Abstract
Th1 cells and gamma interferon (IFN-γ) production play critical roles in protective immunity against genital tract infections by Chlamydia trachomatis. Here we show that inducible costimulatory molecule (ICOS)−/− mice develop greatly augmented host resistance against chlamydial infection. Protection following a primary infection was characterized by strong Th1 immunity with enhanced CD4+ T-cell-mediated IFN-γ production in the genital tract and high expression of T-bet in the draining para-aortic lymph node. This Th1 dominance was associated with low expression of interleukin 10 (IL-10) mRNA in the uteruses of protected ICOS−/− mice. By contrast, CD28−/− mice were severely impaired in their adaptive immune response, demonstrating a lack of CD4+ T cells and IFN-γ in the genital tract, with a substantial delay in bacterial elimination compared to that seen in wild-type (WT) mice. Upon reinfection, WT mice exhibited a transient local infection with evidence of regulatory T-cell (Treg)/Foxp3 mRNA and a more balanced Th1 and Th2 response in the genital tract than ICOS−/− mice, whereas 90% of the latter mice developed sterile immunity, poor expression of local Treg/Foxp3 mRNA, and macroscopic signs of enhanced local immunopathology. Therefore, different requirements for CD28 signaling and ICOS signaling clearly apply to host protection against a genital tract infection by C. trachomatis. Whereas, CD28 signaling is critical, ICOS appears to be dispensable and can have a dampening effect on Th1 development by driving Th2 immunity and anti-inflammation through IL-10 production and promotion of the Foxp3+ Treg populations in the genital tract. Both the CD28-deficient and the ICOS-deficient mice demonstrated poor specific antibody production, supporting the fact that antibodies are not needed for protection against genital tract chlamydial infections.
Chlamydia trachomatis is the causative bacterial agent of an increasing number of genital tract infections worldwide, annually. Although they are treatable with antibiotics, C. trachomatis infections are often asymptomatic, persistent, and recurring. Of the utmost concern are fertility complications resulting from damage to uterine and tubal epithelium, the increased transmission of human immunodeficiency virus, and an association with cervical cancer and neoplasia (2, 39). Given these personal and economic consequences, efforts have been focused on understanding the host immune factors involved in the immunopathology of chlamydial infections and the development of a safe and effective vaccine.
Despite attempts to further understand immune mechanisms that characterize protection, an efficacious vaccine candidate has not yet been identified. Therefore, a better understanding of the relationship between immunopathology and host resistance is much warranted. We and others have shown that adaptive immunity against C. trachomatis is dominated by CD4+ Th1-type cells and that gamma interferon (IFN-γ) is critical for resistance (19, 38). Antibody production during a chlamydial infection has been shown to have a complementary but subordinate role in host protection (35). However, in some infectious disease models, specific antibodies may be involved in the immunopathogenesis of the infection through, e.g., complement activation (9, 22, 43). Thus, paradoxically, the inflammation and antibody production that are raised in response to the infection may be responsible for the development of adverse sequelae. A prophylactic vaccine must not exacerbate inflammation in pursuit of clearance of the pathogen.
Priming of naïve T cells and clonal expansion depend on an antigen-specific signal via the T-cell receptor, in addition to costimulatory interactions through the receptor CD28, constituently expressed on T cells, and the ligands CD80/86, expressed on antigen-presenting cells (14). The activation of T cells also induces the upregulation of other costimulatory molecules including the inducible costimulatory molecule (ICOS), which is expressed at low levels with naïve cells but is upregulated and retained on memory T cells (28). ICOS has been shown to be more highly expressed on Th2 than on Th1 cells and is involved in germinal center formation, somatic hypermutation, and class switch recombination (11, 28, 49). In addition, ICOS signaling is needed for the production of cytokines, including interleukin 10 (IL-10), IL-4, and IFN-γ in particular, whereas CD28 signaling has also been shown to induce IL-2 and promote cell survival and cell cycle progression (36, 44, 49). Moreover, regulatory T cells (Tregs) are thought to develop as a consequence of signaling through costimulatory molecules (50). Currently, two broad categories of Tregs have been described, (i) the natural Foxp3+ CD4+ CD25+ Tregs which develop in the thymus and (ii) the inducible Tregs (Th3 and Tr1 cells) which develop in the periphery (reviewed in reference 3). The importance of CD28 cells in the development, maintenance, and function of Tregs has been established (10, 51). Interestingly, some subsets of Treg cells have been shown to depend on ICOS expression for in vivo and in vitro suppression through the production of IL-10 (24, 33).
Since immunity against C. trachomatis is based primarily on the contribution of Th1 cells, we sought to characterize the generation of protective specific memory responses during infection in the absence of costimulatory molecules. In the present report, we have analyzed the differential requirements of costimulatory signaling through CD28 and ICOS in the course of an adaptive immune response against C. trachomatis infection. Studies using gene knockout mice have implicated signaling via CD28 and ICOS in the host immune response to a number of intracellular infections with organisms such as Leishmania major, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes, by polarization of the immune response toward Th1 or Th2 (8, 31, 32). Recently, studies using Salmonella enterica serovar Typhimurium infections showed delayed adaptive immunity in ICOS−/− mice which were a result of poor CD8+, Th1, and antibody responses in these mice (55). However, here we report that the adaptive immune response to C. trachomatis infection is dependent on CD28 cells, whereas ICOS−/− mice develop greatly augmented Th1 effector cells but impaired Treg populations, which results in sterile immunity and enhanced immunopathology.
MATERIALS AND METHODS
Chlamydia stocks.
A human genital tract clinical isolate of C. trachomatis serovar D was propagated in buffalo-green monkey kidney cells and purified as previously described, before storage at −80°C (7). The infectivity of C. trachomatis stocks was tested by the intravaginal infection of C57BL/6 mice.
Mice.
Female C57BL/6 mice, 6 to 8 weeks old, were purchased from B & K Universal (Sweden) and used as age- and sex-matched wild-type (WT) controls. ICOS−/− and CD28−/− mice were purchased from the Jackson Laboratory, backcrossed for >12 generations on a C57BL/6 background. All experiments included medroxyprogesterone (Depo-Provera, Pharmacia Sverige AB)-treated (2.5 mg in 0.1 ml PBS subcutaneously 7 days prior to infection) naïve controls for each group. Mice were maintained under specific-pathogen-free conditions, according to FELASA-specified guidelines, at the Department of Experimental Biomedicine at Göteborg University, Sweden.
Bacterial infection and challenge protocols.
Mice were given a 2.5-mg subcutaneous injection of medroxyprogesterone acetate (53) 7 days prior to the inoculation of 1 × 106 inclusion forming units (IFU) of C. trachomatis elementary bodies (EBs) intravaginally, a dose which was determined by titration to be the 100% infectious dose. Bacterial shedding was monitored at 8-day intervals, using a commercial MicroTrak II Chlamydia EIA kit (Trinity Biotech, Plc.) according to the manufacturer's instructions, which is a measurement of chlamydial antigen rather than infectious bacteria. Samples with an absorbance greater than the provided cutoff value were considered positive for chlamydial antigen. Four weeks after all mice tested negative for chlamydial shedding, the infection procedure was repeated in a manner identical to that described for the primary infection. Bacterial shedding was monitored at 2, 4, 8, and 16 days postreinfection. 1° represents the primary infection, while 2° represents the secondary infection.
