Abstract
Recent studies have suggested an important role for the B-cell-attracting chemokine CXCL13 in the B-cell-dominated cerebrospinal fluid (CSF) infiltrate in patients with neuroborreliosis (NB). High levels of CXCL13 were present in the CSF of NB patients. It has not been clear, however, whether high CSF CXCL13 titers are specific for NB or are a characteristic of other spirochetal diseases as well. Furthermore, the mechanisms leading to the observed CXCL13 expression have not been identified yet. Here we describe similarly elevated CSF CXCL13 levels in patients with neurosyphilis, while pneumococcal meningitis patient CSF do not have high CXCL13 levels. In parallel, challenge of human monocytes in vitro with two of the spirochetal causative organisms, Borrelia garinii (the Borrelia species most frequently found in NB patients) and Treponema pallidum, but not challenge with pneumococci, induced CXCL13 release. This finding implies that a common spirochetal motif is a CXCL13 inducer. Accordingly, we found that the lipid moiety N-palmitoyl-S-(bis[palmitoyloxy]propyl)cystein (Pam3C) (three palmitoyl residues bound to N-terminal cysteine) of the spirochetal lipoproteins is critical for the CXCL13 induction in monocytes. As the Pam3C motif is known to signal via Toll-like receptor 2 (TLR2) and an anti-TLR2 monoclonal antibody blocked CXCL13 production of human monocytes incubated with B. garinii, this suggests that TLR2 is a major mediator of Borrelia-induced secretion of CXCL13 from human monocytes.
Spirochetes are a group of bacteria that can be distinguished morphologically from other bacteria based on the fact that they are thin, long, and helical or corkscrew shaped. While in the northern hemisphere Borrelia burgdorferi is the predominant spirochete responsible for neuroinfectious diseases, infections of the central nervous system (CNS) with Treponema pallidum are endemic in regions all over the world. Moreover, a dramatic increase in the incidence of syphilis has been noted in several countries (9). In contrast to their high prevalence, the pathogenesis of neuroborreliosis (NB) and neurosyphilis (NS) is not yet well understood. After crossing the blood-brain barrier, both spirochetes elicit an inflammatory response in the cerebrospinal fluid (CSF), predominantly mediated by mononuclear cells. This contrasts with the massive invasion of polymorphonuclear cells into the CSF of patients with pneumococcal meningitis (PM), the most frequent bacterial infection of the adult CNS.
It is assumed that the infiltration and the composition of the immunocompetent cells in the CSF are mainly the result of the intrathecal production of chemokines. Besides being grouped according to common motifs in their amino acid sequences, chemokines can be subdivided according to the cell populations that they predominantly attract. B lymphocytes, in contrast to other leukocytes, show substantial migration in response to only a very few chemokines, namely, CCL19, CCL21, CXCL12, and CXCL13 (3). Since the proportion of B lymphocytes in the CSF infiltrate in NB patients is higher than the proportion resulting from any other CNS infection (6), the chemokines listed above might play an important role in NB-induced inflammatory CNS reactions.
Recently, we demonstrated presence of CXCL13 in the CSF of patients with NB at high levels (19). Until now, the degree of specificity of increased CXCL13 CSF levels for borrelial infection compared to other spirochetal diseases was unknown. Since the two spirochetes (Borrelia and Treponema) share production of specific membrane components eliciting immune responses (18), we (i) examined the CXCL13 concentrations in paired CSF and serum samples from NS patients and compared these concentrations with those measured in samples from NB and PM patients and (ii) analyzed the CXCL13 secretion of human monocytes challenged with the respective bacteria (Borrelia garinii, T. pallidum, and Streptococcus pneumoniae) in vitro in a comparative manner.
MATERIALS AND METHODS
Reagents.
Synthetic N-palmitoyl-S-(bis[palmitoyloxy]propyl)cysteinyl-seryl-(lysyl)3-lysine (Pam3CSK4), outer surface protein A (OspA) (KQNVSSLDEKNSVSV), and OspA carrying a tripalmitoylation (lipidated OspA; Pam3C-KQNVSSLDEKNSVSV) from Borrelia garinii strain PBi were purchased from EMC micro collections, Tuebingen, Germany, and lipopolysaccharide (LPS) (Escherichia coli) was purchased from Sigma, Deisenhofen, Germany. The human-murine cross-reactive Toll-like receptor 2 (TLR2) monoclonal antibody T2.5 (immunoglobulin G1) was isolated as described previously (15).
Bacteria.
B. garinii strain PBi was isolated from the CSF of a patient with lymphocytic meningoradiculitis (24). Low-passage borreliae were killed by ultrasound sonication. T. pallidum Nichols strain was generously provided by BAG (Lich, Germany). S. pneumoniae ATCC 33400 was a gift from B. Grabein, Max von Pettenkofer Institute, Munich, Germany. Both the treponema and the streptococcus were killed by resuspension of a pellet of viable bacteria after washing in 70% ethanol. All bacteria were washed twice in medium prior to use in cell experiments. To exclude any influence of the killing method, alternative bacterial preparations of B. burgdorferi were generated by sonication, which led to equivalent induction of CXCL13 release from monocytes compared to challenge with ethanol-killed B. burgdorferi (data not shown). The number of bacteria is expressed below as the multiplicity of infection (MOI); an MOI of 2 was equivalent to 3,000 bacteria/μl, an MOI of 0.2 was equivalent to 300 bacteria/μl, and an MOI of 0.02 was equivalent to 30 bacteria/μl.
