Abstract
The three skin disorders of Lyme borreliosis in Europe include erythema migrans, an acute, self-limited lesion; borrelial lymphocytoma, a subacute lesion; and acrodermatitis chronica atrophicans, a chronic lesion. Using quantitative reverse transcription-PCR, we determined mRNA expression of selected chemokines, cytokines, and leukocyte markers in skin samples from 100 patients with erythema migrans, borrelial lymphocytoma, or acrodermatitis chronica atrophicans and from 25 control subjects. Chemokine patterns in lesional skin in each of the three skin disorders included low but significant mRNA levels of the neutrophil chemoattractant CXCL1 and the dendritic cell chemoattractant CCL20 and intermediate levels of the macrophage chemoattractant CCL2. Erythema migrans and particularly acrodermatitis lesions had high mRNA expression of the T-cell-active chemokines CXCL9 and CXCL10 and low levels of the B-cell-active chemokine CXCL13, whereas lymphocytoma lesions had high levels of CXCL13 and lower levels of CXCL9 and CXCL10. This pattern of chemokine expression was consistent with leukocyte marker mRNA in lesional skin. Moreover, using immunohistologic methods, CD3+ T cells and CXCL9 were visualized in erythema migrans and acrodermatitis lesions, and CD20+ B cells and CXCL13 were seen in lymphocytoma lesions. Thus, erythema migrans and acrodermatitis chronica atrophicans have high levels of the T-cell-active chemokines CXCL9 and CXCL10, whereas borrelial lymphocytoma has high levels of the B-cell-active chemokine CXCL13.
Lyme borreliosis (LB), a tick-transmitted spirochetal infection, is associated with three skin disorders in Europe (26). Erythema migrans (EM) is an acute, self-limited lesion, often located in or near the groin, axilla, or popliteal fossa. Borrelial lymphocytoma (BL) is a subacute lesion, typically on the ear or breast, that eventually resolves (4, 26). Acrodermatitis chronica atrophicans (ACA) is a slowly progressive lesion located primarily on the extensor (acral) surfaces of the extremities. This chronic lesion, which has inflammatory and atrophic phases, sometimes with fibrotic features, results from persistent spirochetal infection (3, 26).
In Europe, LB is caused by three pathogenic species of Borrelia burgdorferi sensu lato: Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto (hereafter referred to as B. burgdorferi) (5). However, in Europe (and specifically in Austria), B. afzelii is the most common cause of each of these dermatologic manifestations of the infection (2, 26, 30, 39, 40). In the United States, Lyme disease is caused only by B. burgdorferi. There, EM lesions are a feature of the illness, but not BL or ACA lesions (38).
In EM lesions, perivascular infiltrates of macrophages and CD8+ and CD4+ T cells are found along with smaller numbers of B cells and plasma cells (25, 42). ACA lesions are infiltrated throughout the dermis with these cells, including abundant plasma cells (7, 42). In contrast, BL lesions have dense infiltrates of B cells with proportionally fewer T cells, and the B cells often form follicular structures, sometimes with the appearance of germinal centers (11, 42).
Two previous studies have analyzed cytokine responses in human EM lesions. In our initial study of Austrian patients, the major cytokines expressed by infiltrating cells in the lesions were the proinflammatory cytokine gamma interferon (IFN-γ) and the anti-inflammatory cytokine interleukin-10 (IL-10), as determined by in situ hybridization with riboprobes (25). In addition, in patients with extracutaneous signs and symptoms, tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6 were often expressed. In a study of EM lesions in U.S. patients (33), in which flow cytometry was used to characterize cytokines in fluids aspirated from suction blisters raised over EM lesions, the fluids, which were enriched for T cells, macrophages, and dendritic cells, contained predominantly IL-6 and IFN-γ. Thus, in both studies, proinflammatory cytokines, particularly IFN-γ, were predominant.
Chemokines (chemoattractant cytokines) play a crucial role in the homing of inflammatory cells to infected tissues. In a seminal study with B. burgdorferi-infected mice, which develop arthritis but not skin or neurologic abnormalities, CXCL1, a neutrophil chemokine, and CCL2, a macrophage chemokine, were highly expressed in infected joints (9). In tissue culture systems of monocytes, endothelial cells, keratinocytes, fibroblasts, or mast cells, B. burgdorferi sensu lato potently induced the neutrophil chemoattractants CXCL1 and CXCL8; the macrophage chemoattractants CCL2, CCL3, and CCL5; or the T-cell chemoattractants CXCL9 and CXCL10 (10, 15, 16, 36). In human patients or nonhuman primates with neuroborreliosis, elevated levels of the neutrophil chemokine CXCL8; the macrophage chemokines CCL3, CCL4, and CCL5; the T-cell chemokines CXCL10, CXCL11, and CXCL12; or the B-cell chemokine CXCL13, were found in serum or cerebrospinal fluid (18, 22, 29, 32) or in nervous system tissues (27).
Chemokine and cytokine signatures have not yet been defined and compared in lesional skin of the three skin disorders of LB in Europe. In the current study, we determined the mRNA expression of 12 chemokines, 8 cytokines, and 5 leukocyte markers in the lesional skin of 100 patients with EM, BL, or ACA, using high-throughput quantitative reverse transcription-PCR (QRT-PCR) techniques. We found a predominance of IFN-γ-inducible T-cell-active chemokines CXCL9 and CXCL10 in EM and ACA and of the B-cell-active chemokine CXCL13, in BL.
MATERIALS AND METHODS
Patients.
