Abstract
Treatment of AZ-521 cells with Helicobacter pylori VacA increased cyclooxygenase 2 (COX-2) mRNA in a time- and dose-dependent manner. A p38 mitogen-activated protein kinase (MAPK) inhibitor, SB203580, blocked elevation of COX-2 mRNA levels, whereas PD98059, which blocks the Erk1/2 cascade, partially suppressed the increase. Consistent with involvement of p38 MAPK, VacA-induced accumulation of COX-2 mRNA was reduced in AZ-521 cells overexpressing a dominant-negative p38 MAPK (DN-p38). Phosphatidylinositol-specific phospholipase C, which inhibits VacA-induced p38 MAPK activation, blocked VacA-induced COX-2 expression. In parallel with COX-2 expression, VacA increased prostaglandin E2 (PGE2) production, which was inhibited by SB203580 and NS-398, a COX-2 inhibitor. VacA-induced PGE2 production was markedly attenuated in AZ-521 cells stably expressing DN-p38. VacA increased transcription of a COX-2 promoter reporter gene and activated a COX-2 promoter containing mutated NF-κB or NF-interleukin-6 sites but not a mutated cis-acting replication element (CRE) site, suggesting direct involvement of the activating transcription factor 2 (ATF-2)/CREB-binding region in VacA-induced COX-2 promoter activation. The reduction of ATF-2 expression in AZ-521 cells transformed with ATF-2-small interfering RNA duplexes resulted in suppression of COX-2 expression. Thus, VacA enhances PGE2 production by AZ-521 cells through induction of COX-2 expression via the p38 MAPK/ATF-2 cascade, leading to activation of the CRE site in the COX-2 promoter.
Helicobacter pylori is of growing concern today because of its crucial role in the pathogenesis of chronic gastritis, peptic ulcer disease, and gastric cancer (3, 25, 32, 39). Persistent infection with H. pylori causes prolonged inflammation, including intraglandular infiltration in the gastric mucosa by neutrophils, lymphocytes, and plasma cells. Inflammatory reactions mediated by cytokines, adhesion molecules, active oxygen species, nitric oxide, and prostaglandins have been implicated in the pathogenesis of gastric mucosal injury induced by H. pylori (35). Increased expression of the inducible type of nitric oxide synthase and cyclooxygenase 2 (COX-2) (19) and elevated levels of proinflammatory cytokines (25) are also observed in the gastric mucosa of patients with H. pylori infection.
In the stomach, prostaglandins, especially prostaglandin E2 (PGE2), play a cardinal role in maintenance of gastric mucosal integrity via several mechanisms, including regulation of gastric mucosal blood flow, kinetics of epithelial cells, synthesis of mucus, and inhibition of gastric acid secretion (52). On the other hand, PGE2 transactivates epidermal growth factor receptor (EGFR) and triggers mitogenic signaling in gastric epithelial and colon cancer cells as well as in rat gastric mucosa in vivo. Thus, PGE2 exerts trophic actions on gastric and intestinal mucosa, resulting in hypertrophy as well as proliferation of colonic cancers (37). Interestingly, COX-2 inhibitors or nonsteroidal anti-inflammatory drugs may be part of a new therapeutic strategy for protection against colorectal cancer (24). In addition, we recently found that the levels of thrombin-antithrombin complex, epidermal growth factor, and prostaglandin E2 were higher in patients infected with a VacA-producing H. pylori strain than in those with a non-VacA-producing strain (51).
Among the virulence factors produced by H. pylori, vacuolating cytotoxin, VacA, an oligomeric protein complex of about 1,000 kDa (31), has been shown to cause progressive vacuolation and death of epithelial cells (12, 14). VacA induces multiple effects on susceptible cells (e.g., epithelial and lymphatic cells [12]), including vacuolation with alterations of endo-lysosomal function (13, 59), mitochondrial damage (11, 21, 29, 30, 55, 56), and inhibition of primary human CD4+ cell proliferation (49). These effects of VacA appear to result from activation of different signal transduction pathways. VacA-induced activation of the p38/activating transcription factor 2 (ATF-2)-mediated signal pathway is independent of VacA effects on cellular vacuolation, a decrease in mitochondrial membrane potential, or cytochrome c release from mitochondria (33). Further, phosphorylation of Git1 (G protein-coupled receptor kinase interactor 1), which may be responsible for epithelial cell detachment, results from a mechanism different from that leading to vacuolation (20). In addition, consistent with suppression of nuclear translocation of nuclear factor of activated T cells, NFAT, in Jurkat T cells (4, 22), VacA counteracted CagA-induced activation of NFAT in AGS cells, suggesting that the two major H. pylori virulence factors inversely control NFAT activity (60). Moreover, in neutrophils or macrophages treated with VacA, p38 mitogen-activated protein kinase (MAPK) phosphorylation as well as COX-2 expression were observed (4). In addition, more recent work (2) described the activation of p38 MAP kinase by VacA in a human T-cell line (Jurkat) and a murine T-cell line (LBRM-33), but not in primary murine splenocytes and CD4+ T cells.
Here we report that VacA increases PGE2 production by AZ-521 cells by up-regulation of COX-2 expression through a signaling pathway involving the p38 MAP kinase/ATF-2 cascade, leading to activation of the cis-acting replication element (CRE) on the COX-2 promoter.
MATERIALS AND METHODS
Antibodies and other reagents.
SB203580 was purchased from Calbiochem; PD98059 was obtained from Biomol International, L.P.; bafilomycin A1, G418, 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB), NS-398, and phosphatidylinositol-specific phospholipase C (PI-PLC) were from Sigma. Other reagents were of analytical grade.
Cell culture.
AZ-521 human gastric adenocarcinoma cells (Culture Collection of Health Science Resource Bank, Japan Health Science Foundation) were grown in Earle's minimal essential medium (Sigma) containing 10% fetal calf serum (FCS) under a 5% CO2 atmosphere at 37°C.
Purification of VacA.
The toxin-producing H. pylori strain ATCC 49503 was the source of VacA for purification by a modification of our published procedure (33). In brief, after growth of H. pylori in Brucella broth containing 0.1% β-cyclodextrin at 37°C for 3 to 4 days with vigorous shaking in a controlled microaerobic atmosphere of 10% O2 and 10% CO2, VacA was precipitated from culture supernatant with 50% saturated ammonium sulfate. Precipitated proteins were dialyzed against RX buffer (10 mM KCl, 0.3 mM NaCl, 0.35 mM MgCl2, and 0.125 mM EGTA in 1 mM HEPES, pH 7.3) and applied to an anti-VacA-specific immunoglobulin G (IgG) antibody column equilibrated with RX buffer. After washing the column with RX buffer, VacA was eluted with 50 mM glycine-HCl buffer (pH 1.0), which was promptly neutralized with 1 M Tris-HCl (pH 10). After gel filtration on Superose 6HR 10/30 equilibrated with TBS buffer (60 mM Tris-HCl buffer, pH 7.7, containing 0.1 M NaCl), purified VacA was stored at −20°C. This purified VacA was activated by acidic elution from an anti-VacA-specific IgG antibody column.
