/or more readily associate with and be taken up by eukaryotic cells. Indeed, compared to parental bacteria, the ΔcpxR null mutant appeared to more efficiently associate with eukaryotic cells, especially at 37°C (Fig. 4A), consistent with an earlier suggestion (32). In addition, at 26°C, cell-associated bacteria ably invaded these cells (Fig. 4B). This is consistent with the ΔcpxR null mutant producing more invasin, but this could not be demonstrated by Western analysis, presumably because invasin is intrinsically unstable (Fig. 3). However, analysis of inv mRNA did indicate that almost twofold more transcription occurred at 26°C in the ΔcpxR null mutant compared to the parental Yersinia strain (Fig. 6). Moreover, this strain also displayed elevated inv transcription at 37°C. Together, these data suggest that controlled inv expression has been incapacitated in the ΔcpxR null mutant. It follows that the comparatively poor uptake of the ΔcpxR null mutant at 37°C is most likely due to a more pronounced Yop-dependent antiphagocytic response (6, 10, 82) rather then down regulation of invasin production (37, 52, 57, 61).
FIG. 6.
RT-PCR of mRNA isolated from Y. pseudotuberculosis. RNA was isolated from stationary-phase bacterial cultures grown at 26°C or 37°C in LB medium. Samples were subjected to RT-PCR with primers specific for rovA, hns, inv, and cpxR. Amplification of rpoA was used as an internal standard. Cpx regulon members ppiA, degP, and cpxP served as controls to monitor the regulatory influence of Cpx pathway activation. Lanes: parent strain YPIII/pIB102; ΔcpxA null mutant strain YPIII07/pIB102; complemented ΔcpxA/pcpxA+ mutant strain YPIII07/pIB102/pMF581; ΔcpxR null mutant strain YPIII08/pIB102; a ΔcpxR mutant strain producing wild-type CpxR in trans, YPIII08/pIB102/pKEC021; and a ΔcpxR mutant strain producing nonphosphorylatable CpxRD51A in trans, YPIII08/pIB102/pJF015. All images were processed as described in the legend to Fig. 2.
To gain further support for a role for CpxR-P as a repressor of invasin production, we independently introduced into the ΔcpxR null mutant a low-copy-number expression plasmid harboring two versions of IPTG-inducible cpxR. However, in an effort to overcome repercussions associated with CpxR toxicity when it is overproduced in bacteria (6, 17), our phenotypic analysis was performed with strains grown either in the presence or in the absence of the IPTG inducer. The first strain harboring wild-type cpxR in trans would be expected to produce an abundant supply of CpxR able to be phosphorylated, because the high protein levels overwhelm the phosphatase activity of CpxA. Not surprisingly, therefore, even in the absence of IPTG induction inv transcript levels (Fig. 6) and invasin protein levels (Fig. 3) were low and comparable to those of the ΔcpxA null mutant. Moreover, this same strain was severely impaired in the ability to associate with cells at either temperature when grown in the presence of IPTG (Fig. 4A). In addition, these bacteria were only internalized by HeLa cells with moderate efficiency (Fig. 4B). These results are consistent with this strain having lost the capacity for efficient T3S and the ability to intoxicate cell monolayers with T3S-dependent Yop effectors (6). The second strain harbored an isogenic cpxR mutant allele containing a substitution that exchanges aspartate at position 51 with alanine. At least in E. coli, this CpxRD51A variant is not able to be phosphorylated (18). Accordingly, this strain was comparable to the ΔcpxR null mutant in some respects. inv expression (Fig. 6) and invasin production (Fig. 3) were elevated at least in the absence of IPTG. This contrasted with the phenotypes of the same mutant strain producing higher levels of wild-type CpxR in trans. Moreover, at least when grown at 26°C and even in the presence of IPTG, numbers of HeLa cell-associated bacteria more closely resembled infections with the ΔcpxR null mutant alone rather then with this same strain background producing wild-type CpxR from an expression plasmid (Fig. 4A). Importantly, these collective effects were not due to the additional burden of maintaining an expression plasmid with a chloramphenicol resistance cassette because the ΔcpxR null mutant harboring just the empty vector essentially behaved like the ΔcpxR null mutant alone with respect to invasin levels (Fig. 3) and cellular interactions (Fig. 4). We interpret these data, taken together, to favor the notion that CpxR-P actively represses invasin production by Y. pseudotuberculosis.