From June 2001 through February 2004, 128 patients with EM, 12 with BL, and 34 with ACA were recruited for this study at the Department of Dermatology in Graz, Austria. The patients had exposure to ticks in southeastern Austria, where LB is endemic. The study was conducted according to the Declaration of Helsinki principles. All patients gave written informed consent approved by the institutional review board. For inclusion, patients were required to meet previously described clinical and histopathologic criteria for EM, BL, or ACA (3, 4, 7, 12, 35, 37, 42). A faculty dermatologist made the clinical diagnosis, and a faculty dermatopathologist read the skin biopsies, without knowledge of the clinical diagnosis. In addition, all patients were required to have a positive immunoglobulin M or immunoglobulin G antibody response to a purified flagellar antigen of B. afzelii (strain DK-1) in acute- or convalescent-phase serum samples, determined by enzyme-linked immunosorbent assay (Dako, Glostrup, Denmark).
Patients were excluded if they had a history of previous LB; if they had a coincident condition, disease, or medication that might alter immune function; or if they had already received antibiotic therapy for the infection. Finally, they were required to be seronegative for coinfection with Anaplasma phagocytophilum by indirect immunofluorescence assay (MRL Diagnostics, Cypress, CA). Of the 128 patients with EM, 38 were excluded because of a negative serologic result for B. burgdorferi sensu lato, 11 because of inconclusive histopathology, and 15 because of a positive serologic test for A. phagocytophilum. One of the 12 BL patients and 9 of the 34 ACA patients were excluded because of inconclusive histologic findings. Patient recruitment was continued until samples were obtained from 100 evaluable patients.
Skin biopsy samples.
In all patients, two 4-mm punch skin biopsy samples were obtained, one for histologic examination and one for chemokine, cytokine, and leukocyte marker mRNA analyses and B. burgdorferi sensu lato species determinations. Biopsies were taken from the active borders of EM lesions or from the areas of BL or ACA lesions with the most prominent signs of inflammation. The sample for histology was placed in 4% formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. The sample for mRNA and B. burgdorferi sensu lato determinations was placed immediately in a 2-ml tube containing RNAlater (Ambion, Austin, TX), an RNA stabilization/protection reagent. The tubes were refrigerated overnight at 4°C and then stored at −70°C until ready for use.
A control group of 25 healthy subjects was developed, from whom normal skin samples (4 to 6 mm) were obtained at the edges of elliptical excision specimens of atypical melanocytic nevi. These samples were also placed immediately in a 2-ml tube containing RNAlater.
RNA isolation and cDNA synthesis.
Each skin sample was ground mechanically in guanidine isothiocyanate-containing lysis buffer (QIAGEN, Valencia, CA), which immediately inactivates RNases, and total RNA was extracted using the RNeasy kit according to the manufacturer's protocol (QIAGEN). After DNase I treatment, total RNA from each sample (1 μg) was used as the template for the RT reaction. cDNA (100 μl) was synthesized using oligo(dT)15, random hexamers, and Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA) in a Peltier PTC 200 thermal cycler (MJ Research, Ramsey, MN). All samples were reverse transcribed under the same conditions (25°C for 10 min and 48°C for 30 min) and from the same RT master mix to minimize differences in RT efficiency. As a negative control, each experiment included a sample without reverse transcriptase.
Identification of the B. burgdorferi sensu lato species.
The B. burgdorferi sensu lato species was determined from the cDNA by PCR amplification of B. burgdorferi sensu lato ospA transcripts using published primer sets (13, 31). This reaction amplifies a sequence of approximately 300 bp for each of the three pathogenic B. burgdorferi sensu lato species. Each ospA PCR product was subjected to restriction digestion with the BfaI or MseI enzyme (New England Biolabs, Beverly, MA), which yields specific restriction fragment length polymorphisms for each species. For these analyses, the PCR product (10 μl) was mixed with 4 μl of enzyme-specific buffer and 5 units of enzyme in a 40-μl total volume. For MseI digestions, 4 μl of 0.1-mg/ml bovine serum albumin was included in the mixture as a carrier molecule. Digests were incubated overnight at 37°C and visualized on 2% agarose gels (Invitrogen, Carlsbad, CA), with ethidium bromide staining. After digestion with BfaI, B. afzelii was predicted to have a single band of 293 bp; B. burgdorferi, a single band of 245 bp; and B. garinii, two bands of 147 and 146 bp. After digestion with MseI, B. afzelii was predicted to have two bands of 221 and 66 bp, and both B. burgdorferi and B. garinii were predicted to have two bands of 148 and 74 bp.
Quantitative PCR for chemokines, cytokines, and leukocyte markers.
Quantitation of chemokine, cytokine, and leukocyte marker mRNA expression was performed as previously described (23), using the MX4000 multiplex QRT-PCR system (Stratagene, La Jolla, CA). The primers for each reaction (Table 1) were synthesized commercially (Invitrogen). The 25-μl QRT-PCR mixture contained 2 μl of cDNA, 12.5 μl of 2× SYBR green master mix (Stratagene), and 250 nmol (1.625 μl) of sense and antisense primer. The reaction conditions were 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Emitted fluorescence for each reaction was measured during the annealing/extension phase, and amplification plots were analyzed using the MX4000 software version 3.0 (Stratagene). The DNA encoding sequence for each gene analyzed by QRT-PCR was cloned into a plasmid vector. A series of standards were prepared by performing serial 10-fold dilutions of full-length coding DNAs from 20 million copies to two copies per QRT-PCR. The QRT-PCR primer efficiencies were similar for all target and housekeeping genes. Analysis was performed on the data output from the MX4000 software using Excel XP (Microsoft, Redmond, WA). The threshold cycle (the cycle at which the amount of the amplified gene reaches threshold fluorescence) was determined using the adaptive baseline algorithm in the MX4000 analysis software. Quantity values (i.e., copies) for gene expression were generated by comparison of the fluorescence generated by each sample with standard curves of known quantities. To control for variation in the size of the biopsies, the number of copies was then divided by the number of copies of a housekeeping gene, that for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). As a negative control, reactions without cDNA were included on each plate.
TABLE 1.