RNA preparation, reverse transcription-PCR (RT-PCR), and real-time quantitative PCR.
Total RNA was extracted from AZ-521 cells by using ISOGEN (Nippon Gene, Tokyo, Japan) and reverse transcribed into single-strand cDNA (1st strand synthesis kit; Roche Applied Science) using oligo(dT) primers. The resulting cDNA was used as a template for PCR to amplify specific COX-2 cDNA regions with the primers COX-2-sense (5′-CACTTGAGTGGCTATCACTTCAAACTGAAA-3′) and COX-2-antisense (5′-CTACAGTTCAGTCGAACGTTCTTTTAGTAG-3′). After initial denaturation for 2 min at 95°C, 25 cycles of denaturation (1 min, 95°C), annealing (2 min, 55°C), and elongation (2 min, 74°C) were followed by a final elongation for 5 min at 74°C. For control amplifications of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, similar cycling conditions were used. The amplified products were visualized on 1.0% agarose gels. Real-time quantitative PCR assay was carried out with the LightCycler system (Roche Applied Science), using LightCycler Master SYBR Green I (Roche Applied Science). The basic protocol for real-time quantitative PCR was an initial incubation at 95°C for 2 min, followed by 45 cycles of 94°C for 30 s, 55°C for 30 s, and 74°C for 1 min and finally cooling at 4°C. All samples were run in triplicate; the relative expression values were normalized to the expression of GAPDH. To establish the significance of the results, Student's t test was used for numerical data. Fisher's exact test or χ2 test was used for categorical data as appropriate. A P value less than 0.05 was considered statistically significant.
Effect of SB203580, PD98059, PI-PLC, bafilomycin A1, or NPPB on VacA-induced COX-2 mRNA expression.
AZ-521 cells (1 × 106) were pretreated with SB203580 (10 μM for 60 min), PD98059 (10 μM for 60 min), bafilomycin A1 (2.5 nM for 30 min), NPPB (50 μM for 30 min), or PI-PLC (1 U/ml for 60 min) at 37°C prior to incubation with or without VacA (120 nM) in Eagle's minimal essential medium (EMEM) without serum. Total RNA was isolated at 2 h after addition of VacA. The relative levels of COX-2 mRNA compared with GAPDH mRNA were measured by RT-PCR or real-time quantitative PCR.
p38 MAP kinase dominant-negative transfection of AZ-521 cells.
Transfection procedures were performed using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's instructions. AZ-521 cells were seeded in 6-cm2 plates (1 × 106 cells in 4 ml of EMEM), incubated at 37°C for 24 h, and then transfected with pcDNA3 vector as control or pcDNA3/p38(AF) as the dominant-negative p38 construct. The transfected cells were selected with 100 μg/ml G418 for 3 weeks. Individual stable transformants were isolated using cloning cylinders. To examine whether overexpression of dominant-negative p38 MAP kinase inhibited p38 MAP kinase phosphorylation in the presence of VacA, whole-cell lysates were prepared and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blot analysis was performed with a phosphorylation-specific p38 MAP kinase antibody. To test the inhibitory effect of p38 MAP kinase on VacA-induced COX-2 expression by transfection with dominant-negative p38 MAP kinase constructs, COX-2 mRNA in AZ-521 cells transformed with a dominant-negative p38 MAP kinase was compared with wild-type AZ-521 cells transfected with pcDNA3 control vector by using RT-PCR and/or real-time quantitative PCR.
Transfection with ATF-2 siRNA.
RNA interference to lower ATF-2 content was performed as previously reported by Agelopoulos and Thanos (1). In brief, AZ-521 cells were seeded (2.0 × 105 cells in 4 ml of EMEM/dish) in 60-mm culture dishes and grown overnight; ATF-2-silencer small interfering RNA (siRNA; 1 μg) duplexes were introduced into cells using Lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's recommendations. The ATF-2-siRNA and negative control siRNA (NC-siRNA) were purchased from Santa Cruz. Silencing of the ATF-2 gene was determined by measuring ATF-2 protein expression at 24 h after transfection by Western blotting using anti-ATF-2 antibodies.
COX-2 promoter activity by luciferase assay.
To assess COX-2 promoter activity, AZ-521 cells were seeded in 24-well culture plates (7.5 × 104 cells in 1 ml of EMEM containing 10% FCS and cultured at 37°C for 24 h to reach subconfluence. A reporter construct (1.0 μg) (26, 27) was mixed with 20 ng/ml of a control vector, pRL-TK, in 50 μl of EMEM. The solution was mixed with 1 ml of Lipofectamine 2000 reagent, diluted in 150 μl of EMEM, and incubated at room temperature for 20 min; two vectors in 200-μl solutions were cotransfected into AZ-521 cells after the cells were washed twice with EMEM. The cells were incubated at 37°C for 12 h in a 5% CO2 atmosphere. After transfection with plasmid, the medium was replaced with 150 μl of fresh EMEM without FCS. The next day, the cells were treated with 120 nM VacA. After incubation at 37°C for 6 h, cells were washed with 500 μl of phosphate-buffered saline and lysed by adding 100 μl of lysis buffer (Toyo Ink Co.). After incubation for 15 min at room temperature, the lysate was centrifuged (15,000 × g, 5 min, 4°C) and the supernatant was harvested and assayed with a PicaGene dual-luciferase assay kit (PG-DUAL SP; Toyo Ink, Tokyo, Japan) according to the manufacturer's instructions.
PGE2 enzyme immunoassay.
AZ-521 cells were seeded in 24-well culture plates (2 × 105 cells in 1 ml of EMEM containing 10% FCS per well) and incubated at 37°C for 24 h in a 5% CO2 atmosphere. Cells were treated with or without 120 nM VacA for 6 h. The supernatant medium was then collected and assayed for PGE2 using a specific enzyme immunoassay kit according to the manufacturer's instructions (Amersham). Medium alone without cells was incubated under the same conditions and used as a blank control for the enzyme immunoassay.
RESULTS
Effect of VacA on COX-2 mRNA levels in AZ-521 cells.
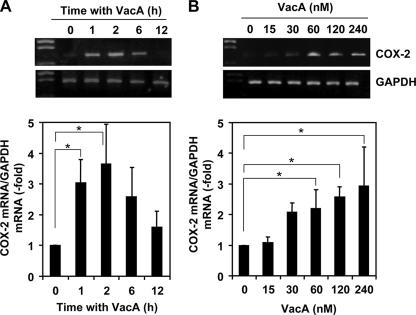
To understand whether VacA affects the levels of COX-2 mRNA, AZ-521 gastric epithelial cells (1 × 106) were incubated with 120 nM VacA for 1, 2, 6, and 12 h. After incubation, COX-2 mRNA was measured from the total isolated RNA by semiquantified RT-PCR and real-time PCR assays (Fig. 1A). COX-2 mRNA levels in VacA-treated cells were significantly elevated within 2 h of incubation and declined thereafter. Cells not exposed to VacA showed low or undetectable levels of COX-2 mRNA. In agreement, quantification of the effects of VacA by real-time PCR indicated a concentration-dependent increase in expression of COX-2 mRNA after 2 h incubation (Fig. 1B).