The CpxRA system controls invasin levels via modulation of rovA transcription.
Optimal inv expression in Y. pseudotuberculosis requires competition between the transcriptional activator RovA and the negative regulatory protein H-NS (31, 52, 80). When levels of RovA are low, it competes poorly with H-NS bound to overlapping binding sites within the rovA and inv promoters. This maintains H-NS mediated promoter silencing. However, this is reversed when levels of RovA are high, because it outcompetes H-NS for promoter binding. Since RovA can suppress the loss of CpxA, it seems logical that activation of a CpxRA response may inhibit rovA transcription. Perhaps CpxR-P acts directly as a repressor by binding to the rovA promoter or indirectly through the transcriptional inhibition of an unknown activator of RovA expression. At the same time, high levels of CpxR-P might even induce transcription from the hns promoter to elevate levels of the H-NS repressor. Together, these factors would have the effect of controlling inv expression.
To shed light on the role of CpxRA in the control of inv expression, we analyzed rovA and hns transcription in bacteria lacking CpxA or CpxR that were cultured to stationary phase at 26°C or 37°C. To monitor for the effect of Cpx system activation, we again analyzed the expression pattern of the cpxP, degP, and ppiA genes, three members of the Cpx regulon in E. coli (11, 13, 17, 64, 78). As expected, the anticipated elevation of CpxR-P levels in the ΔcpxA null mutant increased the expression of these three genes while loss of CpxR generally restricted their expression (Fig. 6). Moreover, compared to that in the parental bacterial strain, rovA transcription was severely reduced at 26°C in the ΔcpxA null mutant (Fig. 6). This could be overcome by trans complementation with cpxA. Consistent with this, rovA expression was elevated threefold in the ΔcpxR null mutant and lowered when wild-type copies of cpxR were provided in trans (Fig. 6). Furthermore, the ΔcpxR null mutant expressing a mutated cpxR allele, which encodes CpxRD51A lacking the aspartate residue normally phosphorylated by activated CpxA, again generated somewhat elevated levels of rovA transcription (Fig. 6). Significantly, this transcription profile was mirrored by mRNA levels derived from inv. It also correlates with the thermoregulated levels of the RovA protein in the ΔcpxA and ΔcpxR null strains (Fig. 3). We interpret these data to reflect an importance of Cpx system activation generating phosphorylated CpxR in the controlled repression of the transcription of both rovA and inv. Moreover, no notable difference in hns transcription (Fig. 6) or H-NS production (Fig. 3) at either temperature was detected in any of the strains. Thus, under the conditions used for these assays, hns transcription and H-NS levels in the bacteria appear to be unaffected by the Cpx system.
In further support for the accumulation of CpxR-P in yersiniae lacking CpxA, it is worth pointing out that cpxR mRNA levels are clearly elevated in this background (Fig. 6). In E. coli, the cpxRA operon is autoactivated by CpxR-P (16, 66). Thus, the cpxRA operon also appears to be autoregulated in yersiniae, such that the elevated cpxR transcription probably coincides with an increase in CpxR-P. Hence, these collective results suggest that CpxR-P regulates invasin production by controlling transcription from the rovA promoter. As RovA is a global regulator of gene expression (7), Y. pseudotuberculosis appears to have adapted the CpxRA signal transduction system to regulate its pathogenic potential via RovA.
Phosphorylated CpxR binds to inv and rovA control regions.