Oligonucleotide primers for the leukocyte antigens, chemokines, and cytokines analyzed
| Leukocyte antigen, chemokine, or cytokine category and namea | Primer
|
|
|---|---|---|
| 3′ (reverse) | 5′ (forward) | |
| Dendritic cell marker CD11c | GGAACTGGCTTATCACAGCTCT | TGTACCTCACCGGACTCTG |
| Macrophage marker CD14 | GCCCAGTCCAGGATTGTCAG | ACTTGCACTTTCCAGCTTGC |
| T-lymphocyte markers | ||
| CD4 | CAAGACTTGGAGGAGGAAGCC | TTGAAAAGCTGACGGGCAGT |
| CD8 | GTCTCCCGATTTGACCACAGG | GCAACCACAGGAACCGAAGA |
| B-lymphocyte marker CD19 | CCATCTGTTGAGAGACGTTGAA | GAGTCCCCGCTTAAACCCTTC |
| Neutrophil chemoattractants | ||
| CXCL8/IL-8 | CCTTGGCAAAACTGCACCTT | CTGGCCGTGGCTCTCTTG |
| CXCL1/GROα | GCCCATTCTTGAGTGTGGCT | ACGTGAAGTCCCCCGGAC |
| Dendritic cell chemoattractant CCL20/MIP-3α | TCAAAGTTGCTTGCTGCTTCTG | TCCTGGCTGCTTTGATGTCA |
| Macrophage chemoattractants | ||
| CCL2/MCP-1 | TGCATCTGGCTGAGCGAG | CTCTGCCGCCCTTCTGTG |
| CCL3/MIP-1α | CGGCTTCGCTTGGTTAGGA | AGCTGACTACTTTGAGACGAGCAG |
| CCL4/MIP-1β | GTAAGAAAAGCAGCAGGCGG | CTGCTCTCCAGCGCTCTCA |
| Th1 lymphocyte chemoattractants | ||
| CXCL9/MIG | GGTGGATAGTCCCTTGGTTGG | TGCAATGAACCCCAGTAGTGA |
| CXCL10/IP-10 | CAGACATCTCTTCTCACCCTTCTTT | TGAAATTATTCCTGCAAGCCAA |
| CXCL11/I-TAC | CCACTTTCACTGCTTTTACCCC | CAAGGCTTCCCCATGTTCA |
| Th2 lymphocyte chemoattractants | ||
| CCL22/MDC | GGTTAGCAACACCACGCCA | TGCGCGTGGTGAAACACT |
| CCL1/I-309 | GTCCACATCTTCCGGCCA | TGCAGATCATCACCACAGCC |
| B-lymphocyte chemoattractant CXCL13/BCA-1 | TTCCAGACTATGATTTCTTTTCTTGG | GACGCTTCATTGATCGAATTCA |
| Proinflammatory macrophage-derived cytokines | ||
| IL-1β | CCATGGCCACAACAACTGAC | ACGAATCTCCGACCACCACT |
| TNF-α | CAGGCAGAAGAGCGTGGTG | GGTGCTTGTTCCTCAGCCTC |
| IL-12p40 | CAAGATGAGCTATAGTAGCGGTCCT | CGGTCATCTGCCGCAAA |
| Proinflammatory Th1 lymphocyte-derived cytokine IFN-γ | CGCTTCCCTGTTTTAGCTGC | CCAACGCAAAGCAATACATGA |
| Anti-inflammatory Th2 lymphocyte-derived cytokines | ||
| IL-4 | GCCTGTGGAACTGCTGTGC | GGCAGTTCTACAGCCACCATG |
| IL-5 | GTGCCAAGGTCTCTTTCACCA | AGCTGCCTACGTGTATGCCA |
| Anti-inflammatory macrophage- or T-lymphocyte-derived cytokines | ||
| TGF-β | CAGCTTGGACAGGATCTGGC | TATCGACATGGAGCTGGTGAAG |
| IL-10 | TCCCCCAGGGAGTTCACA | GGTGATGCCCCAAGCTGA |
| Housekeeping gene product GAPDH | GAAGATGGTGATGGGATTC | GAAGGTGAAGGTCGGAGTC |
GROα, growth-regulated oncogene α; MIP-3α, macrophage inflammatory protein 3α; MCP-1, monocyte chemoattractant protein 1; MIG, monokine induced by IFN-γ; IP-10, IFN-γ-inducible protein 10; I-TAC, interferon-inducible T-cell α chemokine; MDC, macrophage-derived chemokine; BCA-1, B-cell-attracting chemokine 1.
Immunohistochemistry.
Paraffin-embedded, formalin-fixed tissue sections (6 μm) from patients with EM, BL, or ACA were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), deparaffinized with xylene, and rehydrated in graded alcohol solutions. The slides were washed with phosphate-buffered saline and treated with antigen retrieval buffer (BioGenex, San Ramon, CA) at 95 to 99°C for 30 min. After cooling to room temperature, the slides were washed with phosphate-buffered saline and incubated in normal blocking serum for 20 min. The sections from patients with EM and ACA were incubated with purified mouse anti-human monoclonal antibodies to CD3 (10 μg/ml) (Dako USA, Carpinteria, CA) and CXCL9 (0.5 μg/ml) (R&D Systems, Minneapolis, MN), and the sections from patients with BL were incubated with purified mouse anti-human monoclonal antibody to CD20 (2 μg/ml) (BD Pharmingen, San Jose, CA) and purified goat anti-human polyclonal antibody to CXCL13 (2 μg/ml) (R&D Systems) in a moist chamber at 4°C overnight. Other investigators previously showed that these monoclonal antibodies were specific for these targets, using isotype-matched control antibodies (12, 20, 34). Therefore, in our experiments, a negative control section, without the primary antibody, was included for each slide. Subsequent incubations were carried out with Vectastain biotinylated horse anti-mouse or anti-goat secondary antibody (Vector Laboratories, Burlingame, CA), depending on the primary antibody, followed by the Vectastain ABC reagent and the chromogen substrate 3-diaminobenzidine-tetra-HCl (Acros, NJ). After the final rinse with distilled water, the slides were counterstained with hematoxylin (Fisher Scientific) and mounted with VectaMount (Vector Laboratories).