FIG. 1.
Time- and dose-dependent induction of COX-2 by VacA in AZ-521 cells. (A) Confluent AZ-521 cells were incubated with VacA (120 nM) in EMEM without serum. Total RNA was isolated at 0, 1, 2, 6, and 12 h after addition of VacA. The relative levels of COX-2 mRNA expression compared with GAPDH mRNA were measured by RT-PCR (upper panel) and real-time quantitative PCR (lower panel). The data are representative of at least three experiments. Real-time quantitative PCR results are from three independent experiments, which are presented as the mean ± standard deviation (SD). *, P < 0.01. (B) Confluent AZ-521 cells were stimulated with 0, 15, 30, 60, 120, or 240 nM VacA in MEM without serum. Total RNA was isolated at 2 h after addition of VacA. The relative levels of COX-2 mRNA expression compared with GAPDH mRNA were measured by RT-PCR (upper panel) and real-time quantitative PCR (lower panel). The data are representative of at least three experiments. Real-time quantitative PCR results from three independent experiments with assays in duplicate are presented as the mean ± SD. *, P < 0.02.
Effects of p38 and Erk inhibitors on VacA-induced COX-2 mRNA expression.
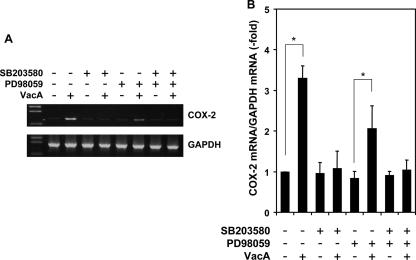
Up-regulation of COX-2 by VacA may be indicative of a strong host cell inflammatory response. Activation of each of the three major MAP kinase pathways, Erk1/2, JNK, and p38 MAP kinase, have been shown to enhance the expression of COX-2 by endotoxin (58). Since VacA activates Erk1/2 and p38 MAP kinase, but not JNK (4), additional studies were carried out with inhibitors of Erk1/2 and p38 MAP kinase to determine the significance of MAP kinase pathways for VacA-induced COX-2 expression. COX-2 mRNA up-regulation induced by VacA was blocked by a specific inhibitor of p38 MAPK activity, SB203580, at 10 μM (Fig. 2). A partial inhibitory effect was achieved in cells treated with a MEK inhibitor, 10 μM PD98059, which is known to block the Erk1/2 signal cascade, prior to treatment with 120 nM VacA for 1 h (Fig. 2). Thus, the p38 MAP kinase signaling pathway may be primarily responsible for the increase in COX-2 mRNA induced by VacA in AZ-521 cells.
FIG. 2.
Effects of p38 MAP kinase and Erk1/2 inhibitors on VacA-induced COX-2 expression in AZ-521 cells. Confluent AZ-521 cells were pretreated with SB203580 (10 μM) or PD98059 (10 μM) or both for 1 h prior to incubation with or without VacA (120 nM) in EMEM without serum. Total RNA was isolated at 2 h after addition of VacA. The relative levels of COX-2 mRNA expression compared with GAPDH mRNA were measured by RT-PCR (A) and real-time quantitative PCR (B). The data are representative of at least three experiments. Real-time PCR results from three independent experiments with assays in duplicate are presented as the mean ± standard deviation (n = 3). *, P < 0.005.
Further evidence of p38 MAPK involvement in COX-2 expression.
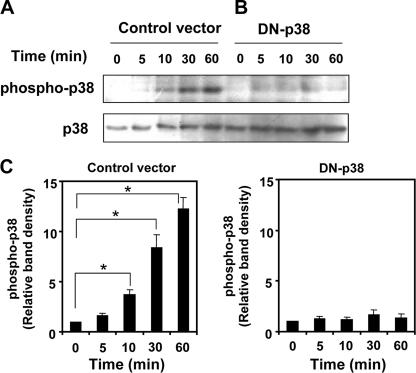
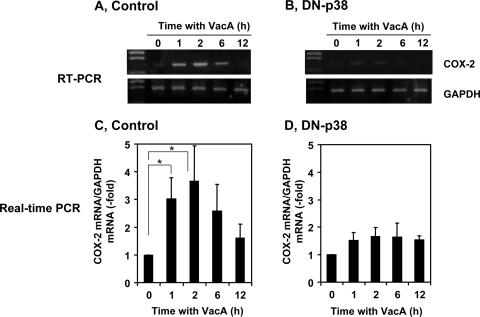
To determine further the role of p38 MAP kinase in regulating COX-2 expression in response to VacA treatment, AZ-521 cells were transformed with a p38 MAP kinase dominant-negative construct. Overexpression of dominant-negative p38 MAP kinase inhibited p38 MAP kinase phosphorylation in the presence of VacA (Fig. 3). VacA-induced COX-2 expression was markedly attenuated in AZ-521 cells transformed with a dominant-negative construct compared to control cells (Fig. 4), consistent with the conclusion that the p38 MAP kinase signaling pathway was critical for VacA-induced COX-2 expression. We found recently that NPPB, which disrupts anion channels, or bafilomycin A1, which is a vacuolar-type H+-ATPase inhibitor, inhibited VacA internalization followed by vacuolation but did not inhibit translocation of VacA to lipid rafts or VacA-induced activation of p38 MAP kinase in AZ-521 cells. In contrast, PI-PLC, which removes glycosylphosphatidylinositol (GPI)-anchored proteins, inhibited VacA translocation to lipid rafts, p38 MAP kinase activation, VacA internalization, and VacA-induced vacuolation (34). Consistent with the inhibition of p38 MAP kinase activation by PI-PLC treatment, PI-PLC inhibited VacA-induced COX-2 expression, whereas NPPB and bafilomycin A1 had no effect (Fig. 5). These results support our hypothesis that p38 MAP kinase activation by VacA is critical for signaling pathways leading to COX-2 expression in AZ-521 cells.
FIG. 3.
Effect of overexpression of dominant-negative p38 on phosphorylation of p38 MAP kinase in AZ-521 cells. AZ-521 cells transfected with pcDNA3 control vector (A) or Flag-tagged dominant-negative p38 construct (B) were incubated with 120 nM VacA for 0, 5, 10, 30, or 60 min. Whole-cell lysates were prepared and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blot analysis was performed with a phosphorylation-specific p38 MAP kinase antibody. The blot was then stripped and reprobed with a p38 MAP kinase antibody to determine equivalent loading of each lane. Data are representative of three separate experiments. (C) Relative densities of phospho-p38 as determined by densitometry scan analysis were compared to densities obtained at 0 min as a control. Data are means ± standard errors of values from triplicate experiments. *, P < 0.01.
FIG. 4.