Evaluation of antibody responses.
Sera and vaginal secretions were analyzed for specific antibodies against EBs from C. trachomatis serovar D by enzyme-linked immunosorbent assay (ELISA). Serum and vaginal secretions were collected following the clearance of the primary infection and again following the clearance of the secondary challenge infection. Blood was collected from the lateral tail vein, while vaginal secretions were collected by using a Polywicks tampon, as previously described (16). Briefly, 96-well microtiter plates (Nunc) were coated with 2.5 μg of C. trachomatis D antigen in Na2CO3 (0.1 M [pH 9.5]) and incubated at 4°C overnight. Serum, diluted 1:100, and vaginal secretions, diluted 1:2, were added, and serial dilutions in subwells were performed before incubation at 4°C overnight. Serum samples were analyzed for immunoglobulin G (IgG), while vaginal secretions were analyzed for both IgG and IgA levels. IgA and IgG were detected by adding anti-mouse IgA/IgG conjugated to alkaline phosphatase (Southern Biotechnology Associates, Inc.), followed by 0.1 mg of p-nitrophenyl phosphate substrate (Sigma-Aldrich). The absorbance was measured at 405 nm.
In vitro assessment of T-cell proliferation.
Para-aortic lymph node (PALN) cells from two mice per group were seeded into 96-well culture plates (Nunc) at 1 × 106 cells/ml and cultured in Iscove's medium (Biochrom) supplemented with 10% heat-inactivated fetal calf serum (Biochrom), 50 μM 2-mercaptoethanol (Sigma-Aldrich), 1 mM l-glutamine (Biochrom), and 50 μg/ml gentamicin (Iscove's complete medium; Sigma-Aldrich) for 72 h at 37°C in 5% CO2, either alone or with 100 μl of 1× 105 IFU/ml heat-inactivated C. trachomatis serovar D. EBs were confirmed to be inactivated by their inability to infect BGMK cells. Proliferation was assessed after the addition of 1 μCi/well [3H]thymidine (Amersham Biosciences) for the last 6 h of culturing. [3H]thymidine uptake was determined using a beta scintillation counter (Beckman Coulter).
PCR analysis.
Total mRNA was extracted from whole-tissue samples by using TRIzol, and 4 μg of the resulting extraction was used for cDNA synthesis using oligo(dT) primer and SuperScript reverse transcriptase (RT; Invitrogen Life Technologies) and analyzed by real-time RT-PCR. For analysis of cytokine mRNA levels, PCR amplification was undertaken using commercially available kits (Search-LC) according to manufacturers' instructions. Primers (MWG-Biotech AG, Ebersberg, Germany) used for the determination of transcription factor mRNA levels were as follows: T-bet forward (5′-GCCAGGGAACCGCTTATAT-3′), T-bet reverse (5′-GAGTGATCTCTGCGTTCTGGT-3′), GATA-3 forward (5′-TGACGGAAGAGGTGGACGTA-3′), GATA-3 reverse (5′-TGGATGGACGTCTTGGAGAA-3′), Foxp3 forward (5′-AATATGCGACCCCCTTTCA-3′), Foxp3 reverse (5′-CAGGGATTGGAGCACTTGTT-3′), CD3-γ forward (5′-ATC TCT CTT CTT CAA GGC ACT-3′), and CD3-γ reverse (5′-CCT ATG TTT AGC TCA ATG CAG T-3′). Real-time RT-PCR was performed using a LightCycler system and software (Roche Diagnostics GmbH). Results were expressed as a normalized ratio of the target mRNA to the housekeeping mRNA (HPRT or CD3-γ). For statistical analysis, results were expressed as the normalized ratio of individual samples minus the average naïve normalized ratio for each group. All samples were run in duplicate.
Immunohistochemistry.
The reproductive tract including the uterus and uterine horns were removed, snap-frozen in TissueTek OCT compound (Histolab Products AB), and stored at −80°C within 2 h. Some tissues were embedded in paraffin, and sections were fixed with formalin for 10 min before staining with hematoxylin and eosin (H&E). The oviduct area was measured from H&E sections by using Leica IM1000 version 4.0 software. Cryostat sections of 5 μm were fixed in acetone before blocking with 0.3% H2O2, using an avidin-biotin blocking kit (Vector Laboratories) when required, followed by 20% normal horse serum. For CD4+ T-cell singular staining, sections were incubated with anti-CD4 (BD PharMingen) and developed using peroxidase-conjugated avidin (DAKO Cytomation) and a commercial peroxidase 3-amino-9-ethylcarbazole (AEC) substrate (Sigma-Aldrich). Sections were counterstained with hematoxylin and mounted with Faramount (Histolab Products AB). For identification of IFN-γ+/CD4+ T cells, sections were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, anti-IFN-γ conjugated to biotin (BD PharMingen), and streptavidin-conjugated Texas Red (Vector Laboratories). Negative controls were stained with isotype-matched irrelevant antibodies or the secondary antibody in the absence of a primary antibody. Sections were visualized using a Leica LSC microscope.
Statistical analysis.
Analysis of variance followed by Dunnett's C test was used to evaluate significance. P values of <0.05 and P values of <0.01 denote statistically significant differences between the WT and either CD28−/− or ICOS−/− groups, while P values of <0.05 and <0.01 denote statistical significance between the CD28−/− and ICOS−/− groups.
RESULTS
ICOS−/− mice, but not CD28−/− mice, develop protective immunity to C. trachomatis infection.
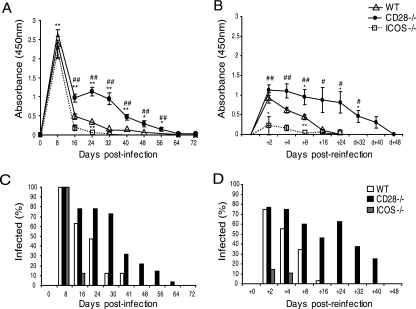
Previous studies have shown that costimulatory molecules play an important role in the development of adaptive immune responses to intracellular bacteria and have been shown to be critical in the immune response to Chlamydia lung infection (15, 31, 32). To determine whether defects in the ICOS ligand (ICOSL) and CD28-CD80/86 signaling pathways alter the immune response of mice to genital tract infection with Chlamydia, we compared the courses of primary infection in CD28−/− and ICOS−/− mice with that in WT mice following intravaginal inoculation with 1 × 106 C. trachomatis EBs. We found distinctly different patterns of bacterial shedding in CD28−/− mice compared to that in WT mice. Whereas ICOS−/− mice resolved their infection with kinetics that were similar or faster than that observed for WT mice, the CD28−/− mice exhibited higher levels of bacterial shedding and prolonged progression of the infection (Fig. 1A). As many as 32% of the CD28−/− mice remained positive for infection, even at day 41, while only 12% of WT and no ICOS−/− mice shed bacteria by that time (Fig. 1C).