Statistics.
Differences in mRNA expression of chemokines, cytokines, and leukocyte markers among patients with EM, BL, or ACA or normal control subjects were determined by the Kruskal-Wallis test. All P values were two tailed. P values of ≤0.05 were considered statistically significant.
RESULTS
Clinical characteristics.
Of the 100 study patients, 64 had EM, 11 had BL, and 25 had ACA. In each group, the female-to-male ratio was about 2:1, and the median ages ranged from 42 to 56 years. EM lesions, which were biopsied at a median of 1 week (range, 0.2 to 30 weeks) after onset, were usually solitary macular or annular erythemas, often located in or near intertriginous areas such as the groin, axilla, or popliteal fossa. One-third of these patients had extracutaneous signs or symptoms, including headache, arthralgias, myalgias, elevated temperature, stiff neck, malaise, or fatigue. BL lesions, which were biopsied at a median of 6 weeks (range, 3 to 20 weeks) after onset, were small, solitary nodules or plaques on the ear or breast. These patients rarely had other signs or symptoms. ACA lesions, which were biopsied at a median of 26 weeks (range, 4 to 432 weeks) after onset, ranged from early inflammatory to chronic atrophic lesions. They were located on the extensor (acral) surfaces of the extremities. These patients often had pain or dysesthesias in the region of the lesions, but they rarely had systemic symptoms.
Analysis of B. burgdorferi sensu lato DNA in the skin biopsy samples showed that 77% or more of the patients with each of the skin disorders were infected with B. afzelii, about 10% had B. burgdorferi, and the remaining patients had B. garinii or mixed infection (Table 2). By definition, all patients were seropositive for B. burgdorferi sensu lato infection. Most patients with EM were treated with oral doxycycline for 2 or 3 weeks, and those with BL or ACA were usually given 4 weeks of this medication. A small number of patients received other, comparable regimens. All patients responded to therapy, but in several patients with ACA, skin atrophy persisted or neuropathy resolved slowly.
TABLE 2.
Borrelia burgdorferi sensu lato species in lesional skin from patients with skin disorders of Lyme borreliosis in Austria
| Species (no. of patient skin biopsies positive for ospA by PCR) (n = 76a) | No. (%) positive
|
||
|---|---|---|---|
| EM (n = 48) | BL (n = 9) | ACA (n = 19) | |
| B. afzelii (60) | 37 (77) | 8 (88) | 15 (79) |
| B. burgdorferi (9) | 6 (13) | 1 (12) | 2 (10.5) |
| Mixed B. afzelii/ B. burgdorferi (6) | 4 (8) | 0 (0) | 2 (10.5) |
| B. garinii (1) | 1 (2) | 0 (0) | 0 (0) |
Of the 76 ospA-positive samples whose Borrelia species were identified using restriction fragment length polymorphisms analysis, four B. afzelii isolates, three B. burgdorferi isolates, and the single B. garinii isolate were confirmed by sequencing.
Chemokine expression.
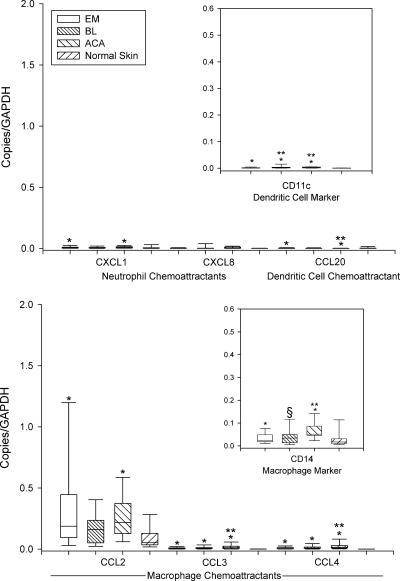
As a part of the innate immune response, low mRNA levels of the neutrophil chemoattractant CXCL1, the dendritic cell chemoattractant CCL20, and the dendritic cell marker CD11c were found in lesional skin in each of the three skin disorders (Fig. 1). In addition, moderate amounts of mRNAs for macrophage chemoattractants, particularly CCL2, and the CD14 macrophage marker were identified. In most instances, mRNA expression for each of these chemokines and leukocyte markers were significantly greater in lesional skin than in normal skin.
FIG. 1.
mRNA expression of neutrophil, dendritic cell, and macrophage chemoattractants and their cell markers in 64 EM lesions, 11 BL lesions, 25 ACA lesions, and 25 normal skin samples relative to the GAPDH gene. Data are shown in box plots in which the boxes represent the 25th and 75th percentiles, the lines within the boxes represent the median values, and the lines outside the boxes represent the 5th and 95th percentiles. A difference between normal and lesional skin (*), between EM and BL or EM and ACA lesions (**), or between BL and ACA lesions (§) at the 0.05 level is indicated above the bars. Low mRNA levels of neutrophil and dendritic cell chemoattractants and moderate mRNA levels of macrophage chemoattractants, primarily CCL2, were found.