Induction of COX-2 mRNA by VacA in AZ-521 cells transformed with dominant-negative p38. Confluent AZ-521 cells transformed with dominant-negative p38 or pcDNA3 control vector were incubated with VacA (120 nM) in EMEM without serum. Total RNA was isolated at 0, 1, 2, 6, and 12 h after addition of VacA. The relative levels of COX-2 mRNA expression compared with GAPDH mRNA were measured by RT-PCR (A and B) and real-time quantitative PCR (C and D). The data are representative of at least three experiments. Real-time PCR results from three independent experiments with assays in duplicate are presented as the means ± standard deviations (n = 3). *, P < 0.01.
FIG. 5.
Treatment with PI-PLC, but not bafilomycin A1 or NPPB, inhibited VacA-induced COX-2 expression in AZ-521 cells. Confluent AZ-521 cells were pretreated with bafilomycin A1 (2.5 nM for 30 min), NPPB (50 μM for 30 min), or PI-PLC (1 U/ml for 60 min) at 37°C prior to incubation with or without VacA (120 nM) in EMEM without serum. Total RNA was isolated at 2 h after addition of VacA. The relative levels of COX-2 mRNA compared with GAPDH mRNA were measured by RT-PCR (A) and real-time quantitative PCR (B). The data are representative of at least three experiments. Real-time PCR results from three independent experiments with assays in duplicate are presented as the means ± standard deviations. *, P < 0.02; **, P < 0.04.
VacA up-regulates COX-2 through a CRE site in the COX-2 promoter and ATF-2 function.
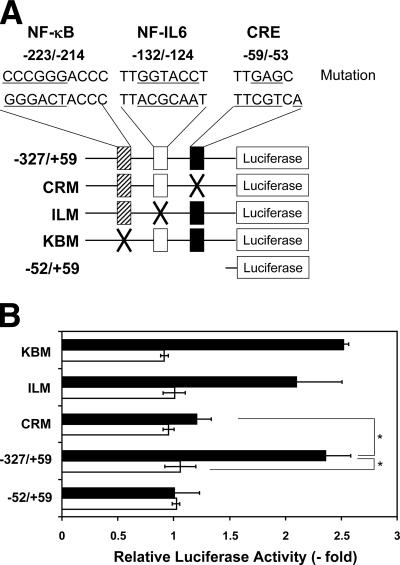
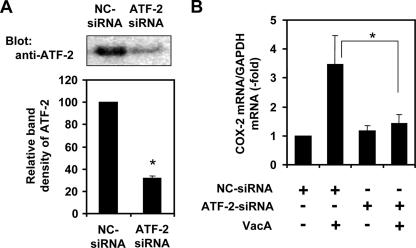
Among known positive COX-2 transcriptional regulators, such as NF-κB (41), NF-interleukin-6 (NF-IL-6) (41), AP-1 (41), NFAT (17), or ATF-2/CREB (38), we found that VacA induced activation of ATF-2 through p38 MAP kinase stimulation in AZ-521 cells (33). To examine which site in the COX-2 promoter was responsible for COX-2 expression, we next transfected AZ-521 cells with the COX-2 reporter plasmid WTCOX-2 (−327/+59) and evaluated the effect of VacA treatment on AZ-521 cells transfected with the COX-2 gene promoter with a site-specific mutation of the CRE region, which is a binding site for ATF-2 and CREB (Fig. 6A). VacA increased luciferase activity by more than 2.5-fold, and this effect was blocked by a CRE mutation (Fig. 6B), suggesting that the effect was mediated at least in part through the CRE region. On the other hand, the effects of VacA were not altered on AZ-521 cells transfected with a COX-2 promoter plasmid mutated at either the NF-κB or NF-IL-6 site (Fig. 6B). These data demonstrate direct involvement of the ATF-2/CREB binding region in VacA-induced activation of the COX-2 promoter. In agreement, the reduction of ATF-2 expression in AZ-521 cells transformed with ATF-2-siRNA resulted in suppression of COX-2 expression (Fig. 7).
FIG. 6.
Transcriptional activation of the COX-2 promoter by VacA in AZ-521 cells. (A) Schematic representation of the 5′ regulatory region of the COX-2 gene with mutations (26, 27). Rectangles indicate the location of the NF-κB, NF-IL-6, and CRE sites. The luciferase reporter driven by the COX-2 promoter region (−327/+59) was mutated at the NF-κB, NF-IL-6, or CRE site. The mutated sequences are as follows: NF-κB site (−233/−214), changed from GGGACTACCC to cccgggACCC; NF-IL-6 site (−132/−124), changed from TTACGCAAT to TTggtaccT; CRE site (−59/−53), changed from TTCGTCA to TTgagCt. Promoter activity is not contained in the short type of reporter vector containing the 5′-flanking region of the COX-2 gene promoter (−52/+59). Distances are given as nucleotide positions relative to the transcriptional start site, which is +1. (B) AZ-521 cells were transiently transfected with COX-2 promoter-luciferase reporter plasmids with the −327/+59, CRM, ILM, KBM, or −52/+59 promoters. Cells were either untreated or treated with 120 nM VacA (0 or 6 h) at 37°C. Relative changes in luciferase expression were measured. Open bars, activities without VacA; solid bars, activities with VacA incubation. Data are means ± standard deviations of values from three independent experiments with assays in duplicate. *, P < 0.02.
FIG. 7.
Effect of ATF-2 silencing on VacA-induced COX-2 mRNA expression in AZ-521 cells. AZ-521 cells were grown overnight, and silencing of the ATF-2 gene was performed with ATF-2-siRNA or NC-siRNA as described in Materials and Methods. After a 24-h transfection, cells were suspended in EMEM without serum and treated with VacA for 2 h. (A) Reduction of the ATF-2 protein level was confirmed by Western blotting with anti-ATF-2 antibodies (upper panel), and relative densities determined by densitometry scan analysis (bottom panel) were compared to densities obtained by NC-siRNA transfection. The data are representative of at least two experiments. *, P < 0.002 compared with the control siRNA. (B) The relative levels of COX-2 mRNA expression were compared with GAPDH mRNA as measured by real-time quantitative PCR. The data are representative of at least three experiments. *, P < 0.02.
VacA-induced p38 MAP kinase-mediated activation of the COX-2 promoter.
To determine whether VacA-induced p38 MAP kinase-mediated activation was involved in the COX-2 promoter activation, we evaluated the effect of VacA treatment on AZ-521 cells expressing a dominant-negative p38 MAP kinase or control cells transfected with empty vector after transfection with the −327/+59 promoter. VacA did not increase luciferase expression in AZ-521 cells transformed with a p38 MAP kinase dominant-negative construct compared to empty vector, suggesting that functional p38 MAPK kinase is required for VacA-induced activation of the COX-2 promoter (Fig. 8).
FIG. 8.