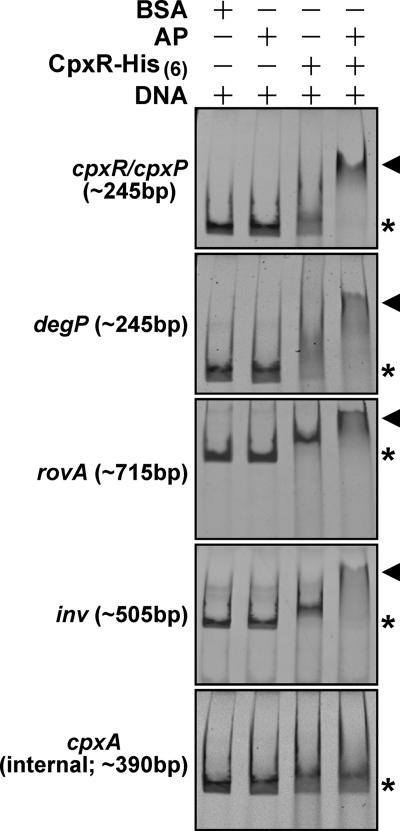
To better understand how invasin and RovA regulation is influenced by Cpx pathway activation, we first purified from E. coli the Yersinia CpxR protein containing a C-terminal His6 tag. Purified CpxR-His6 was then phosphorylated in vitro by incubation with acetyl phosphate. We performed an electrophoretic mobility shift assay with CpxR-P targeting control regions upstream of the inv and rovA genes. In particular, the ∼505-bp inv-specific fragment incorporated nucleotide positions −473 through to +32 relative to the translational start codon, while the ∼715-bp rovA-specific fragment encompassed nucleotides −673 to +40. We also included ∼245-bp amplified fragments of the cpxR/cpxP divergent promoter and the degP promoter, both of which are known to bind CpxR-P (16, 64, 86). While nonphosphorylated CpxR did appear to weakly bind all four promoters, this binding was dramatically enhanced by prior in vitro phosphorylation of CpxR with acetyl phosphate (Fig. 7). CpxR-P specifically bound these DNA targets in our assay, because no mobility shift was observed for DNA amplified from within cpxR (encompassing nucleotides +32 through to +420) (Fig. 7). Hence, Cpx pathway activation exerts its influence on invasin and RovA levels by direct binding of CpxR-P to the inv and rovA promoters.
FIG. 7.
Y. pseudotuberculosis CpxR-His6 binds to rovA and inv control regions. Mobility shift assays were performed with purified CpxR-His6 (26 μg/ml) and agarose gel-extracted PCR fragments harboring the regulatory regions of cpxR/cpxP, degP, rovA, and inv (40 to 80 μg/ml, depending on the fragment). Where indicated, CpxR-His6 was phosphorylated with acetyl phosphate (AP). An internal fragment of CpxR was used as a negative control. Constituents of each lane are indicated by plus signs. The approximate size of each amplified PCR fragment is given in parentheses. The electrophoretic mobility of these DNA fragments in the absence of protein is indicated by asterisks, while DNA-CpxR-P complexes are indicated by arrowheads.
DISCUSSION
Enteropathogenic Y. pseudotuberculosis serves as an ideal model to study bacterium-host cell interactions. This bacterium has evolved multiple independent adhesins, such as invasin, YadA, pH 6 antigen, and Ail, to establish close contact with eukaryotic cells (38). Although it triggers multiple host cell signaling events by engaging β1 integrins, an important function of invasin is to advance Yersinia infections via intestinal translocation through M cells overlying Peyer's patches (reviewed in reference 29). YadA binds to immobilized extracellular matrix proteins to promote cell uptake and is also a potent inhibitor of the classical pathway of complement (reviewed in reference 25). The pH 6 antigen binds to constituents of glycosphingolipids (55) that might be responsible for thermoinducible binding to cells (87). However, interactions with cell membranes and also with plasma lipoproteins (45) could rather aid pH 6 antigen's function as an antiphagocytic factor (33). In contrast, Ail (YPTB2867) has no known host receptor but is associated with uptake and conferring serum resistance (reviewed in reference 38). A role for the other three Ail paralogues has not yet been described. In addition, flagella (89), lipopolysaccharide (LPS) (reviewed in references 49 and 81), and LcrV of the Ysc-Yop T3SS (77) also potentially contribute to the ability of yersiniae to interact with mammalian cells.