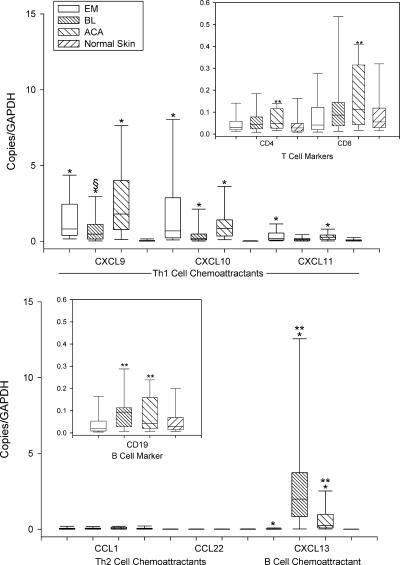
As a part of the adaptive immune response, high mRNA levels of the IFN-γ-inducible chemokines CXCL9 and CXCL10, which are strong chemoattractants for effector CD8+ and Th1-type CD4+ T cells, were found in lesional skin in EM, BL, and particularly ACA (Fig. 2). Consistent with these findings, high mRNA levels were identified for both the CD8 and CD4 T-cell markers; the CD8/CD4 ratios were 1.3 in EM, 1.9 in BL, and 2.0 in ACA. In contrast, mRNAs for the Th2 cell chemokines CCL1 and CCL22 were virtually absent. Whereas EM and ACA had only low mRNA levels of the B-cell chemoattractant CXCL13, BL had significantly higher levels of this chemokine and the CD19 B-cell marker and proportionately lower levels of CXCL9 and CXCL10. In each of the three skin disorders of the infection, mRNA levels for Th1 and B-cell chemoattractants were greater in lesional skin than in normal skin.
FIG. 2.
mRNA expression of T- and B-cell chemoattractants and their cell markers in 64 EM lesions, 11 BL lesions, 25 ACA lesions, and 25 normal skin samples relative to the GAPDH gene. Data are shown in box plots in which the boxes represent the 25th and 75th percentiles, the lines within the boxes represent the median values, and the lines outside the boxes represent the 5th and 95th percentiles. A difference between normal and lesional skin (*), between EM and BL or EM and ACA lesions (**), or between BL and ACA lesions (§) at the 0.05 level is indicated above the bars. High levels of the CD8+ and Th1-type CD4+ T-cell chemoattractants CXCL9 and CXCL10 were found in EM and ACA lesions, whereas BL lesions had high levels of the B-cell chemoattractant CXCL13 and proportionally lower levels of CXCL9 and CXCL10.
Immunohistochemistry.
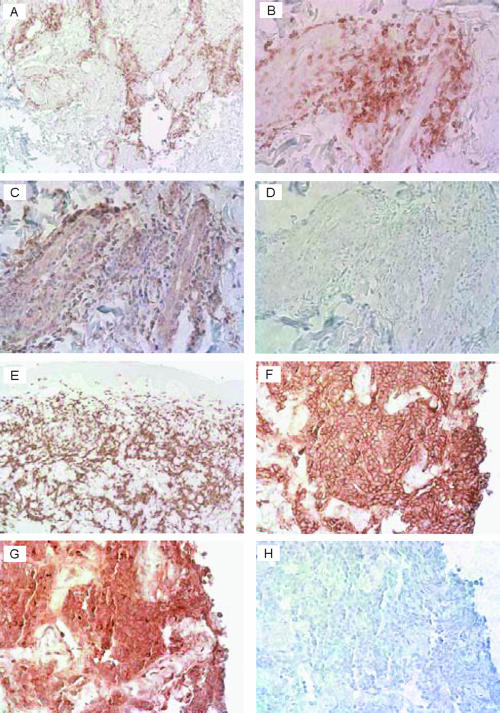
Because of the predominance of mRNA for CXCL9 in EM and ACA and of mRNA for CXCL13 in BL, these chemokines and the cells that they attract were studied in skin biopsy samples from five patients each with EM, ACA, or BL, using immunohistochemical techniques. Similar pictures were seen among the patients with EM or ACA and among those with BL. In the patients with EM, infiltrates of CD3+ T cells were visualized in perivascular and periglandular locations throughout the dermis and in the subcutaneous fat, as shown for one representative patient in Fig. 3A and B. Consistent with these findings, CXCL9 was seen in dermal and subcutaneous areas of T-cell accumulation (Fig. 3C). In patients with ACA, denser infiltrates of CD3+ T cells were often seen, and CXCL9 was found in these areas (data not shown). In patients with BL, dense, sometimes clustered perivascular and interstitial or nodular infiltrates of B cells were visualized, as shown for one representative patient in Fig. 3E and F, and intense staining for CXCL13 was seen in these areas (Fig. 3G).
FIG. 3.
Immunohistochemistry of skin biopsy samples from a representative patient with EM (A to D) or BL (E to H). At low power (A), infiltrating CD3+ T cells are seen around sweat glands in the reticular dermis of the EM lesion, and at higher power (B), they are seen in perivascular locations in the papillary dermis. In panel C, with the microscope centered on the same location, staining for CXCL9 is visualized in the area of the T-cell infiltrate, and in panel D, the same area is stained without primary antibody. In the biopsy of the central portion of a BL lesion, intense staining of CD20+ B-cell clusters is seen at low power, particularly within the mid- and deeper dermis (E), and at higher power (F), a single aggregate of B cells is seen. In panel G, with the microscope centered on the same location, staining for CXCL13 is visualized in the area of the B-cell aggregate, and in panel H, the same area is stained without primary antibody. In each section, the specific stain is diaminobenzidine (brown) and the nonspecific background stain is hematoxylin (purple). Magnifications, ×100 (A and E) and ×400 (B to D and F to H).
Cytokine expression.
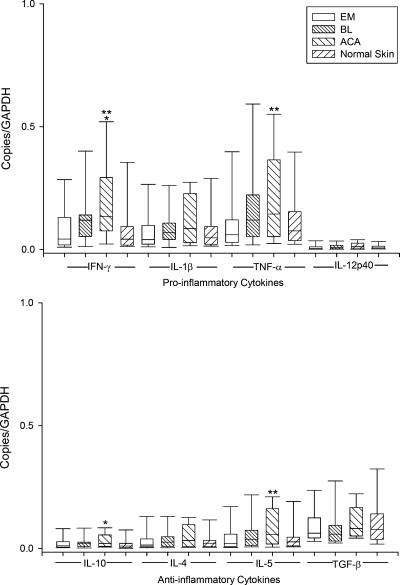
In lesional skin in each of the three skin disorders, mRNA expression of the proinflammatory cytokines IL-1β and TNF-α and the Th1 cytokine IFN-γ was greater than that for the anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) and the Th2 cytokines IL-4 and IL-5, but mRNA levels of the proinflammatory cytokine IL-12p40 were low (Fig. 4). Cytokine mRNA expression was usually lowest and similar to that in normal skin in EM lesions, intermediate in BL, and highest in ACA, which had significantly greater levels of IFN-γ, TNF-α, IL-10, and IL-5 than normal skin or EM lesions.