Effect of dominant-negative p38 MAP kinase on VacA-dependent COX-2 promoter activation. AZ-521 cells transfected with the pcDNA3 control vector or a dominant-negative p38 construct (DN-p38) were transiently transfected with the luciferase reporter plasmid containing the COX-2 promoter (−327/+59) construct. Cells were incubated with 120 nM VacA for 0 or 6 h at 37°C. Relative changes in luciferase expression were measured. Open bars, activities without VacA; solid bars, activities with VacA incubation. Data are means ± standard deviations of values from three independent experiments with assays in duplicate. *, P < 0.02.
VacA up-regulates PGE2 production.
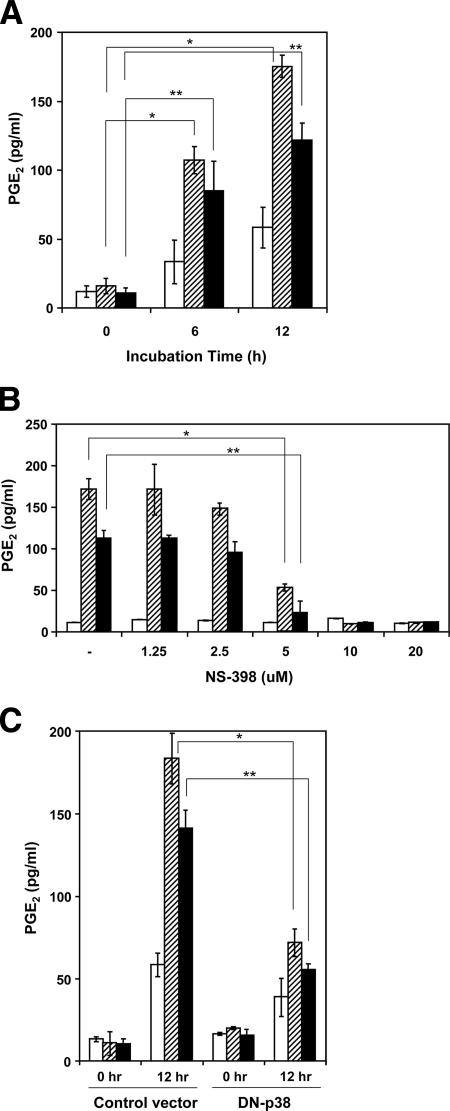
We also examined whether VacA-induced COX-2 expression affected PGE2 production by AZ-521 cells. As shown in Fig. 9A, VacA induced PGE2 production by AZ-521 cells in a time-dependent manner. In addition, NS-398, a COX-2 inhibitor, reduced VacA- or EGF-induced PGE2 production by AZ-521 cells in a concentration-dependent manner (Fig. 9B). Further, VacA-induced PGE2 production was reduced in AZ-521 cells overexpressing a dominant-negative p38 MAP kinase construct, suggesting that VacA increased PGE2 production through a signaling pathway that included p38 MAP kinase (Fig. 9C).
FIG. 9.
Production of PGE2 in AZ-521 cells by VacA, its inhibition by NS-398, and overexpression of a DN-p38. (A) Confluent AZ-521 cells were incubated without (open bars) or with 25 ng/ml EGF (hatched bars) or 120 nM VacA (solid bars) for 0, 6, or 12 h at 37°C. The medium was collected, and PGE2 was measured by enzyme immunoassay. *, P < 0.001; **, P < 0.02. (B) To assay the effects of NS-398, cells were preincubated with the indicated amount of NS-398 for 30 min, followed by incubation without (open bars) or with 25 ng/ml EGF (hatched bars) or 120 nM VacA (solid bars) for 12 h at 37°C. *, P < 0.05; **, P < 0.01. (C) AZ-521 cells transfected with pcDNA3 vector or a Flag-tagged DN-p38 construct were incubated with control solution (open bars), 120 nM VacA (solid bars), or EGF (25 ng/ml) (hatched bars) for 0 or 12 h. The medium was collected, and PGE2 was measured by enzyme immunoassay. Data are means ± standard deviations of values from three separate experiments. *, P < 0.008; **, P < 0.002.
DISCUSSION
Two COX isoforms initiate the conversion of arachidonate to prostaglandins and thromboxanes. COX-1 is constitutively expressed in almost all cells and is important for the maintenance of homeostatic functions, whereas COX-2 is transiently induced by proinflammatory cytokines, growth factors, and tumor promoters (16). Indeed, COX-2 is overexpressed in cancers of the colon (18), stomach (43), lung (5), and breast (48). Alterations in PGE2 production controlled by COX-2 have been implicated in many pathological processes, including acute and chronic inflammation, angiogenesis, cancer, and allergic diseases (36). It has been argued that up-regulation of COX-2 plays a central role in the inflammatory changes and tissue damage associated with chronic H. pylori infection and is also involved in gastric tumorigenesis (24, 51). Three publications are relevant to COX-2 expression induced by VacA. Caputo et al. (7) reported that H. pylori up-regulated vascular endothelial growth factor (VEGF) expression in MKN 28 gastric cells: this effect was specifically related to VacA and appeared to depend on the activation of an EGFR-, Erk-, and COX-2-mediated pathway. Romano et al. (44) reported that H. pylori up-regulated COX-2 mRNA expression and PGE2 synthesis in MKN 28 cells independent of VacA, CagA, and urease-generated ammonia; this group found that H. pylori γ-glutamyltranspeptidase was involved in the upregulation of COX-2, heparin binding EGF, and amphiregulin in MKN 28 and AGS cells (6). Jüttner et al. (28) reported that H. pylori stimulated COX-2 gene transcription, indicating the critical importance of MEK/Erk-dependent activation of upstream stimulatory factors 1 and 2 and CREB transcription factors in MKN 28 cells, independent of VacA gene- and cagPAI-encoded virulence factors. In these publications, however, instead of using purified VacA, a mutated strain of H. pylori lacking a VacA gene was used, and two research groups concluded that H. pylori-stimulated COX-2 expression in MKN 28 and AGS cells was independent of the VacA gene (6, 28), although Erk appears to mediate COX-2 expression responsible for induction of VEGF in MKN 28 cells by a VacA-positive H. pylori strain (7). Thus, the mechanism whereby VacA contributed to COX-2 expression and PGE2 synthesis was obscured by the presence of H. pylori γ-glutamyltranspeptidase, which also affects COX-2 expression. In addition, recent work showed that COX-2 expression was induced by EGF (47), which is found at 0.2 to 1 ng/ml in serum (45). Therefore, we simplified the experiment by using purified VacA without serum and examined the potential roles of VacA as an inducer of COX-2 expression and prostaglandin production to determine its importance in the pathogenesis of disease caused by H. pylori infection.