In this study, we have demonstrated that the CpxRA ECS-responsive pathway, which in E. coli is associated with the control of at least 100 genes, some of which encode factors involved in posttranscriptional regulatory control (17), is also required for the controlled production of at least one Y. pseudotuberculosis adhesin, invasin. In part, this regulatory control appears to be mediated through the global transcriptional activator RovA. Thus, the Cpx pathway is involved in the efficiency of bacterium-host cell contact (this study) and the subsequent T3SS-dependent translocation of Yop effectors into the cytosol of infected mammalian cells (this study; 6). However, it needs to be remembered that the background strain for this study lacked a functional YadA adhesin. Hence, it would be interesting to investigate the relevance of the Cpx pathway in wild-type yersiniae producing a full complement of cellular adhesins.
While a considerable amount is known about the role of invasin in Yersinia infections, only more recently has information about inv regulation surfaced. Expression of inv occurs maximally at ambient temperatures (37, 52, 57, 61), but repression at elevated temperatures can be relieved by other environmental parameters such as low pH (57). The molecular mechanism underlying this environmental control involves an antagonism for binding sites at the inv promoter between the RovA transcriptional activator (52, 68) and the repressive effects of the nucleoid regulatory protein H-NS (23, 31, 80) and its interaction partner YmoA in Y. enterocolitica (22, 23). On the basis of our data, we present a model that also implicates the Cpx pathway in the regulation of rovA and inv expression (Fig. 8). However, rather then being an indirect effect of the function of other CpxR-P regulon members, we believe that CpxR-P probably acts as a direct repressor of the transcription of both inv and rovA. The resultant low levels of RovA would further silence the inv promoter by enabling the repressive action of H-NS to take effect. Reduction of CpxR-P levels would then allow autoactivation of rovA transcription. CpxR-P apparently exerts this repressive effect by binding to DNA harboring control regions upstream of inv and rovA, thereby preventing their transcription. However, despite demonstrating this direct binding, a scan of the inv and rovA promoter regions only revealed partial consensus binding sites for CpxR-P based on the sequence 5′-GTAAA(N)5GTAAA-3′ (17, 86). Thus, a future aspect of this work concerns mapping of the precise location of CpxR-P binding upstream of inv and rovA.
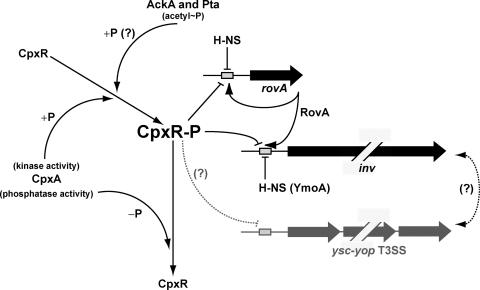
FIG. 8.
Model of the effect of CpxR-P on rovA and inv expression. Activated CpxA acts as a kinase phosphorylating CpxR (+P). CpxR-P can then bind directly to rovA and inv control regions to ultimately repress transcription (line with a horizontal bar). When homeostasis is achieved, CpxR-P is quenched by the intrinsic phosphatase activity of CpxA (−P). This relieves the repression, allowing the production of an elevated level of RovA that, in turn, activates its own synthesis, as well as that of invasin (line with arrows). However, in the absence of CpxA, CpxR-P levels will remain high, forcing continued repression of inv and rovA transcription. This may even be further amplified by the CpxA-independent phosphorylation of CpxR via second-messenger phosphodonors such as acetyl phosphate (acetyl∼P) (indicated by a question mark). Cpx system activation also diminishes efficient T3S by yersiniae by an undisclosed mechanism (dotted line) (6). Additionally, the production of surface-located organelles can affect the activity of other similarly located virulence determinants, but such a connection between invasin and Ysc-Yop T3S remains unexplored (also indicated by a question mark).
To confirm that hyperphosphorylated CpxR is a repressor of Yersinia sp.-host cell interactions, we expressed two cpxR allelic variants in trans in a ΔcpxR null background, the wild-type allele and one that encodes a nonphosphorylatable D51A substitution variant. Western blotting and RT-PCR assays did indicate that CpxRD51A behaved more like the ΔcpxR null mutant with respect to the phenotypes produced by inv and rovA. This reinforces our idea that CpxR-P acts as a negative regulator of inv and rovA. However, producing elevated levels of CpxR can be toxic to bacteria (6, 17), which means that one needs to bear this in mind when analyzing these data. Therefore, we are currently generating a series of defined point mutations in cis in the Cpx two-component system to specifically disrupt the phosphorelay mechanism. In parallel, we are also investigating the contribution of the low-molecular-weight phosphodonor acetyl phosphate (reviewed in reference 85) in the regulation of Yersinia pathogenicity. Derived from the phosphotransacetylase (Pta)—acetate kinase (AckA) pathway, acetyl phosphate is proposed to donate phosphoryl groups to response regulators of two-component signal transduction systems (47, 83), including CpxR when CpxA is absent (11, 13). Together, these ongoing studies should eventually provide definitive proof that CpxR-P influences the virulence properties of yersiniae, while also permitting the assessment of any impact the global signaling molecule acetyl phosphate may have on this regulation.