FIG. 1.
ICOS−/− mice, but not CD28−/− mice, develop protective immunity in the genital tract to C. trachomatis infection. Bacterial shedding was determined at given intervals after a primary infection (A) or after reinfection (B) with 1 × 106 IFU of C. trachomatis EBs given intravaginally to WT, CD28−/−, and ICOS−/− mice. (C) The percentages of infected mice per group at given time intervals after the primary infection or postreinfection (D) were calculated. Results represent mean absorbances ± standard errors of the mean (A and B), and the percentages (C and D) of mice positive for chlamydial antigen shedding, as determined by Chlamydia Mikrotrak EIA. Values given are the combined means of three independent experiments, each including at least 10 mice per group. * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between the WT and either CD28−/− or ICOS−/− groups, while # (P < 0.05) and ## (P < 0.01) are statistically significant differences between the CD28−/− and ICOS−/− groups.
Next, we asked whether adaptive immunity had developed in mice recovering from a primary genital tract infection. To this end, shedding-negative mice were reinfected with 1 × 106 EBs, and bacterial shedding was determined at the indicated intervals on days 2, 4, 8, and 16 postinoculation. In agreement with previous work, highly immune WT mice exhibited strong resistance against reinfection, suffering only a transient infection (Fig. 1B) (27). Interestingly, all groups displayed lower bacterial shedding than that observed for the primary infection, suggesting partial resistance to reinfection. More importantly though, CD28−/− mice demonstrated a significant lack of protective immunity, displaying much higher bacterial shedding on days 8 (P < 0.05) and 24 (P < 0.05) postreinfection than WT mice did. In fact, on day 16 postreinfection, 46% of CD28−/− mice were still positive for chlamydial shedding compared to only 3% of WT mice. In striking contrast, ICOS−/− mice were significantly better protected than WT mice, and a majority (86%) of these mice exhibited sterile immunity (P < 0.05), as determined within the limits of the method of detection. In the 14% of ICOS−/− mice that demonstrated some bacterial shedding, eradication of bacteria from the genital tract was already complete within 8 days (Fig. 1D). This indicates that ICOS−/− mice develop stronger protective immunity following a primary infection than WT mice do, suggesting a quantitatively or qualitatively better adaptive immune response than WT mice. On the contrary, CD28−/− mice largely failed to develop adaptive immunity against genital tract Chlamydia infection.
Antibody production does not correlate to protection.
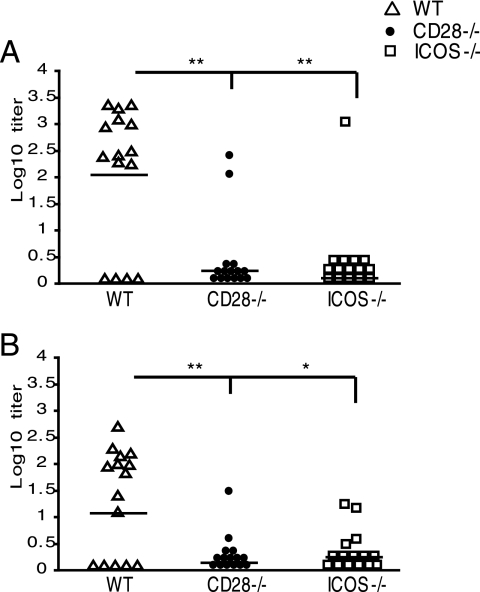
The formation of antigen-specific antibodies is dependent on effector functions of CD4+ T cells in providing B-cell help, with costimulatory signaling being central to this process (23). Therefore, we assessed antigen-specific antibody responses in sera and vaginal secretions from ICOS−/−, CD28−/−, and WT mice after the clearance of a primary infection and again after clearance of a challenge infection. Strong Chlamydia-specific IgG antibodies were detectable in the sera of WT mice following the clearance of the primary infection (Fig. 2A) and remained high after the challenge, but no distinct booster effect was observed for reinfection (data not shown). In contrast, both ICOS−/− and CD28−/− mice failed to respond with significant antigen-specific IgG in either serum (Fig. 2A) or genital tract secretions (Fig. 2B). These data show that ICOS−/− mice develop strong protection against infection, despite an almost complete lack of specific serum IgG response. Of note, specific IgA antibodies were not detected in any of the groups (data not shown). Thus, ICOS signaling is critically required to generate helper functions for specific antibody production, but it is clearly dispensable for the development of protective immunity following a primary infection. By contrast, CD28 signaling is required not only for specific antibody formation but also for the generation of immune protection against a genital tract infection with C. trachomatis bacteria.
FIG. 2.
Greatly impaired systemic and local Chlamydia-specific antibody production in ICOS−/− and CD28−/− mice. Serum IgG (A) and vaginal IgG (B) from WT, CD28−/−, and ICOS−/− mice were measured by ELISA after the clearance of a primary infection. Responses are given as individual log10 titers, with the combined mean of the group indicated by a line. Results represent two independent experiments. * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between the WT and either CD28−/− or ICOS−/− groups, while # (P < 0.05) and ## (P < 0.01) denote statistically significant differences between CD28−/− and ICOS−/− groups.
Protection in ICOS−/− mice is concurrent with enhanced CD4+ T-cell IFN-γ production in the genital tract.