The p38 MAP kinase phosphorylation and COX-2 expression induced by VacA were reported first using human neutrophils or macrophages (4). Algood et al. (2) more recently reported activation of p38 MAP kinase by VacA in T-cell lines. It is clear that VacA activates p38 MAP kinase in not only human blood cells, (neutrophils and macrophages [4] and T cells [2]), but also in gastric epithelial cells (33). With regard to the function of p38 MAP kinase, it has been found that the p38 MAP kinase pathway affects the stability of interleukin-1-induced COX-2 mRNA (42). p38 MAP kinase substrates can be divided into two categories, e.g., transcription factors (Sap1, CHOP, p53, MEF2, C/EBPb, NFATp, STAT4, and ATF-2) and protein kinases (MK2. MK3, MNK1, PRAK, and MSK1/2). It appears that MK2 is one of the substrates of p38 MAP kinase involved in mRNA stabilization in T cells (15). Interleukin-1-induced increases in COX-2 mRNA are NF-κB and MAPK independent, but the translation of COX-2 protein is NF-κB dependent in intestinal epithelial cells (57). Therefore, the signaling pattern due to interleukin-1 demonstrates the complexities of regulating COX-2 gene in T cells and intestinal epithelial cells. To investigate the molecular mechanism of COX-2 expression in gastric epithelial cells, we examined whether the p38 MAP kinase/ATF-2 cascade is responsible for COX-2 expression in VacA-treated cells.
The MAPKs are a family of highly evolutionarily conserved protein kinases (Erk, JNK, and p38 MAP kinase) connecting cell surface receptors to critical regulatory targets within cells. They regulate important cellular processes, including gene expression, cell proliferation, and cell motility (8). Interestingly, Erk, JNK, and p38 MAP kinase play a role in COX-2 expression in a cell-specific manner. Our previous studies in AZ-521 cells demonstrated that VacA activates the p38 MAP kinase/ATF-2 and Erk1/2 cascades, but not the JNK pathway (33).
To determine the significance of MAP kinase pathways for VacA-induced COX-2 expression in AZ-521 cells, we examined whether VacA induced COX-2 expression (Fig. 1) and if its induction was affected by the presence of inhibitors of p38 MAPK and Erk1/2 (Fig. 2). VacA increased amounts of COX-2 mRNA in a time- and dose-dependent manner. Consistent with our previous findings, which showed that p38 MAPK phosphorylation in AZ-521 cells treated with VacA was completely inhibited by addition of anti-VacA IgG (33), VacA-stimulated COX-2 induction was blocked by addition of anti-VacA IgG (data not shown), suggesting that the induction of p38 MAP kinase and subsequent effects are not due to the presence of contaminants in the purified VacA (e.g., lipopolysaccharide). SB203580, an inhibitor of p38 MAP kinase, completely blocked the elevation of COX-2 mRNA levels, whereas PD98059, which is known to block the Erk1/2 signal cascade, only partially suppressed it, consistent with the activation of an EGFR-, Erk-, and COX-2-mediated pathway for VEGF induction (7) or, as an alternate possibility, that the inhibitory effects of PD98059 are not limited to the Erk1/2 signaling cascade. In addition, data from studies on overexpression of a dominant-negative p38 MAP kinase (Fig. 3, 4, and 9C) suggested that VacA increased COX-2 expression followed by PGE2 production in AZ-521 cells through a signaling pathway that included p38 MAP kinase. This action of VacA is similar to that of endothelin-1, which stimulates COX-2 expression through p38 MAP kinase activation (40). It has been shown that there are significant differences in the signaling pathway between angiotensin II (Ang II) and EGF. Inhibition of Erk activation by PD98059 or U0126 significantly decreased EGF-dependent COX-2 expression but did not affect Ang II-dependent COX-2 expression. Inhibition of p38 MAP kinase by SB202190 or PD169316 blocked COX-2 expression by Ang II but did not inhibit COX-2 induction by EGF. In addition, Ang II induced phosphorylation of p38 MAP kinase, followed by an increase in the phosphorylation of ATF-2, whereas EGF stimulated COX-2 expression through Erk activation (47). Activated Erk1/2 is able to directly phosphorylate transcription factors such as Elk-1 and Sap-1, inducing transcription from c-fos via AP-1 (46, 54) and stimulates AP-1 binding to the CRE site on the COX-2 promoter (23, 48). As shown in Fig. 6, we found that VacA increased luciferase expression in AZ-521 cells transfected with the −327/+59 promoter whereas VacA had no effect in AZ-521 cells transfected with a COX-2 promoter containing a mutated CRE site, but a promoter having mutated NF-κB or NF-IL-6 sites did not show an altered VacA response. VacA-induced COX-2 expression was partially suppressed by PD98059 (Fig. 2), suggesting the involvement of the Erk1/2 signal cascade in VacA-induced COX-2 expression, although major induction of COX-2 expression and PGE2 production appears to be due to p38 MAP kinase activation.
Induction of COX-2 expression is strongly correlated with the extent of inflammation and the severity of gastric disease (50). Levels of the COX-2 protein were significantly higher in gastric cancer patients infected with H. pylori than in those with nonulcer dyspepsia (53), and the up-regulation of COX-2 in H. pylori-associated gastric cancer is related to vascular invasion (10), consistent with a crucial role of COX-2 expression in H. pylori-associated gastric cancer in addition to gastric inflammation. These findings raised the possibility that H. pylori isolates from patients with different disease status vary in their capacity to induce COX-2 expression; in vitro studies revealed that H. pylori strains isolated from gastric cancer patients induced higher expression levels of COX-2 protein. There was no difference in the genotypes (e.g., hrg, iceA, babA2, cagA, and vacA) among the H. pylori isolates from patients with different disease status, despite the higher COX-2 induction capability of the strains isolated from gastric cancer patients (9). Chang et al. (9) postulated that the signaling pathways involved in regulation of the NF-κB, NF-IL-6, and CRE were important in H. pylori-induced COX-2 expression in AGS cells, i.e., H. pylori acts through Toll-like receptor 2 (TLR2) and TLR9 and then activates PI-PLC-γ to induce protein kinase C and c-Src activation, leading to tyrosine phosphorylation of IKKα/β, resulting in the phosphorylation and degradation of IkBα, stimulation of NF-κB in the COX-2 promoter and, finally, initiation of COX-2 expression. However, since VacA did not bind to either TLR2 or TLR9 (data not shown), it appears not to induce COX-2 expression via signaling pathways using TLR2 or TLR9 as a receptor.
GPI-anchored proteins are tethered to eukaryotic plasma membranes and associate with lipid rafts, specialized regions of elevated cholesterol and sphingolipid content, where they are involved in the modulation of signaling mechanisms and cellular functions involving protein/lipid sorting. Treatment of AZ-521 cells with PI-PLC reduced subsequent VacA-induced COX-2 expression (Fig. 5). Since PI-PLC did not affect VacA binding to cells, this effect of PI-PLC may be due to inhibition of receptor-dependent translocation of VacA to lipid rafts, which is critical for signaling pathways leading to p38 MAP kinase/ATF-2 activation and vacuolation (34). The presence of the anchor appears to impose conformational restraints, and its removal may alter the catalytic properties, structure, and localization of a GPI-anchored protein. Release of GPI-anchored proteins from the cell surface by PI-PLC may result in changes of functional properties of the cell. In addition, as shown in Fig. 7, transfection of AZ-521 cells with ATF-2-siRNA resulted in reduction of COX-2 expression, suggesting that ATF-2 is the transcription factor involved in regulation of COX-2 expression in VacA-treated AZ-521 cells.