Our data indicate that transcription from psaA, which encodes the tip adhesin of pH 6 antigen, is dependent on a functional CpxA protein. Two membrane-associated proteins, PsaE and PsaF, are known to be directly associated with the transcriptional activation of psaA (88). Although the molecular mechanism responsible for PsaE/PsaF-mediated regulation of psaA is unknown, it appears to require a functional CpxA pathway for activity. In light of a recent report (7), we suspect that the Cpx system indirectly influences the regulation of the psa locus through an effect on RovA. This is consistent with the ability of RovA to bind to both the psaA and psaE promoter regions (7). It is also noteworthy that the pH 6 antigen belongs to a class of adhesins whose biogenesis follows the chaperone/usher pathway (34). Interestingly, E. coli P pilus subunits misassembled in the periplasm by a prototypic chaperone/usher pathway trigger an ECS response perceived by the Cpx pathway (35, 41). CpxRA responds by regulating the phase-variable expression of all of the genes required for pilus biogenesis (35). It is therefore tempting to speculate that Cpx responsiveness is commonly adapted by bacterial pathogens to oversee the function of the chaperone/usher pathway during pilus biogenesis.
It is less clear how the Cpx pathway may influence the regulation of ail (YPTB2867) expression. Regulated by temperature and growth phase (62), ail transcription is somehow modulated by ClpP, a subunit of the Clp protease (56). Because this protease also degrades the Yersinia YmoA repressor (40), YmoA was suggested to be a molecular connector in the Clp-mediated regulation of ail (5). We did not examine if YmoA levels were influenced by the Cpx pathway, nor are we aware of any known link between the Clp and Cpx regulatory pathways. However, the ECS-responsive alternate sigma factor σE (RpoE) is activated by a cascade of proteolytic events that culminates in the action of the ClpXP protease (27). Perhaps it is here that the overlap resides since the Cpx pathway modulates rpoE expression (17).
Another interesting outcome of this work concerned the activity of RovA expressed in trans in a ΔcpxA null mutant. The extent of bacterial internalization even at 37°C was reproducibly higher than for the parental Yersinia strain. This suggests that RovA's function as a transcriptional regulator is not limited to inv and rovA expression. Additional gene targets appear to encode facilitators of bacterial uptake into eukaryotic cells and/or repressors of the antiphagocytic properties of the Ysc-Yop T3SS. Such a global regulatory role for RovA is not without precedent, because a rovA mutant is comparatively more attenuated then an inv mutant in the mouse model of infection (68) and a transcriptome analysis of yersiniae has identified additional RovA-targeted genes (7).
It is interesting that Cpx pathway activation also reduces the efficiency of Yop effector T3S and translocation (6). As previously discussed, this may well be due to any number of CpxR-P regulon members that in E. coli alter protein levels by a posttranscriptional mechanism (6). On the basis of data presented here, however, we are also keen to investigate another possible consequence of Cpx pathway activation—does the down-regulation of one or more Yersinia adhesins (invasin, pH 6 antigen, or Ail) affect T3S or vice versa (Fig. 8)? This interrelationship of surface-anchored components is not uncommon. The length of the YadA adhesin affects the performance of the T3SS in Y. enterocolitica (51). However, it is equally noteworthy that LPS assembly status also influences T3S efficiency in S. flexneri (84), Pseudomonas aeruginosa (1), and Y. enterocolitica (58), along with modulating invasin production (3) and the entry of Y. enterocolitica into eukaryotic cells (63). Even more intriguing is the idea that the CpxRA system plays a role in LPS regulation in yersiniae (2). In this sense, perhaps it is not the loss of any given adhesin(s) during Cpx pathway activation that affects Yersinia T3S but rather an altered LPS status. Examination of the LPS structure in our Cpx-defective bacteria is currently under way. Such observations would further support the contention that outer membrane biogenesis and assembly of virulence factors at the bacterial surface are inextricably linked (69). Similar signals may report to ECS-responsive pathways on the assembly status of outer membrane components and virulence factors alike, permitting bacterial pathogens the opportunity to rapidly respond to ever-changing environments encountered during infection. Indeed, this offers a plausible explanation as to why Cpx pathway activation generally influences the expression of multiple virulence factors in pathogenic yersiniae.