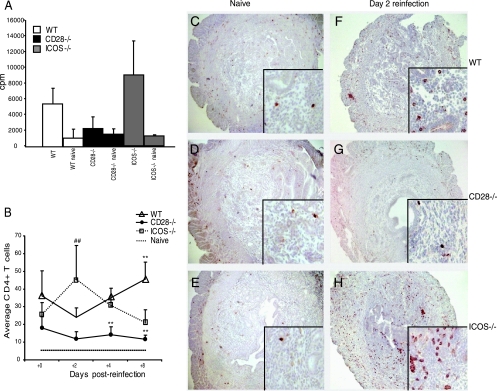
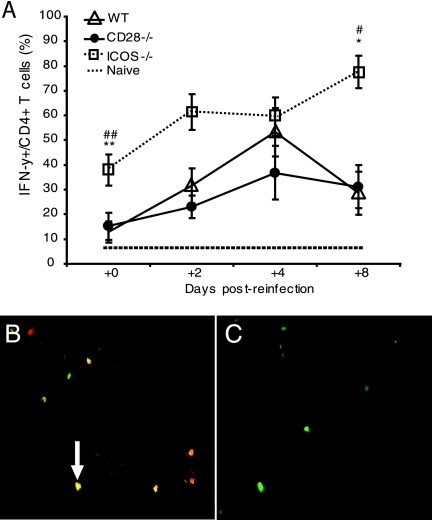
We and others have previously shown that CD4+ T cells are crucial for the clearance of a primary infection with C. trachomatis from the genital tract and the development of protective immunity (17, 19, 47). To assess the level of T-cell responsiveness to recall antigen (Ag), we prepared single-cell suspensions from the PALN, which were incubated with heat-inactivated chlamydial Ag. We found no differences in T-cell proliferative responses from naïve WT, CD28−/−, and ICOS−/− mice when cultured in either medium alone (data not shown) or with heat-inactivated chlamydial Ag (Fig. 3A). Postinfection, we found that T-cell proliferative responses in cultures from ICOS−/− mice were increased compared to those in cultures from WT mice (Fig. 3A). However, quite in contrast, CD28−/− T cells failed to respond to recall Ag, suggesting a failure to prime antigen-specific T cells (Fig. 3A). Since CD4+ T cells, infiltrating the genital tract mucosa, have been associated with resistance against infection, we quantified the number of CD4+ T cells in the uterine mucosa (47). There were no significant differences in the numbers of naive CD4+ T cells between the WT and the CD28−/− mice or the ICOS−/− mice (Fig. 3B and C to E). Postreinfection (Fig. 3B), WT and CD28−/− mice demonstrated an initial decrease in CD4+ T-cell numbers. However, whereas CD4+ T-cell numbers in CD28−/− mice remained low, the WT mice exhibited a doubling of CD4+ T cells in the uterus from day 2 to 8 (Fig. 3B). In contrast, CD4+ T-cell numbers in the uteruses of ICOS−/− mice increased rapidly and had already peaked by day 2, after which the numbers declined (Fig. 3B). Furthermore, as CD4+ T cells exert their protective effects against Chlamydia infection predominantly through the production of IFN-γ, we undertook dual immunolabeling of tissue sections with antibodies against CD4 and IFN-γ (19, 56). Numbers of CD4 and IFN-γ double-positive cells were low in all groups of naïve mice (Fig. 4A). Strikingly, we found CD4+ T cells expressing IFN-γ in the uterus of reinfected WT mice at all time points after the challenge, with a 50% peak level of IFN-γ+/CD4+ cells observed on day 4 of reinfection (Fig. 4A). However, the uterine mucosa of ICOS−/− mice displayed even higher frequencies of IFN-γ-producing CD4+ T cells, peaking at 80% on day 8 (Fig. 4A). By contrast, at early time points, CD28−/− mice had both much lower frequencies (20%) (Fig. 4A) and absolute numbers of IFN-γ-producing CD4+ T cells than ICOS−/− mice (data not shown). Thus, in agreement with previous studies, protection against C. trachomatis infection was concurrent with an increase in IFN-γ-producing CD4+ T cells in the uterine mucosa (6, 20).
FIG. 3.
Augmented CD4+ T-cell responses in ICOS−/− mice but impaired responses in CD28−/− mice to genital tract infection with C. trachomatis. (A) Cell proliferation of PALN cells isolated 10 days after clearance of infection and cultured with or without heat-inactivated C. trachomatis EBs. Results are given as the mean cpm ± standard error of the mean above background in cultures with medium alone and represent pooled pairs from three independent experiments with 6 to 10 mice per group. (B) CD4+ T-cell infiltrates in the genital tract mucosa of naïve, WT, CD28−/−, and ICOS−/− mice during a secondary challenge infection were determined. Cross sections of the uterus were peroxidase labeled with biotin-conjugated anti-CD4 monoclonal antibodies, followed by a commercial ABC/AEC kit. Mean CD4+ T-cell counts of three fields (each 1,250 μm2 at a magnification of ×20), from the left and right uterine horns of at least three mice per group, per experiment were performed. Data represent the means + standard errors of the means of three independent experiments. Naïve represents the maximum CD4+ T-cell count in all groups. Representative sections of CD4+ T-cell infiltrates in naïve WT mice (C), naïve CD28−/− mice (D), and naïve ICOS−/− mice (E) are shown. Right panels (F to H) represent reinfected WT (F) mice, CD28−/− (G) mice, and ICOS−/− (H) mice on day 2 of the reinfection. Large photographs at a magnification of ×10, with a magnification of ×40 for the insets. * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between the WT and either CD28−/− or ICOS−/− groups, while # (P < 0.05) and ## (P < 0.01) denote statistically significant differences between CD28−/− and ICOS−/− groups.
FIG. 4.
Early and enhanced IFN-γ production in ICOS−/− mice in response to reinfection with C. trachomatis. T cells containing IFN-γ were enumerated in frozen cross sections of the uteruses of infected mice. Results are given as average percentages ± standard errors of the means of total CD4+ T cells that double stained for IFN-γ+ at the indicated time points postreinfection. Cells were counted in at least three fields (1,250 μm2 each at ×20 magnification), from left and right uterine horns from three independent experiments with at least three mice per group. (A) Frozen sections were colabeled with anti-IFN-γ (red, Texas Red) and anti-CD4+(green, FITC) and double-labeled of IFN-γ+ CD4+ T cells (yellow) were counted (B). Negative controls include the uteruses from Chlamydia-infected IFN-γ−/− mice (C) or isotype controls (not shown) labeled and scored identically to tissues from the WT, ICOS−/−, or CD28−/− mice. Photographs shown at a magnification of ×40. * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between the WT and either CD28−/− or ICOS−/− groups, while # (P < 0.05) and ## (P < 0.01) denote statistically significant differences between CD28−/− and ICOS−/− groups.
ICOS deficiency promotes Th1 effector cells in the genital tract mucosa.
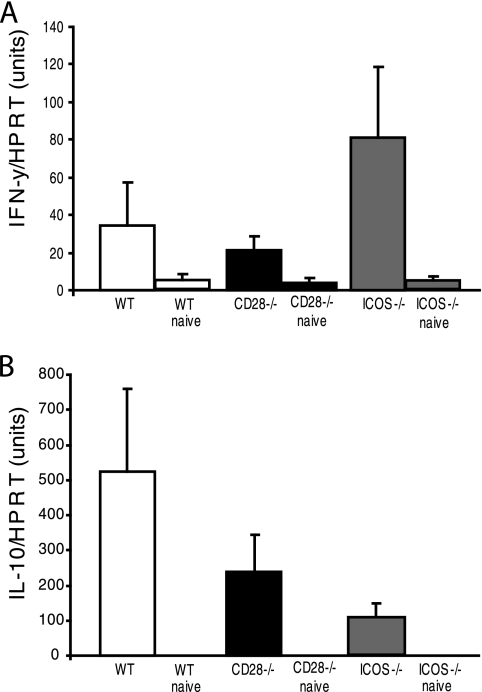
Cytokine production in situ plays a central role in modulating the effector immune response during infection (3). Therefore, we examined whether signaling through ICOS or CD28 had influenced the cytokine production pattern in the uterus during a C. trachomatis infection. Tissue samples were collected from the uterus, and IFN-γ or IL-10 mRNA expression was determined by quantitative PCR assay on various days after infection. The expression of both IFN-γ and IL-10 was low in naïve controls from all groups (Fig. 5A and B). We found that the level of mRNA expression of the Th1-type cytokine IFN-γ was highest on day 2 in all groups, with higher levels, although not significant, in ICOS−/− mice than in WT mice. On the contrary, CD28−/− mice had impaired IFN-γ mRNA levels compared to that of WT mice (Fig. 5A). However, IL-10 mRNA expression was strongest in WT mice, with much lower levels observed for both CD28−/− and ICOS−/− mice (Fig. 5B). Thus, the local uterine tissue was strikingly different with regard to cytokine production in ICOS−/− mice as opposed to CD28−/− and WT mice. Whereas, the former exhibited a relative dominance of IFN-γ and weak IL-10 mRNA expression, the opposite was found for WT mice, while CD28−/− mice demonstrated poor production of both cytokine mRNAs.