The identification of the virulence factor, signaling pathways, and regulatory elements that control COX-2 expression in inflammatory cells, as well as gastric cells in particular, is a subject of major interest for H. pylori infection. As a whole, the present study has helped us to gain insights into the possible contribution of VacA to gastric inflammation, ulceration and, perhaps, gastric cancer.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Waksman Foundation, and Cooperative Research Grants of the Institute of Tropical Medicine, Nagasaki University.
We thank K. Maeda and K. Tamura for skillful assistance and I. Kato (Medical School of Chiba University) for helpful discussions. We thank M. Vaughan of the P-CCMB, NHLBI, National Institutes of Health (Bethesda, MD), for helpful discussions and critical review of the manuscript. J. Moss was supported by the Intramural Research Program, NHLBI, NIH.
Editor: F. C. Fang
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Agelopoulos, M., and D. Thanos. 2006. Epigenetic determination of a cell-specific gene expression program by ATF-2 and the histone variant macroH2A. EMBO J. 25:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algood, H. M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2007. Resistance of primary murine CD4+ T cells to Helicobacter pylori vacuolating cytotoxin. Infect. Immun. 75:334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axon, A. T. 1999. Are all Helicobacters equal? Mechanisms of gastroduodenal pathology and their clinical implications. Gut 45:11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boncristiano, M., S. R. Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M. M. D'Elios, J. L. Telford, and C. T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 198:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, J. R., and R. N. DuBois. 2004. Cyclooxygenase as a target in lung cancer. Clin. Cancer Res. 10:4266s-4269s. [DOI] [PubMed] [Google Scholar]
- 6.Busiello, I., R. Acquaviva, A. Di Popolo, T. G. Blanchard, V. Ricci, M. Romano, and R. Zarrilli. 2004. Helicobacter pylori gamma-glutamyltranspeptidase upregulates COX-2 and EGF-related peptide expression in human gastric cells. Cell. Microbiol. 6:255-267. [DOI] [PubMed] [Google Scholar]
- 7.Caputo, R., C. Tuccillo, B. A. Manzo, R. Zarrilli, G. Tortora, V. Blanco Cdel, V. Ricci, F. Ciardiello, and M. Romano. 2003. Helicobacter pylori VacA toxin up-regulates vascular endothelial growth factor expression in MKN 28 gastric cells through an epidermal growth factor receptor-, cyclooxygenase-2-dependent mechanism. Clin. Cancer Res. 9:2015-2021. [PubMed] [Google Scholar]
- 8.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y. J., M. S. Wu, J. T. Lin, B. S. Sheu, T. Muta, H. Inoue, and C. C. Chen. 2004. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-κB activation. Mol. Pharmacol. 66:1465-1477. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C. N., C. T. Sung, M. T. Lin, P. H. Lee, and K. J. Chang. 2001. Clinicopathologic association of cyclooxygenase 1 and cyclooxygenase 2 expression in gastric adenocarcinoma. Ann. Surg. 233:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover, T. L., U. S. Krishna, D. A. Israel, and R. M. Peek, Jr. 2003. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63:951-957. [PubMed] [Google Scholar]
- 12.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320-332. [DOI] [PubMed] [Google Scholar]
- 13.de Bernard, M., B. Arico, E. Papini, R. Rizzuto, G. Grandi, R. Rappuoli, and C. Montecucco. 1997. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol. Microbiol. 26:665-674. [DOI] [PubMed] [Google Scholar]
- 14.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 15.Dodeller, F., and H. Schulze-Koops. 2006. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res. Ther. 8:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBois, R. N., J. Awad, J. Morrow II, L. J. Roberts, and P. R. Bishop. 1994. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-a and phorbol ester. J. Clin. Investig. 93:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duque, J., M. Fresno, and M. A. Iniguez. 2005. Expression and function of the nuclear factor of activated T cells in colon carcinoma cells: involvement in the regulation of cyclooxygenase-2. J. Biol. Chem. 280:8686-8693. [DOI] [PubMed] [Google Scholar]
- 18.Eberhart, C. E., R. J. Coffey, A. Radhika, F. M. Giardiello, S. Ferrenbach, and R. N. DuBois. 1994. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183-1188. [DOI] [PubMed] [Google Scholar]
- 19.Fu, S., K. S. Ramanujam, A. Wong, G. T. Fantry, C. B. Drachenberg, S. P. James, S. J. Meltzer, and K. T. Wilson. 1999. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116:1319-1329. [DOI] [PubMed] [Google Scholar]
- 20.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukuda, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, H. Fukamachi, and M. M. Noda. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33:375-381. [DOI] [PubMed] [Google Scholar]
- 21.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 23.Guo, Y. S., M. R. Hellmich, X. D. Wen, and C. M. Townsend, Jr. 2001. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J. Biol. Chem. 276:22941-22947. [DOI] [PubMed] [Google Scholar]
- 24.Harris, R. E., J. Beebe-Donk, H. Doss, and D. Burr Doss. 2005. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade. Oncol. Rep. 13:559-583. [PubMed] [Google Scholar]
- 25.Houghton, J., and T. C. Wang. 2005. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology 128:1567-1578. [DOI] [PubMed] [Google Scholar]
- 26.Inoue, H., C. Yokoyama, S. Hara, Y. Tone, and T. Tanabe. 1995. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. J. Biol. Chem. 270:24965-24971. [DOI] [PubMed] [Google Scholar]
- 27.Inoue, H., Y. Taba, Y. Miwa, C. Yokota, M. Miyagi, and T. Sasaguri. 2002. Transcriptional and posttranscriptional regulation of cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 22:1415-1420. [DOI] [PubMed] [Google Scholar]
- 28.Jüttner, S., T. Cramer, S. Wessler, A. Walduck, F. Gao, F. Schmitz, C. Wunder, M. Weber, S. M. Fischer, W. E. Schmidt, B. Wiedenmann, T. F. Meyer, M. Naumann, and M. Hocker. 2003. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell. Microbiol. 5:821-834. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, M., S. Goto, A. Wada, K. Yahiro, T. Niidome, T. Hatakeyama, H. Aoyagi, T. Hirayama, and T. Kondo. 1999. Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microbiol. Pathog. 26:45-52. [DOI] [PubMed] [Google Scholar]