CSF samples.
Blood was drawn and lumbar puncture was performed for diagnostic purposes after the patient's informed consent was obtained. Paired CSF and blood samples were obtained from the following groups. (i) The first group consisted of 20 patients with noninflammatory CNS disease (NIND); 65% of these patients were male, the mean age was 61 years, the mean CSF cell count was 0.8 cells/μl, and the mean total CSF protein level was 5.2 g/liter. Five of these patients suffered from polyneuropathy, four suffered from somatoformic disorders, three suffered from a peripheral cranial neuritis, two had a lumbar puncture because of a first epileptic seizure, and the remaining patients suffered from either a transient paraparesis or Guillain-Barré syndrome, Parkinson's disease, leucoencephalopathy, amyotrophic lateral sclerosis, or low CSF pressure. In none of these patients was an elevated CSF cell count or spirochete-specific intrathecal antibody detected. (ii) The second group consisted of 19 acute NB patients; 58% of these patients were male, the mean age was 55 years, the mean CSF cell count was 176.1 cells/μl, and the mean total CSF protein level was 13.2 g/liter. The diagnosis was based on a typical clinical picture (meningoradiculitis, meningitis, or cranial neuritis), CSF lymphocytic pleocytosis (more than 5 leukocytes per μl), and intrathecal B. burgdorferi-specific antibody production. All CSF samples were drawn before therapy. (iii) The third group consisted of 15 NS patients; 87% of these patients were male, the mean age was 31.3 years, the mean CSF cell count was 16.9 cells/μl, and the mean total CSF protein level was 3.3 g/liter. The diagnosis was based on serological proof of a syphilitic infection (positive Treponema particle agglutination and fluorescent treponemal antibody-absorbed tests) and an elevated CSF white blood cell count according to the criteria described by Marra et al. (14). (iv) The fourth group consisted of six patients with syphilis without CNS involvement; all of these patients were male, the mean age was 32.2 years, the mean CSF cell count was 1.8 cells/μl, and the mean total CSF protein level was 3.2 g/liter. These patients had serological proof of a syphilitic infection as described above but a normal CSF white blood cell count. All patients with syphilis were human immunodeficiency virus negative. (v) The fifth group consisted of 10 PM patients; 50% of these patients were male, the mean age was 49.7 years, the mean CSF cell count was 3,745.1 cells/μl, and the mean total CSF protein level was 39.5g protein/liter. The diagnosis was based on cultivation of S. pneumoniae from the CSF.
The CSF and blood samples were removed and frozen at −30°C until they were assayed. Measurement of CXCL13 in the CSF and serum was done by an enzyme-linked immunosorbent assay (ELISA) (R&D) performed according to the recommendations of the manufacturer. The CXCL13 levels measured in the CSF were expressed in relation to the amount of total CSF protein to account for the disruption of the blood-brain barrier and therefore the influx of cytokines from the systemic circulation.
Human monocytes.
Human peripheral blood mononuclear cells were isolated from whole blood obtained from a healthy adult volunteer by venous puncture as previously described (13). The monocytes in isolated mononuclear cells were enriched with a magnetic bead application system (Miltenyi-Biotech, Bergisch-Gladbach, Germany) used according to the recommendations of the manufacturer, leading to a purity of up to >90%.
Incubation.
After purification, the monocytes were resuspended in RPMI containing 5% fetal calf serum (both obtained from Sigma), plated in a plastic dish for 24 h, and incubated at 37°C with 5% CO2. Thereafter, monocytes were pelleted again and incubated in 96-well plates at a concentration of 150,000 cells/well with either the medium alone or medium and the appropriate stimulating agent for 24 h at 37°C with 5% CO2. For inhibition experiments, the cells were preincubated with TLR2 antibody or an isotype control (R&D, Minneapolis, MN) at a concentration of 12.5 μg/ml at 37°C with 5% CO2 for 30 min. Finally, the supernatant was collected and its CXCL13 concentration was determined by an ELISA (R&D). For experiments in which cells were exposed to bacteria, the viability of monocytes was analyzed by trypan blue staining, and no significant differences between the cells incubated with B. burgdorferi, the cells incubated with T. pallidum, and the cells incubated with S. pneumoniae were apparent; the mean viabilities (based on the total number of cells counted) were 78%, 75%, and 81%, respectively.
Murine cells.
Bone marrow-derived macrophages from C3H/HeN mice were prepared as described previously (25). Briefly, macrophages were recovered from bone marrow cells, plated in 96-well dishes (150,000 cells/well), and incubated with either medium alone, B. garinii (MOI, 0.02 to 2), T. pallidum (MOI, 0.04 to 4), 1 μg/ml Pam3CSK4, 100 to 1,000 ng/ml lipidated OspA, or 1 μg/ml LPS for 24 h.