Acknowledgments
This work, performed within the framework of the Umeå Centre for Microbial Research, was supported by grants from the Carl Tryggers Foundation for Scientific Research (M.S.F.), the Umeå University Basic Science-Oriented Biotechnology Research Fund, the Swedish Research Council (M.S.F.), the Foundation for Medical Research at Umeå University (M.S.F.), and the J. C. Kempes Memorial Fund (K.E.C., P.J.E.). J.L. is sponsored by a Carl Tryggers Foundation postdoctoral fellowship.
We also express gratitude to Hans Wolf-Watz and Bernt Eric Uhlin for the gift of anti-invasin and anti-H-NS antibodies, respectively; Roland Rosqvist for the inv expression construct; and Virginia Miller for the Yersinia inv yadA double mutant. Thanks also to Petra Dersch for helpful discussion, the anti-RovA antibodies, and the rovA expression construct. Additionally, Monika Åhlund and R. Rosqvist are acknowledged for assistance with eukaryotic cell imaging, as are Sara Bengtsson and Tina Enghardt for help with some aspects of this work.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Augustin, D. K., Y. Song, M. S. Baek, Y. Sawa, G. Singh, B. Taylor, A. Rubio-Mills, J. L. Flanagan, J. P. Wiener-Kronish, and S. V. Lynch. 2007. Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. J. Bacteriol. 189:2203-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengoechea, J. A., L. Zhang, P. Toivanen, and M. Skurnik. 2002. Regulatory network of lipopolysaccharide O-antigen biosynthesis in Yersinia enterocolitica includes cell envelope-dependent signals. Mol. Microbiol. 44:1045-1062. [DOI] [PubMed] [Google Scholar]
- 3.Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52:451-469. [DOI] [PubMed] [Google Scholar]
- 4.Bölin, I., and H. Wolf-Watz. 1984. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect. Immun. 43:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, S. M., R. A. Festa, M. J. Pearce, and K. H. Darwin. 2006. Self-compartmentalized bacterial proteases and pathogenesis. Mol. Microbiol. 60:553-562. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson, K. E., J. Liu, P. J. Edqvist, and M. S. Francis. 2007. Extracytoplasmic stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect. Immun. 75:3913-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathelyn, J. S., S. D. Crosby, W. W. Lathem, W. E. Goldman, and V. L. Miller. 2006. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc. Natl. Acad. Sci. USA 103:13514-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis, G. R., C. Sluiters, I. Delor, D. Geib, K. Kaniga, C. Lambert de Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 12.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 13.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danese, P. N., G. R. Oliver, K. Barr, G. D. Bowman, P. D. Rick, and T. J. Silhavy. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol. 180:5875-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Wulf, P., O. Kwon, and E. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 18.DiGiuseppe, P. A., and T. J. Silhavy. 2003. Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 185:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorel, C., P. Lejeune, and A. Rodrigue. 2006. The Cpx system of Escherichia coli, a strategic signaling pathway for confronting adverse conditions and for settling biofilm communities? Res. Microbiol. 157:306-314. [DOI] [PubMed] [Google Scholar]
- 20.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 21.Edqvist, P. J., M. Aili, J. Liu, and M. S. Francis. 2007. Minimal YopB and YopD translocator secretion by Yersinia is sufficient for Yop-effector delivery into target cells. Microbes Infect. 9:224-233. [DOI] [PubMed] [Google Scholar]
- 22.Ellison, D. W., B. Young, K. Nelson, and V. L. Miller. 2003. YmoA negatively regulates expression of invasin from Yersinia enterocolitica. J. Bacteriol. 185:7153-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison, D. W., and V. L. Miller. 2006. H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J. Bacteriol. 188:5101-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellison, D. W., and V. L. Miller. 2006. Regulation of virulence by members of the MarR/SlyA family. Curr. Opin. Microbiol. 9:153-159. [DOI] [PubMed] [Google Scholar]