- 30.Kuck, D., B. Kolmerer, C. Iking-Konert, P. H. Krammer, W. Stremmel, and J. Rudi. 2001. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 69:5080-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manetti, R., P. Massari, D. Burroni, M. de Bernard, A. Marchini, R. Olivieri, E. Papini, C. Montecucco, R. Rappuoli, and J. L. Telford. 1995. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect. Immun. 63:4476-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall, B., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama, M., M. Kimura, A. Wada, K. Yahiro, K. Ogushi, T. Niidome, A. Fujikawa, D. Shirasaka, N. Aoyama, H. Kurazono, M. Noda, J. Moss, and T. Hirayama. 2004. Helicobacter pylori VacA activates the p38/activating transcription factor 2-mediated signal pathway in AZ-521 cells. J. Biol. Chem. 279:7024-7028. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama, M., J. Hisatsune, E. Yamasaki, Y. Nishi, A. Wada, H. Kurazono, J. Sap, K. Yahiro, J. Moss, and T. Hirayama. 2006. Helicobacter pylori VacA clustering in lipid rafts, mediated by its receptor, receptor-like protein tyrosine phosphatase β, is required for intoxication in AZ-521 cells. Infect. Immun. 74:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen, H., and L. P. Andersen. 1992. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology 103:1747-1753. [DOI] [PubMed] [Google Scholar]
- 36.Noble, S. L., D. S. King, and J. I. Olutade. 2000. Cyclooxygenase-2 enzyme inhibitors: place in therapy. Am. Fam. Physician 61:3669-3676. [PubMed] [Google Scholar]
- 37.Pai, R., B. Soreghan, I. L. Szabo, M. Pavelka, D. Baatar, and A. S. Tarnawski. 2002. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 8:289-293. [DOI] [PubMed] [Google Scholar]
- 38.Park, S. W., M. W. Sung, D. S. Heo, H. Inoue, S. H. Shim, and K. H. Kim. 2005. Nitric oxide upregulates the cyclooxygenase-2 expression through the cAMP-response element in its promoter in several cancer cell lines. Oncogene 24:6689-6698. [DOI] [PubMed] [Google Scholar]
- 39.Parsonnet, J., G. P. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, and N. Orentriech. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 40.Pratt, P. F., D. Bokemeyer, M. Foschi, A. Sorokin, and M. J. Dunn. 2003. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J. Biol. Chem. 278:51928-51936. [DOI] [PubMed] [Google Scholar]
- 41.Ramsay, R. G., D. Ciznadija, M. Vanevski, and T. Mantamadiotis. 2003. Transcriptional regulation of cyclo-oxygenase expression: three pillars of control. Int. J. Immunopathol. Pharmacol. 16:59-67. [PubMed] [Google Scholar]
- 42.Ridley, S. H., J. L. E. Dean, S. J. Sarsfield, M. Brook, A. R. Clark, and J. Saklatvala. 1998. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 439:75-80. [DOI] [PubMed] [Google Scholar]
- 43.Ristimaki, A., N. Honkanen, H. Jankala, P. Sipponen, and M. Harkonen. 1997. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 57:1276-1280. [PubMed] [Google Scholar]
- 44.Romano, M., V. Ricci, A. Memoli, C. Tuccillo, A. Di Popolo, P. Sommi, A. M. Acquaviva, C. Del Vecchio Blanco, C. B. Bruni, and R. Zarrilli. 1998. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J. Biol. Chem. 273:28560-28563. [DOI] [PubMed] [Google Scholar]
- 45.Savage, A. P., V. K. Chatterjee, H. Gregory, and S. R. Bloom. 1986. Epidermal growth factor in blood. Regul. Pept. 16:199-206. [DOI] [PubMed] [Google Scholar]
- 46.Sinnett-Smith, J., E. Zhukova, N. Hsieh, X. Jiang, and E. Rozengurt. 2004. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in Swiss 3T3 cells. J. Biol. Chem. 279:16883-16893. [DOI] [PubMed] [Google Scholar]
- 47.Slice, L. W., T. Chiu, and E. Rozengurt. 2004. Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J. Biol. Chem. 279:1582-1593. [DOI] [PubMed] [Google Scholar]
- 48.Subbaramaiah, K., N. Telang, J. T. Ramonetti, R. Araki, B. DeVito, B. B. Weksler, and A. J. Dannenberg. 1996. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 56:4424-4429. [PubMed] [Google Scholar]
- 49.Sundrud, M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. USA 101:7727-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung, J. J., W. K. Leung, K. F. To, A. S. Cheng, E. K. Ng, and F. K. Chan. 2000. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am. J. Pathol. 157:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki, Y., E. C. Gabazza, I. Imoto, R. F. James, T. Hirayama, A. Wada, N. Horiki, M. Nakamura, H. Inoue, M. Kuroda, A. Ogura, Y. Taguchi, Y. Yano, O. Taguchi, K. Suzuki, and Y. Adachi. 2005. Vacuolating cytotoxin A is associated with increased thrombin generation in gastric mucosa. Helicobacter 10:323-331. [DOI] [PubMed] [Google Scholar]
- 52.Tanigawa, T., T. Watanabe, M. Hamaguchi, E. Sasaki, K. Tominaga, Y. Fujiwara, N. Oshitani, T. Matsumoto, K. Higuchi, and T. Arakawa. 2004. Anti-inflammatory effect of two isoforms of COX in H. pylori-induced gastritis in mice: possible involvement of PGE2. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G148-G156. [DOI] [PubMed] [Google Scholar]
- 53.Wambura, C., N. Aoyama, D. Shirasaka, T. Sakai, T. Ikemura, M. Sakashita, S. Maekawa, K. Kuroda, T. Inoue, S. Ebara, M. Miyamoto, and M. Kasuga. 2002. Effect of Helicobacter pylori-induced cyclooxygenase-2 on gastric epithelial cell kinetics: implication for gastric carcinogenesis. Helicobacter 7:129-138. [DOI] [PubMed] [Google Scholar]
- 54.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 55.Willhite, D. C., T. L. Cover, and S. R. Blanke. 2003. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 278:48204-48209. [DOI] [PubMed] [Google Scholar]
- 56.Yamasaki, E., A. Wada, A. Kumatori, I. Nakagawa, J. Funao, M. Nakayama, J. Hisatsune, M. Kimura, J. Moss, and T. Hirayama. 2006. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J. Biol. Chem. 281:11250-11259. [DOI] [PubMed] [Google Scholar]
- 57.Yan, S. R., R. R. Joseph, J. Wang, and A. W. Stadnyk. 2006. Differential pattern of inflammatory molecule regulation in intestinal epithelial cells stimulated with IL-1. J. Immunol. 177:5604-5611. [DOI] [PubMed] [Google Scholar]
- 58.Yan, Z., P. P. Stapleton, T. A. Freeman, M. Fuortes, and J. M. Daly. 2004. Enhanced expression of cyclooxygenase-2 and prostaglandin E2 in response to endotoxin after trauma is dependent on MAPK and NF-kB mechanisms. Cell. Immunol. 232:116-126. [DOI] [PubMed] [Google Scholar]
- 59.Ye, D., D. C. Willhite, and S. R. Blanke. 1999. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 274:9277-9282. [DOI] [PubMed] [Google Scholar]
- 60.Yokoyama, K., H. Higashi, S. Ishikawa, Y. Fujii, S. Kondo, H. Kato, T. Azuma, A. Wada, T. Hirayama, H. Aburatani, and M. Hatakeyama. 2005. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl. Acad. Sci. USA 102:9661-9666. [DOI] [PMC free article] [PubMed] [Google Scholar]