FIG. 5.
Striking Th1 skewing of protective immunity in ICOS−/− mice. Uterine tissues were collected from WT, CD28−/−, and ICOS−/− mice at different time points after reinfection for mRNA extraction. Quantitative analyses using real-time RT-PCR were undertaken to determine the relative levels of (A) IFN-γ or (B) IL-10 mRNA in the effector tissues of reinfected mice at peak expression, on day 2 postreinfection. Values represent the means (WT, CD28−/−, or ICOS−/− mice) or means naive (WT, CD28−/−, or ICOS−/− mice) from three independent experiments with two to three mice per group in each experiment. Values were normalized against mRNA expression levels for the HRPT gene, the housekeeping gene.
Differential influence of ICOS and CD28 signaling on the priming of genital tract T-cell immunity.
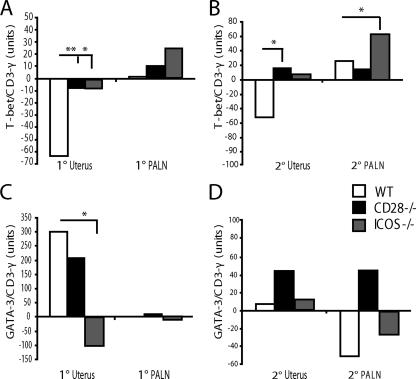
Because the well-protected ICOS-deficient mice demonstrated a strong Th1 cytokine dominance in the uterus, we investigated to what extent signaling through ICOS or CD28 had influenced the priming of Chlamydia-specific naïve CD4+ T cells in the genital tract. We assessed the balance between Th1 and Th2 cells by determining expression of the transcription factors T-bet and GATA-3 in the draining lymph node and uterus by using quantitative PCR and CD3-γ mRNA levels to account for total T cells in the tissue samples. In WT mice, we found no upregulation of T-bet or GATA-3 in the PALN following a primary infection (Fig. 6A and C). The level of T-bet mRNA in ICOS−/− mice exceeded that of WT levels, while only low levels of T-bet were detected in the PALN of CD28−/− mice (Fig. 6A). Moreover, after the secondary challenge infection, T-bet mRNA levels were raised in the uteruses of CD28−/− and ICOS−/− mice (Fig. 6B). In contrast, GATA-3 mRNA expression was downregulated from that of naïve levels or expressed at very low levels in ICOS−/− mice but was prominent in the uteruses of both WT and CD28−/− mice after the primary infection. Taken together, these results demonstrate that ICOS−/− mice develop much-augmented Th1 immunity compared to WT mice, whereas CD28−/− mice are dominated by GATA-3 mRNA expression and skew toward Th2-type immunity.
FIG. 6.
Detection of Th1 and Th2 transcription factors provide compelling evidence for Th1 dominance in protective immunity in ICOS−/− mice. PALN and uterine tissues from WT, CD28−/−, and ICOS−/− mice were collected on day 8 of the primary infection (1°; A and C) and at peak expression on day 2 of the reinfection (2°; B and D). Real-time RT-PCR was used to determine the T-bet (A and B) and GATA-3 (C and D) mRNA transcription factor expression levels. Values are calculated as mean postinfection (WT, CD28−/−, or ICOS−/− mice) minus mean naive (WT, CD28−/− or ICOS−/− mice, respectively) from one representative experiment of three with two to three mice per group. Values were normalized against mRNA expression levels for CD3-γ. * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between WT and either CD28−/− or ICOS−/− groups.
Impaired Treg development in response to genital tract infection in ICOS−/− mice.
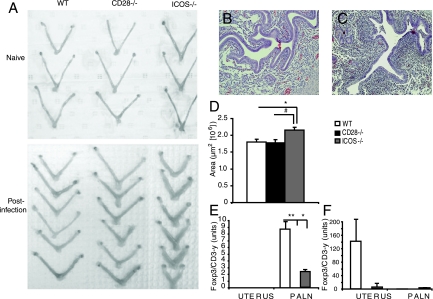
As we observed augmented Th1 differentiation and effector function for ICOS−/− mice compared to that of WT mice, we hypothesized that such a difference could involve impaired development of Treg cells in these mice (46, 52). Macroscopically, the uteruses of ICOS−/− mice exhibited clear evidence of increased inflammation of the uterine horns following the clearance of the primary infection compared to that of WT or CD28−/− mice (Fig. 7A). This was confirmed by H&E staining of the uterine horns on day 8 of the primary infection. The uterine horns of ICOS−/− mice clearly showed increased cellular infiltration compared to those of WT mice (Fig. 7B and C). Additionally, oviduct dilation was significantly increased in ICOS−/− mice compared to that of WT or CD28−/− mice (Fig. 7D). The uterus and PALN were excised and analyzed for the level of Foxp3 mRNA transcription on day 8 of the primary infection and on day 2 of the reinfection by using quantitative PCR. WT mice had dominant Foxp3 expression in the PALN on day 8 of the primary infection compared to the PALN of uninfected mice (Fig. 7E). However, following the reinfection, WT mice showed a strong expression of Foxp3 mRNA in uterus and detectable but low levels in the PALN (Fig. 7F). By contrast, in ICOS−/− and CD28−/− mice, the Foxp3 mRNA levels in the PALN were low or absent compared to that of WT mice (Fig. 7F). In the uterus, Foxp3 mRNA expression was not upregulated in any group following the primary infection, with only WT mice upregulating Foxp3 mRNA levels after the reinfection (Fig. 7F). Thus, signaling through the CD28 or ICOS costimulatory pathways results in a differential development and regulation of host protective immunity against genital tract C. trachomatis infection. Concurrent with the augmented Th1 response in ICOS−/− mice, these mice also appeared to be impaired in their development of Treg activity in the genital tract compared to that seen with WT mice. Thus, exacerbated immunopathology exhibited by Chlamydia-infected ICOS−/− mice may have been a consequence of reduced Treg activity. Foxp3 mRNA levels in CD28−/− mice were always lower than those in uninfected controls, also suggesting an inability to develop the Treg responses to genital tract Chlamydia infection.
FIG. 7.