FIG. 4.
mRNA expression of proinflammatory and anti-inflammatory cytokines in 64 EM lesions, 11 BL lesions, 25 ACA lesions, and 25 normal skin samples relative to the GAPDH gene. Data are shown in box plots in which the boxes represent the 25th and 75th percentiles, the lines within the boxes represent the median values, and the lines outside the boxes represent the 5th and 95th percentiles. A difference between normal and lesional skin (*) or between EM and ACA lesions (**) at the 0.05 level is indicated above the bars. mRNA expression of the proinflammatory cytokines IFN-γ, IL-1β, and TNF-α was greater than of the anti-inflammatory cytokines IL-10, IL-4, IL-5, and TGF-β.
When the cytokine data for the patients with EM, ACA, or BL were stratified by age (quartiles) or sex, no significant differences were found. In addition, the 22 EM patients with extracutaneous signs and symptoms and the 42 patients who lacked such symptoms had no significant differences in cytokine mRNA levels. Finally, the 10 ACA patients with atrophic lesions (present for ≥6 months) had significantly higher mRNA levels of the potentially profibrotic cytokine TGF-β than the 15 patients with early inflammatory lesions (median values, 0.09 versus 0.06 copies/GAPDH; P = 0.05). However, there were no other significant differences between these two groups.
DISCUSSION
In this study, EM and particularly ACA lesions had high mRNA levels of the IFN-γ-inducible CD8+ and Th1-type CD4+ T-cell chemoattractants CXCL9 and CXCL10, intermediate levels of the macrophage chemokine CCL2, and low levels of the B-cell chemokine CXCL13. In contrast, BL lesions had high mRNA levels of CXCL13 and proportionally lower levels of the T-cell chemokines. This chemokine pattern was consistent with leukocyte marker mRNA in lesional skin. Moreover, using immunohistologic techniques, T cells and CXCL9 were visualized directly in EM and ACA, and B cells and CXCL13 were seen in BL. The major purpose of these T- and B-cell responses in LB seems to be to promote antibody production, since opsonization of spirochetes with antibodies results in more effective spirochetal killing (24).
Among the current patients, 77% or more with each skin disorder had B. afzelii infection, about 10% had B. burgdorferi, and the remaining patients had B. garinii or mixed infection. Similar distributions of these three Borrelia species (72% B. afzelii, 15% B. burgdorferi, 6% B. garinii, 5% mixed infection, and 2% Borrelia valaisiana) were found in a survey of Ixodes ricinus ticks from southeastern Austria, where the current patients resided (R. R. Müllegger and M. Glatz, unpublished data). Although B. afzelii is the most common cause of ACA, each of these Borrelia species has now been recovered from ACA lesions (21, 30). Thus, in southeastern Austria, the three skin disorders of LB are primarily but not exclusively associated with B. afzelii infection.
In EM lesions, cytokine protein levels or cytokine mRNA expression have been measured by flow cytometry (33), in situ hybridization (25), and, in the current analysis, by QRT-PCR techniques. QRT-PCR does not distinguish mRNA of infiltrating cells from that of constituent cells, and mRNA expression does not prove that a biologically active protein is produced. However, as shown here for CXCL9 and CXCL13 and as documented in the literature (8), mRNA expression correlates well with actual cytokine and chemokine responses at sites of inflammation. Thus, QRT-PCR has become the method of choice for the quantification and comparison of immune mediators in tissues (17, 41). In the current study, mRNAs for a large number of chemokines, cytokines, and leukocyte markers were measured in skin biopsy samples from 100 patients and 25 control subjects at one time, thereby limiting experimental variables and facilitating the comparison of immune reactants within and among the groups.
Consistent with previous cytokine analyses (25, 33), the current chemokine data provide unequivocal evidence for a dominant Th1 response in lesional skin of each of the three skin disorders of LB. In addition, CD8+ T cells, which presumably serve as a source of IFN-γ, are often more abundant than CD4+ T-helper cells. The major difference in this study compared with past experience concerns ACA. This chronic skin lesion was originally thought to occur predominately in older women (3). However, with more awareness of the disease and better diagnostic testing, it is becoming clear that younger adults, including both men and women, may also develop this lesion (26). In the current study, the age ranges of EM and ACA patients were similar, and the sex ratios in both groups were only 2 to 1 in favor of women. In our previous analysis, ACA lesions had a more restricted cytokine profile than EM lesions, with little or no expression of IFN-γ, as determined by in situ hybridization (25). We hypothesized that this cytokine response may be less effective in spirochetal killing, thereby explaining in part the chronicity of the lesion. However, in the current analysis, similar but higher mRNA expression of chemokines and cytokines, including IFN-γ, was found in ACA compared with EM lesions, and these values did not differ by age or sex. In another study, high levels of IFN-γ were found in the blood of ACA patients (43). Nevertheless, the microenvironment of ACA is primarily acral surfaces of the extremities, where the immune response may be modulated so that complete spirochetal killing is inhibited.