- 25.El Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 26.Fällman, M., K. Andersson, S. Håkansson, K. E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flynn, J. M., I. Levchenko, R. T. Sauer, and T. A. Baker. 2004. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 18:2292-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 29.Grassl, G. A., E. Bohn, Y. Muller, O. T. Buhler, and I. B. Autenrieth. 2003. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 293:41-54. [DOI] [PubMed] [Google Scholar]
- 30.Han, Y. W., and V. L. Miller. 1997. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect. Immun. 65:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heroven, A. K., G. Nagel, H. J. Tran, S. Parr, and P. Dersch. 2004. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol. Microbiol. 53:871-888. [DOI] [PubMed] [Google Scholar]
- 32.Heusipp, G., K. M. Nelson, M. A. Schmidt, and V. L. Miller. 2004. Regulation of htrA expression in Yersinia enterocolitica. FEMS Microbiol. Lett. 231:227-235. [DOI] [PubMed] [Google Scholar]
- 33.Huang, X. Z., and L. E. Lindler. 2004. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect. Immun. 72:7212-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung, D. L., and S. J. Hultgren. 1998. Pilus biogenesis via the chaperone/usher pathway: an integration of structure and function. J. Struct. Biol. 124:201-220. [DOI] [PubMed] [Google Scholar]
- 35.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 37.Isberg, R. R., A. Swain, and S. Falkow. 1988. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect. Immun. 56:2133-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isberg, R. R. 1996. Uptake of enteropathogenic Yersinia by mammalian cells. Curr. Top. Microbiol. Immunol. 209:1-24. [DOI] [PubMed] [Google Scholar]
- 39.Isberg, R. R., Z. Hamburger, and P. Dersch. 2000. Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect. 2:793-801. [DOI] [PubMed] [Google Scholar]
- 40.Jackson, M. W., E. Silva-Herzog, and G. V. Plano. 2004. The ATP-dependent ClpXP and Lon proteases regulate expression of the Yersinia pestis type III secretion system via regulated proteolysis of YmoA, a small histone-like protein. Mol. Microbiol. 54:1364-1378. [DOI] [PubMed] [Google Scholar]
- 41.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langen, G. R., J. R. Harper, T. J. Silhavy, and S. P. Howard. 2001. Absence of the outer membrane phospholipase A suppresses the temperature-sensitive phenotype of Escherichia coli degP mutants and induces the Cpx and σE extracytoplasmic stress responses. J. Bacteriol. 183:5230-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavander, M., L. Sundberg, P. J. Edqvist, S. A. Lloyd, H. Wolf-Watz, and Å. Forsberg. 2002. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J. Bacteriol. 184:4500-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindler, L. E., M. S. Klempner, and S. C. Straley. 1990. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect. Immun. 58:2569-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makoveichuk, E., P. Cherepanov, S. Lundberg, A. Forsberg, and G. Olivecrona. 2003. pH6 antigen of Yersinia pestis interacts with plasma lipoproteins and cell membranes. J. Lipid Res. 44:320-330. [DOI] [PubMed] [Google Scholar]
- 46.Marceau, M. 2005. Transcriptional regulation in Yersinia: an update. Curr. Issues Mol. Biol. 7:151-177. [PubMed] [Google Scholar]
- 47.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mileykovskaya, E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake, K. 2004. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 50.Morales, V., A. Bäckman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 51.Mota, L. J., L. Journet, I. Sorg, C. Agrain, and G. R. Cornelis. 2005. Bacterial injectisomes: needle length does matter. Science 307:1278. [DOI] [PubMed] [Google Scholar]
- 52.Nagel, G., A. Lahrz, and P. Dersch. 2001. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol. Microbiol. 41:1249-1269. [DOI] [PubMed] [Google Scholar]
- 53.Nakayama, S., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naktin, J., and K. G. Beavis. 1999. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin. Lab. Med. 19:523-536. [PubMed] [Google Scholar]
- 55.Payne, D., D. Tatham, E. D. Williamson, and R. W. Titball. 1998. The pH 6 antigen of Yersinia pestis binds to β1-linked galactosyl residues in glycosphingolipids. Infect. Immun. 66:4545-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pederson, K. J., S. Carlson, and D. E. Pierson. 1997. The ClpP protein, a subunit of the Clp protease, modulates ail gene expression in Yersinia enterocolitica. Mol. Microbiol. 26:99-107. [DOI] [PubMed] [Google Scholar]