ICOS−/− mice exhibit a reduced ability to generate Treg cells in response to a genital tract chlamydial infection. Using quantitative real-time RT-PCR, we determined the Foxp3 mRNA expression levels in uterine and PALN tissues after a primary and a secondary challenge infection with Chlamydia. (A) A macroscopic picture of the degree of tissue inflammation is given as comparisons between the uninfected tissue and tissue at day 7 after clearance of a primary infection, from a representative experiment with WT, CD28−/−, and ICOS−/− mice. Representative H&E staining (magnification ×20) reflects the degree of inflammatory infiltrates in the oviducts of WT mice (B) and ICOS−/− mice (C) after clearance of a primary infection. (D) Oviduct area reflects the degree of inflammation. The area was measured from left and right uterine horns from three independent experiments with at least three mice per group on day 8 of the primary infection. PALN and uterine tissues from WT, CD28−/−, and ICOS−/− mice were collected on day 8 of the primary infection (E) or on day 2 of the reinfection (F), and real time RT-PCR values for Foxp3 mRNA levels were calculated for uterus and PALN in relation to the total CD3-γ mRNA content in each sample. Values are calculated as mean postinfection (WT, CD28−/−, or ICOS−/− mice), mean naive (WT, CD28−/−, or ICOS−/− mice, respectively). * (P < 0.05) and ** (P < 0.01) denote statistically significant differences between WT and either CD28−/− or ICOS−/− groups.
DISCUSSION
To our knowledge, this is the first report to investigate the role of costimulatory molecules in host resistance against genital tract infection with Chlamydia trachomatis. The emerging picture of CD28 and ICOS costimulation ascribes very similar functions to these regulatory pathways in T-cell priming, with surprisingly similar effects on gene transcription and cytokine production (42, 54). Therefore, unexpectedly, we found striking differences between CD28 signaling and ICOS signaling in the development of protective immunity following a primary genital tract infection with C. trachomatis. Whereas CD28 was required for the development of adaptive immunity and protection, the lack of ICOS signaling promoted rather than prevented protection, with substantially enhanced Th1-dominated immunity compared to that of WT mice. This Th1 domination emanated from a strong skewing of the priming of CD4+ T cells coupled with an impaired development of Tregs in the genital tracts and draining lymph nodes of ICOS−/− mice. It thus appears that signaling through ICOS can have a dampening effect on Th1 development in normal individuals and also helps to establish Foxp3+ Treg populations in the genital tract. These latter cells may play a role in reducing the immunopathology of genital tract Chlamydia infections, as we observed enhanced tissue inflammation in ICOS−/− mice. Also, whereas WT mice demonstrated prominent IL-10 mRNA expression levels in the uterus relative to those of IFN-γ, the complete opposite was seen with ICOS−/− mice, with little IL-10 mRNA and dramatically augmented IFN-γ mRNA levels, suggesting exaggerated Th1 immunity with little anti-inflammatory regulation in ICOS−/− mice. This augmented Th1 responsiveness resulted in sterile immunity against reinfection in the majority of ICOS−/− mice, a phenomenon which is rarely observed for this mouse model (27). Moreover, and in agreement with our previous work and that of others, full protection was achieved, despite no or very low specific anti-Chlamydia antibody levels (21, 40).
Several studies of host protection against Chlamydia infection have found that CD4+ T cells are critically required, albeit both CD8+ T cells and to some extent specific antibodies may contribute to host resistance against infection (26, 30). Costimulatory molecules are key elements in shaping the adaptive immune response toward Th1, Th2, or Treg cells (10, 45, 50, 51). We found that CD28−/− mice were unable to develop strong adaptive immune responses against C. trachomatis infection. Similar observations have been made for infections caused by other bacteria, such as S. enterica serovar Typhimurium (8, 31). However, our findings in ICOS−/− mice are the complete opposite of those recently reported by Vidric et al., using an ICOS−/− mouse model of systemic infection with Salmonella enterica serovar Typhimurium (55). Since both S. enterica serovar Typhimurium and Chlamydia infections are strictly intracellular, it was surprising to note that ICOS−/− mice demonstrated poor Th1 immunity and impaired clearance of S. enterica serovar Typhimurium bacteria. In striking contrast, we found that ICOS−/− mice cleared C. trachomatis from the genital tract more rapidly than WT mice did and that protection was associated with an enhanced skewing toward Th1 immunity. We also observed that CD4+ T cells in situ in the infected genital tract tissue were producers of high levels of IFN-γ, whereas in the S. enterica serovar Typhimurium model, tissue-resident CD4+ T cells failed to make IFN-γ.
Although there may be several explanations to the discrepant results, we believe the observations are of fundamental importance to our understanding of T-cell-mediated immunity against intracellular bacterial infections in general. First, mucosal as opposed to systemic inoculation with pathogenic bacteria may stimulate vastly different types of cell-mediated immunity. Whereas we inoculated bacteria through the intravaginal route, Vidric et al. injected the bacteria intraperitoneally. It is possible that the ICOS signaling pathway is more important, in relative terms, for the development of Th2 immunity at mucosal sites. Support for such a notion could be found in our previous study with CD28−/− mice, which showed almost intact mucosal IgA immunity while exhibiting strongly impaired systemic immunity (12). Although this assumption agrees well with earlier studies, which indicated that ICOS is more important for Th2 differentiation, recent papers have also pointed to its key function in Th1 development (13, 25). Furthermore, mucosal IFN-γ production has been found to correlate positively with ICOS expression, as reported for intestinal CD4+ T cells from Toxoplasma gondii-infected mice (5). Taking these results together, it appears that ICOS may not directly control Th1 or Th2 differentiation but rather may play a distinct role for effector CD4+ T-cell responses in different tissues depending on the antigen and/or the type of infection (57).
Second, chlamydial infection has recently been reported to augment IL-10 production and ICOSL expression by dendritic cells, and in this regard, Chlamydia infection may differ from that of S. enterica serovar Typhimurium (15). Hence, immune evasion may potentially be established by chlamydia in the genital tract through enhancing the drive for Th2 development via ICOS signaling. In this way, lower Th1 immunity and, thus, prolonged infection could be achieved at the mucosal site. Moreover, the IL-10 production by chlamydia-exposed dendritic cells may directly favor Th2 differentiation and indirectly support Treg1 development/function for the control of effector T cells (1, 4). Thus, in the absence of ICOS signaling, immune evasion may be obstructed, allowing for enhanced bacterial clearance secondarily to an impaired production of IL-10 and Foxp3 Treg cells in the genital tract.
We also observed augmented tissue inflammation in the genital tracts of Chlamydia-infected ICOS−/− mice, which is also supported in the study by Vidric et al., where S. enterica serovar Typhimurium-infected ICOS−/− mice appeared to develop more intense inflammation resulting in, e.g., splenomegaly (55). The lack of local IL-10 production and the infiltration of more CD4+ T cells in the tissue are likely the cause of the enhanced inflammation observed for our study. Nevertheless, apart from CD4+ effector Th1 cells, the level of tissue inflammation may be influenced by macrophages, granulocytes, and other regulatory factors (55, 57). In the mouse models of colitis, Treg cells have recently been found to play important roles in dampening the inflammation (41). We found that Foxp3 Treg development was reduced in ICOS−/− mice, which could have dramatically influenced the ability to counteract inflammation in situ. Such a notion agrees well with other reports linking Treg development to ICOS expression (18).