Although BL lesions had inflammatory mediators similar to those of EM and ACA lesions, mRNA expression of the B-cell chemoattractant CXCL13 and the B-cell marker CD19 were dominant in BL. Presumably, this finding relates to the almost exclusive location of such lesions on the ear or the nipple or areola of the breast (4, 26). Inflammatory responses may depend on tissue microenvironments (e.g., stromal cells) and their interactions with infiltrating immune cells (14). Lymphotoxin-α or -β induces the secretion of CXCL13 (19, 28), which is responsible for the formation of lymphoid aggregates with germinal center-like structures in ectopic locations (ectopic lymphoid organogenesis) (6, 14). As shown again here, this morphological characteristic is found in most BL lesions (11). In addition, B-cell aggregates occur in some synovial lesions in patients with Lyme arthritis (1), and the finding of CXCL13 in cerebrospinal fluid in patients with neuroborreliosis suggests that lymphoid aggregates with germinal centers might be found in inflamed meninges (32).
In conclusion, the three skin disorders of European LB had similar chemokine and cytokine signatures, but they differed in the amounts of these inflammatory mediators. Chemoattractants for cells of the innate immune response, neutrophils, dendritic cells, and macrophages, were present in all three skin disorders. However, it was chemoattractants for cells of the adaptive immune response that differed most. EM and ACA lesions had high mRNA expression of the T-cell-active chemokines CXCL9 and CXCL10, whereas BL lesions had especially high levels of CXCL13. In southeastern Austria, where B. afzelii is the primary cause of these infections, the microenvironment of the skin (and perhaps as-yet-unidentified host immune factors) seems to influence these differences. Thus, depending on the site of the infection, B. afzelii or occasionally other B. burgdorferi sensu lato species may cause an acute, self-limited EM lesion particularly in or near intertriginous sites, a subacute BL lesion on the nipple or ear, or a chronic persistent ACA lesion primarily on extensor surfaces of the extremities.
Acknowledgments
We thank Monika Joch and Daniela Oppitz for performing Borrelia serology and Doris Stünzner for performing Anaplasma serology at the Institute of Hygiene, Medical University, Graz, Austria. In addition, we thank Thompson Flotte and Margaret Sherwood at Massachusetts General Hospital in Boston for help with immunochemistry studies.
This work was supported by the National Institutes of Health (grant CA-69212); the Centers for Disease Control and Prevention (grant C1000157); the English, Bonter, Mitchell Foundation; the Eshe Fund; and the Lyme/Arthritis Research Fund at Massachusetts General Hospital. J.J.S. and K.L.J. received scholarships from the Walter J. and Lille A. Berbecker Foundation for the study of Lyme disease and were also supported by rheumatology training grant AR T32-07258 from the National Institutes of Health.
The authors state no conflict of interest.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Akin, E., J. Aversa, and A. C. Steere. 2001. Expression of adhesion molecules in synovia of patients with treatment-resistant Lyme arthritis. Infect. Immun. 69:1774-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthonissen, F. M., M. De-Kesel, P. P. Hoet, and G. H. Bigaignon. 1994. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res. Microbiol. 145:327-331. [DOI] [PubMed] [Google Scholar]
- 3.Åsbrink, E., and A. Hovmark. 1988. Early and late cutaneous manifestations in Ixodes-borne borreliosis (erythema migrans borreliosis, Lyme borreliosis). Ann. N. Y. Acad. Sci. 539:4-15. [DOI] [PubMed] [Google Scholar]
- 4.Åsbrink, E., A. Hovmark, and I. Olsson. 1989. Lymphadenosis benigna cutis solitaria—borrelia lymphocytoma in Sweden. Zentralbl. Bakteriol. 18(Suppl):156-163. [Google Scholar]
- 5.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS 461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 6.Bodolay, E., A. E. Koch, J. Kim, G. Szegedi, and Z. Szekanecz. 2002. Angiogenesis and chemokines in rheumatoid arthritis and other systemic inflammatory rheumatic diseases. J. Cell. Mol. Med. 6:357-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brehmer-Andersson, E., A. Hovmark, and E. Åsbrink. 1998. Acrodermatitis chronica atrophicans: histopathologic findings and clinical correlations in 111 cases. Acta Derm. Venereol. (Stockholm) 78:207-213. [DOI] [PubMed] [Google Scholar]
- 8.Brennan, F. M., R. N. Maini, and M. Feldman. 1995. Cytokine expression in chronic inflammatory disease. Br. Med. Bull. 51:368-384. [DOI] [PubMed] [Google Scholar]
- 9.Brown, C. R., V. A. Balho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlated with KC and monocyte chemoattractant protein-1 in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 10.Burns, M. J., T. J. Sellati, E. I. Teng, and M. B. Furie. 1997. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect. Immun. 65:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colli, C., B. Leinweber, R. R. Müllegger, A. Chott, H. Kerl, and L. Cerroni. 2004. Borrelia burgdorferi-associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J. Cutan. Pathol. 31:232-240. [DOI] [PubMed] [Google Scholar]
- 12.Corcione, A., S. Casazza, E. Ferretti, D. Giunti, E. Zappia, A. Pistorio, C. Gambini, G. L. Mancardi, A. Uccelli, and V. Pistoia. 2004. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 101:11064-11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaerschalck, I., A. Ben Messaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas, M. R., K. E. Morrison, M. Salmon, and C. D. Buckley. 2002. Why does inflammation persist: a dominant role for the stromal microenvironment? Exp. Rev. Mol. Med. 4:1-18. [DOI] [PubMed] [Google Scholar]