- 57.Pepe, J. C., J. L. Badger, and V. L. Miller. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 11:123-135. [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Gutiérrez, C., C. M. Llompart, M. Skurnik, and J. A. Bengoechea. 2007. Expression of Yersinia enterocolitica pYV-encoded type III secretion system is modulated by lipopolysaccharide O-antigen status. Infect. Immun. 75:1512-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 61.Pierson, D. E., and S. Falkow. 1990. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect. Immun. 58:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierson, D. E., and S. Falkow. 1993. The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect. Immun. 61:1846-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierson, D. E. 1994. Mutations affecting lipopolysaccharide enhance ail-mediated entry of Yersinia enterocolitica into mammalian cells. J. Bacteriol. 176:4043-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 65.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raivio, T. L. 2005. Envelope stress responses and gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119-1128. [DOI] [PubMed] [Google Scholar]
- 68.Revell, P. A., and V. L. Miller. 2000. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol. Microbiol. 35:677-685. [DOI] [PubMed] [Google Scholar]
- 69.Rhodius, V. A., W. C. Suh, G. Nonaka, J. West, and C. A. Gross. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 4:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosqvist, R., M. Skurnik, and H. Wolf-Watz. 1988. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334:522-524. [DOI] [PubMed] [Google Scholar]
- 71.Rosqvist, R., Å. Forsberg, M. Rimpiläinen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 72.Rosqvist, R., Å. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rowley, G., M. Spector, J. Kormanec, and M. Roberts. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383-394. [DOI] [PubMed] [Google Scholar]
- 75.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:787-796. [Google Scholar]
- 76.Simonet, M., S. Richard, and P. Berche. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strozen, T. G., G. R. Langen, and S. P. Howard. 2005. Adenylate cyclase mutations rescue the degP temperature-sensitive phenotype and induce the sigma E and Cpx extracytoplasmic stress regulons in Escherichia coli. J. Bacteriol. 187:6309-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran, H. J., A. K. Heroven, L. Winkler, T. Spreter, B. Beatrix, and P. Dersch. 2005. Analysis of RovA, a transcriptional regulator of Yersinia pseudotuberculosis virulence that acts through antirepression and direct transcriptional activation. J. Biol. Chem. 280:42423-42432. [DOI] [PubMed] [Google Scholar]
- 81.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 83.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 84.West, N. P., P. Sansonetti, J. Mounier, R. M. Exley, C. Parsot, S. Guadagnini, M. C. Prevost, A. Prochnicka-Chalufour, M. Delepierre, M. Tanguy, and C. M. Tang. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313-1317. [DOI] [PubMed] [Google Scholar]
- 85.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto, K., and A. Ishihama. 2006. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 70:1688-1695. [DOI] [PubMed] [Google Scholar]
- 87.Yang, Y., J. J. Merriam, J. P. Mueller, and R. R. Isberg. 1996. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect. Immun. 64:2483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang, Y., and R. R. Isberg. 1997. Transcriptional regulation of the Yersinia pseudotuberculosis pH6 antigen adhesin by two envelope-associated components. Mol. Microbiol. 24:499-510. [DOI] [PubMed] [Google Scholar]
- 89.Young, G. M., J. L. Badger, and V. L. Miller. 2000. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68:4323-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zahrl, D., M. Wagner, K. Bischof, and G. Koraimann. 2006. Expression and assembly of a functional type IV secretion system elicit extracytoplasmic and cytoplasmic stress responses in Escherichia coli. J. Bacteriol. 188:6611-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]