The differentiation of naïve T cells during priming events into functionally distinct cell populations is directed by the expression of transcription factors. Th1 cells are produced following IL-2 stimulation of naïve T cells and the expression of T-bet, which in turn mediates IFN-γ production (48), which is essential for protection against C. trachomatis infection. We demonstrate here that ICOS−/− mice develop a striking Th1 expansion during infection, as shown by higher T-bet expression levels in the draining lymph node. In contrast, CD28−/− mice have substantial T-bet expression when normalized to CD3+ T cells but a relative lack of CD3+ T cells. On the other hand, GATA-3 is the transcription factor that controls Th2 commitment and mediates the secretion of Th2-type cytokines such as IL-4, IL-5, and IL-10 (37). Our results indicate that while ICOS−/− mice clearly have a strong Th1 bias and a relative lack of Th2 expansion, WT mice develop both Th1 and Th2 cells during infection, since both T-bet and GATA-3 were expressed in the uterine tissue as well as Th1/Th2-like cytokines.
We observed that CD28−/− mice were severely impaired in all types of T-cell responses to a genital tract infection with C. trachomatis. Previous studies have clearly demonstrated that CD28−/− mice largely fail to develop adaptive immunity against pathogenic microorganisms and impaired systemic CD4+ T-cell immunity to protein Ags (reviewed in reference 29). We found that the local production of both IFN-γ and IL-10 was reduced compared to that of WT levels and probably reflects the lack of adaptive T-cell immunity in CD28−/− mice. While T-bet assay results indicate that CD28−/− mice are impaired in the differentiation of naïve T cells into Th1 cells during infection, the relative lack of abundance of CD4+ T cells in the uterine tissue is likely responsible for the poor clearance of bacteria and the lack of protective immunity in the genital tract.
We and others have shown that CD4+ T cells are an absolute requirement for protection against infection (19, 47). However, a much-debated question is the role of specific antibodies (reviewed in references 21, 34 and 40, 58). Recent studies addressed this topic and found evidence suggesting that antibodies may indeed play a role in protection (34, 35), whereby Chlamydia-specific antibodies were found to convey protection against reinfection in a CD4+ T-cell-independent manner (34). This could occur through direct neutralization of EB attachment, enhanced complement-mediated lysis, or FcR-mediated cytotoxicity. However, ICOS−/− mice have impaired antibody production and lack germinal center development and hence are also impaired in class switch recombination and somatic hypermutations (11, 28, 49). In agreement with this, we found that ICOS−/− mice showed no or highly impaired serum and secretory Chlamydia-specific IgG and no IgA production in response to infection. Therefore, we would argue that antibodies play a subordinate role in host resistance against genital tract chlamydial infection based on our previous work and the present findings of sterile immunity in a majority of ICOS−/− mice lacking completely specific antibodies while exhibiting enhanced Th1 type of immunity.
Acknowledgments
This study was supported by the Swedish Foundation for Strategic Research, the Mucosal Immunobiology and Vaccine Center (MIVAC), the Swedish Research Council, the Swedish Cancer Foundation, the Ellen AB stipendiet, the Sahlgrenska University Hospital Foundation, and EU grants QLK2-CT-2001-01702, QLK2-CT-199-00228, and LSHP-CT-2003-503240, SAREC.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Akbari, O., R. H. DeKruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725-731. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoresen, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212:287-300. [DOI] [PubMed] [Google Scholar]
- 4.Bilsborough, J., T. C. George, A. Norment, and J. L. Viney. 2003. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonhagen, K., O. Liesenfeld, M. J. Stadecker, A. Hutloff, K. Erb, A. J. Coyle, M. Lipp, R. A. Kroczek, and T. Kamradt. 2003. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur. J. Immunol. 33:392-401. [DOI] [PubMed] [Google Scholar]
- 6.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton, H. L., and J. P. Farrell. 2002. CD28 costimulation and parasite dose combine to influence the susceptibility of BALB/c mice to infection with Leishmania major. J. Immunol. 168:1302-1308. [DOI] [PubMed] [Google Scholar]
- 9.Czermak, B. J., A. B. Lentsch, N. M. Bless, H. Schmal, H. P. Friedl, and P. A. Ward. 1998. Role of complement in in vitro and in vivo lung inflammatory reactions. J. Leukoc. Biol. 64:40-48. [DOI] [PubMed] [Google Scholar]
- 10.de Jong, Y. P., S. T. Rietdijk, W. A. Faubion, A. C. Abadia-Molina, K. Clarke, E. Mizoguchi, J. Tian, T. Delaney, S. Manning, J. C. Gutierrez-Ramos, A. K. Bhan, A. J. Coyle, and C. Terhorst. 2004. Blocking inducible co-stimulator in the absence of CD28 impairs Th1 and CD25+ regulatory T cells in murine colitis. Int. Immunol. 16:205-213. [DOI] [PubMed] [Google Scholar]
- 11.Dong, C., U. A. Temann, and R. A. Flavell. 2001. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166:3659-3662. [DOI] [PubMed] [Google Scholar]
- 12.Gardby, E., J. Wrammert, K. Schon, L. Ekman, T. Leanderson, and N. Lycke. 2003. Strong differential regulation of serum and mucosal IgA responses as revealed in CD28-deficient mice using cholera toxin adjuvant. J. Immunol. 170:55-63. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalo, J. A., J. Tian, T. Delaney, J. Corcoran, J. B. Rottman, J. Lora, A. Al-garawi, R. Kroczek, J. C. Gutierrez-Ramos, and A. J. Coyle. 2001. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat. Immunol. 2:597-604. [DOI] [PubMed] [Google Scholar]
- 14.Gross, J. A., E. Callas, and J. P. Allison. 1992. Identification and distribution of the costimulatory receptor CD28 in the mouse. J. Immunol. 149:380-388. [PubMed] [Google Scholar]
- 15.Han, X., S. Wang, Y. Fan, J. Yang, L. Jiao, H. Qiu, and X. Yang. 2006. Chlamydia infection induces ICOS ligand-expressing and IL-10-producing dendritic cells that can inhibit airway inflammation and mucus overproduction elicited by allergen challenge in BALB/c mice. J. Immunol. 176:5232-5239. [DOI] [PubMed] [Google Scholar]
- 16.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 62:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Q., T. T. Moore, F. O. Eko, D. Lyn, G. A. Ananaba, A. Martin, S. Singh, L. James, J. Stiles, C. M. Black, and J. U. Igietseme. 2005. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. J. Immunol. 174:4860-4869. [DOI] [PubMed] [Google Scholar]