- 15.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 158:3285-3292. [PubMed] [Google Scholar]
- 16.Gergel, E. I., and M. B. Furie. 2004. Populations of human T lymphocytes that traverse the vascular endothelium stimulated by Borrelia burgdorferi are enriched with cells that secrete gamma interferon. Infect. Immun. 72:1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giulietti, A., L. Overbergh, D. Valckx, B. Decallonne, R. Bouillon, and C. Mathieu. 2001. An overview of real-time quantitative PCR. Applications to quantify cytokine gene expression. Methods 25:386-401. [DOI] [PubMed] [Google Scholar]
- 18.Grygorczuk, S., S. Pancewicz, J. Zajkowska, M. Kondrusik, R. Rwierzbinska, and T. Hermanowska-Szpakowicz. 2004. Concentrations of macrophage inflammatory proteins MIP-1alpha and MIP-1beta and interleukin 8 (IL-8) in Lyme borreliosis. Infection 32:350-355. [DOI] [PubMed] [Google Scholar]
- 19.Hjelmstrom, P., J. Fjell, T. Nakagawa, R. Sacca, C. A. Cuff, and N. H. Ruddle. 2000. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am. J. Pathol. 156:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohlgruber, N., M. Gröger, P. Meraner, E. Kriehuber, P. Petzelbauer, S. Brandt, G. Stingl, A. Rot, and D. Maurer. 2004. Plasmocytoid dendritic cell recruitment by immobilized CXCR3 ligands. J. Immunol. 173:6592-6602. [DOI] [PubMed] [Google Scholar]
- 21.Lebech, A. M. 2002. Polymerase chain reaction in diagnosis of Borrelia burgdorferi infections and studies on taxonomic classification. APMIS Suppl. 105:1-40. [PubMed] [Google Scholar]
- 22.Lepej, S. Z., O. D. Rode, T. Jeren, A. Vince, A. Remenar, and B. Barsic. 2005. Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J. Neuroimmunol. 163:128-134. [DOI] [PubMed] [Google Scholar]
- 23.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165-5175. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery, R. R., D. Lusitani, C. A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 25.Müllegger, R. R., G. McHugh, R. Ruthazer, B. Binder, H. Kerl, and A. C. Steere. 2000. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Investig. Dermatol. 115:1115-1123. [DOI] [PubMed] [Google Scholar]
- 26.Müllegger, R. R. 2004. Dermatological manifestations of Lyme borreliosis. Eur. J. Dermatol. 14:296-309. [PubMed] [Google Scholar]
- 27.Narayan, K., D. Dail, L. Li, D. Cadavid, S. Amrute, P. Fitzgerald-Bocarsly, and A. R. Pachner. 2005. The nervous system as ectopic germinal center: CXCL13 and IgG in Lyme neuroborreliosis. Ann. Neurol. 57:813-823. [DOI] [PubMed] [Google Scholar]
- 28.Ngo, V. N., H. Korner, M. D. Gunn, K. N. Schmidt, D. S. Riminton, M. D. Cooper, J. L. Browning, J. D. Sedgwick, and J. G. Cyster. 1999. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pashenkov, M., N. Teleshova, M. Kouwenhoven, T. Smirnova, Y. P. Jin, V. Kostulas, Y. M. Huang, B. Pinegin, A. Boiko, and H. Link. 2002. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunol. 122:106-116. [DOI] [PubMed] [Google Scholar]
- 30.Picken, R. N., F. Strle, M. M. Picken, E. Ruzic-Sabljic, V. Maraspin, S. Lotric-Furlan, and J. Cimperman. 1998. Identification of three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) among isolates from acrodermatitis chronica atrophicans lesions. J. Investig. Dermatol. 110:211-214. [DOI] [PubMed] [Google Scholar]
- 31.Rauter, C., R. Oehme, I. Diterich, M. Engele, and T. Hartung. 2002. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J. Clin. Microbiol. 40:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupprecht, T. A., H. W. Pfister, B. Angele, S. Kastenbauer, B. Wilske, and U. Koedel. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65:448-450. [DOI] [PubMed] [Google Scholar]
- 33.Salazar, J. C., C. D. Pope, T. J. Sellati, H. M. Feder Jr., T. G. Kiely, K. R. Dardick, R. L. Buckman, M. W. Moore, M. J. Caimano, J. G. Pope, P. J. Krause, J. D. Radolf, et al. 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171:2660-2670. [DOI] [PubMed] [Google Scholar]
- 34.Salomonsson, S., M. V. Jonsson, K. Skarstein, K. A. Brokstad, P. Hjelmström, M. Wahren-Herlenius, and R. Jonsson. 2003. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 48:3187-3201. [DOI] [PubMed] [Google Scholar]
- 35.Smith, R. P., R. T. Schoen, D. W. Rahn, V. K. Sikand, J. Nowakowski, D. L. Parenti, M. S. Holman, D. H. Persing, and A. C. Steere. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 136:421-428. [DOI] [PubMed] [Google Scholar]
- 36.Sprenger, H., A. Krause, A. Kaufmann, S. Priem, D. Fabian, G. R. Burmester, D. Gemsa, and M. G. Rittig. 1997. Borrelia burgdorferi induces chemokines in human monocytes. Infect. Immun. 65:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanek, G., S. O'Connell, M. Cimmino, E. Aberer, W. Kristoferitsch, M. Granstrom, E. Guy, and J. Gray. 1996. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien. Klin. Wochenschr. 108:741-747. [PubMed] [Google Scholar]
- 38.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 39.Van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 40.Wang, G., A. P. van Dam, L. Spanjaard, and J. Dankert. 1998. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J. Clin. Microbiol. 36:768-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, T., and M. J. Brown. 1999. mRNA quantification by real time TaqMan polymerase chain reaction: validation and comparison with RNase protection. Anal. Biochem. 269:198-201. [DOI] [PubMed] [Google Scholar]
- 42.Weger, W., and R. R. Müllegger. 2001. Histopathology and immunohistochemistry of dermatoborreliosis. Acta Dermatoven. APA 10:135-142. [Google Scholar]
- 43.Widhe, M., S. Jarefors, C. Ekerfelt, M. Vrethem, S. Bergstrom, P. Forsberg, and J. Ernerudh. 2004. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J. Infect. Dis. 189:1881-1891. [DOI] [PubMed] [Google Scholar]