- 18.Herman, A. E., G. J. Freeman, D. Mathis, and C. Benoist. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199:1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 65:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for humans? Scand. J. Immunol. 46:546-552. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, M., M. Ward, and N. Lycke. 1997. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology 92:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappes, D. J., D. M. Lawrence, M. M. Vaughn, V. P. Dave, A. R. Belman, and G. F. Rall. 2000. Protection of CD3 delta knockout mice from lymphocytic choriomeningitis virus-induced immunopathology: implications for viral neuroinvasion. Virology 269:248-256. [DOI] [PubMed] [Google Scholar]
- 23.Klaus, S. J., L. M. Pinchuk, H. D. Ochs, C. L. Law, W. C. Fanslow, R. J. Armitage, and E. A. Clark. 1994. Costimulation through CD28 enhances T cell-dependent B cell activation via CD40-CD40L interaction. J. Immunol. 152:5643-5652. [PubMed] [Google Scholar]
- 24.Kohyama, M., D. Sugahara, S. Sugiyama, H. Yagita, K. Okumura, and N. Hozumi. 2004. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc. Natl. Acad. Sci. USA 101:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopf, M., A. J. Coyle, N. Schmitz, M. Barner, A. Oxenius, A. Gallimore, J. C. Gutierrez-Ramos, and M. F. Bachmann. 2000. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladel, C. H., I. E. Flesch, J. Arnoldi, and S. H. Kaufmann. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153:3116-3122. [PubMed] [Google Scholar]
- 27.Lyons, J. M., S. A. Morre, L. P. Airo-Brown, A. S. Pena, and J. I. Ito. 2005. Acquired homotypic and heterotypic immunity against oculogenital Chlamydia trachomatis serovars following female genital tract infection in mice. BMC Infect. Dis. 5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdam, A. J., T. T. Chang, A. E. Lumelsky, E. A. Greenfield, V. A. Boussiotis, J. S. Duke-Cohan, T. Chernova, N. Malenkovich, C. Jabs, V. K. Kuchroo, V. Ling, M. Collins, A. H. Sharpe, and G. J. Freeman. 2000. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 165:5035-5040. [DOI] [PubMed] [Google Scholar]
- 29.McAdam, A. J., A. N. Schweitzer, and A. H. Sharpe. 1998. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165:231-247. [DOI] [PubMed] [Google Scholar]
- 30.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 31.Mittrucker, H. W., A. Kohler, T. W. Mak, and S. H. Kaufmann. 1999. Critical role of CD28 in protective immunity against Salmonella typhimurium. J. Immunol. 163:6769-6776. [PubMed] [Google Scholar]
- 32.Mittrucker, H. W., M. Kursar, A. Kohler, D. Yanagihara, S. K. Yoshinaga, and S. H. Kaufmann. 2002. Inducible costimulator protein controls the protective T cell response against Listeria monocytogenes. J. Immunol. 169:5813-5817. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto, K., C. I. Kingsley, X. Zhang, C. Jabs, L. Izikson, R. A. Sobel, H. L. Weiner, V. K. Kuchroo, and A. H. Sharpe. 2005. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J. Immunol. 175:7341-7347. [DOI] [PubMed] [Google Scholar]
- 34.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175:7536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noel, P. J., L. H. Boise, J. M. Green, and C. B. Thompson. 1996. CD28 costimulation prevents cell death during primary T cell activation. J. Immunol. 157:636-642. [PubMed] [Google Scholar]
- 37.Pai, S. Y., M. L. Truitt, and I. C. Ho. 2004. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl. Acad. Sci. USA 101:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 39.Plummer, F. A., J. N. Simonsen, D. W. Cameron, J. O. Ndinya-Achola, J. K. Kreiss, M. N. Gakinya, P. Waiyaki, M. Cheang, P. Piot, A. R. Ronald, et al. 1991. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 163:233-239. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey, K. H., L. S. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley, J. L., M. Mao, S. Kobayashi, M. Biery, J. Burchard, G. Cavet, B. P. Gregson, C. H. June, and P. S. Linsley. 2002. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc. Natl. Acad. Sci. USA 99:11790-11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rupprecht, T. A., B. Angele, M. Klein, J. Heesemann, H. W. Pfister, M. Botto, and U. Koedel. 2007. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J. Immunol. 178:1861-1869. [DOI] [PubMed] [Google Scholar]
- 44.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 45.Smith, K. M., J. M. Brewer, P. Webb, A. J. Coyle, C. Gutierrez-Ramos, and P. Garside. 2003. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J. Immunol. 170:2310-2315. [DOI] [PubMed] [Google Scholar]
- 46.Stassen, M., H. Jonuleit, C. Muller, M. Klein, C. Richter, T. Bopp, S. Schmitt, and E. Schmitt. 2004. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J. Immunol. 173:267-274. [DOI] [PubMed] [Google Scholar]
- 47.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabo, S. J., S. T. Kim, G. L. Costa, X. Zhang, C. G. Fathman, and L. H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655-669. [DOI] [PubMed] [Google Scholar]
- 49.Tafuri, A., A. Shahinian, F. Bladt, S. K. Yoshinaga, M. Jordana, A. Wakeham, L. M. Boucher, D. Bouchard, V. S. Chan, G. Duncan, B. Odermatt, A. Ho, A. Itie, T. Horan, J. S. Whoriskey, T. Pawson, J. M. Penninger, P. S. Ohashi, and T. W. Mak. 2001. ICOS is essential for effective T-helper-cell responses. Nature 409:105-109. [DOI] [PubMed] [Google Scholar]
- 50.Tai, X., M. Cowan, L. Feigenbaum, and A. Singer. 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6:152-162. [DOI] [PubMed] [Google Scholar]
- 51.Tang, Q., K. J. Henriksen, E. K. Boden, A. J. Tooley, J. Ye, S. K. Subudhi, X. X. Zheng, T. B. Strom, and J. A. Bluestone. 2003. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 171:3348-3352. [DOI] [PubMed] [Google Scholar]
- 52.Thornton, A. M., and E. M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuffrey, M., and D. Taylor-Robinson. 1981. Progesterone treatment as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol. Lett. 12:111-115. [Google Scholar]
- 54.van Berkel, M. E., and M. A. Oosterwegel. 2006. CD28 and ICOS: similar or separate costimulators of T cells? Immunol. Lett. 105:115-122. [DOI] [PubMed] [Google Scholar]
- 55.Vidric, M., A. T. Bladt, U. Dianzani, and T. H. Watts. 2006. Role for inducible costimulator in control of Salmonella enterica serovar Typhimurium infection in mice. Infect. Immun. 74:1050-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, S., Y. Fan, R. C. Brunham, and X. Yang. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur. J. Immunol. 29:3782-3792. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, E. H., C. Zaph, M. Mohrs, A. Welcher, J. Siu, D. Artis, and C. A. Hunter. 2006. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J. Immunol. 177:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wira, C. R., C. Kaushic, and J. Richardson. 1999. Role of sex hormones and cytokines in regulating the miucosal immune system in the female reproductive tract, p. 1449-1461. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, CA.