The murine macrophage cell line RAW 264.7 was cultured in RPMI 1640 supplemented with 5% fetal calf serum and 1% penicillin/streptomycin. For the experiments, the cells were plated in 96-well dishes (160,000 cells/well) and incubated with either medium alone, B. garinii at an MOI of 0.6 or 6, or 1 μg/ml LPS for 24 h.
Whole blood samples were isolated from C57BL/6 mice by transcardial puncture, resuspended 1:2 in RPMI 1640, plated in 96-well plates (100 μl/well), and incubated for 24 h with either medium alone, 10 to 1,000 B. garinii cell/μl, or 1 pg/ml, 1 ng/ml, or 1 μg/ml LPS.
All supernatants were stored at −20°C until they were assayed (by an interleukin-6 [IL-6] or CXCL13 ELISA [R&D]).
Statistics.
Statistical calculation was performed by application of the Student t test. For a comparison of two groups (see Fig. 2, 4, and 5), a P value of <0.05 was considered statistically significant, while for a comparison of four groups (see Fig. 1 and 3), according to an alpha-correction for multiple testing, a P value of <0.017 was considered statistically significant.
FIG. 2.
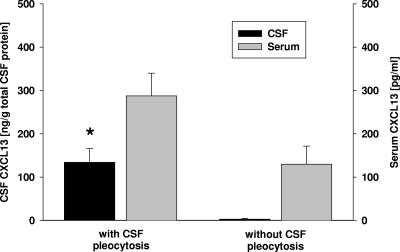
CSF (black bars) and serum (gray bars) concentrations of CXCL13 in patients with a syphilis infection, either with (n = 15) or without (n = 6) CSF pleocytosis. The asterisk indicates that the P value is ≤0.001 for a comparison with patients without CSF pleocytosis. The CXCL13 values for the CSF were expressed in relation to the amount of total CSF protein to account for the disruption of the blood-brain barrier. All error bars indicate the standard error of the mean.
FIG. 4.
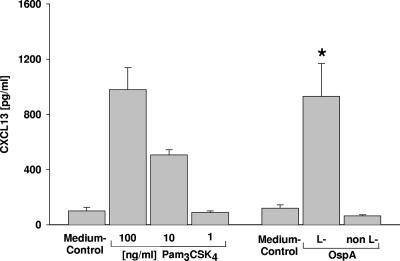
CXCL13 concentrations in the supernatants of monocytes incubated with different concentrations of Pam3CSK4 or 1 μg/ml lipidated (L-) or unlipidated (non L-) OspA. The asterisk indicates that the P value is <0.05 for comparisons with lipidated OspA and the medium control.
FIG. 5.
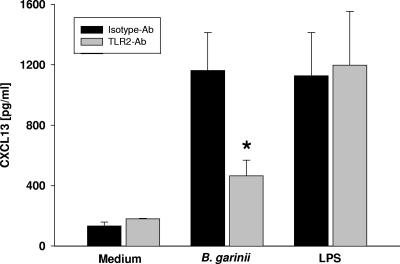
CXCL13 concentrations in the supernatants of monocytes preincubated either with a neutralizing TLR2 antibody (Ab) (gray bars) or with the corresponding isotype control (black bars) (both at 12.5 μg/ml) and then for 24 h with either medium, B. garinii (MOI, 2), or LPS (1 ng/ml). The asterisk indicates that the P value is <0.05 for a comparison with the isotype control. The bars indicate the means of at least three independent experiments done in duplicate, and the error bars indicate the standard errors of the means of the independent experiments.
FIG. 1.
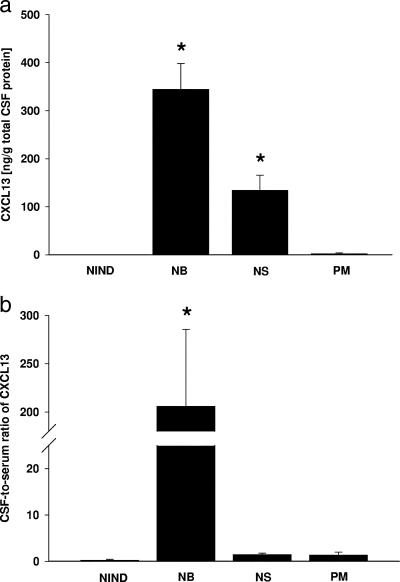
(a) CXCL13 concentrations measured in the CSF of patients with NIND (n = 20), Lyme NB (n = 19), NS (n = 15), and PM (n = 10). An asterisk indicates that the P value is ≤0.001 for a comparison with NIND and PM patients. The P value for NB compared to NS is <0.01. The CXCL13 values for the CSF were expressed in relation to the amount of total CSF protein to account for the disruption of the blood-brain barrier. (b) CSF-to-serum ratios calculated by dividing the CXCL13 concentration (pg/ml) in the CSF by the serum concentration for each patient. The asterisk indicates that the P value is ≤0.001 for comparisons with all other patient groups. All error bars indicate the standard error of the mean.
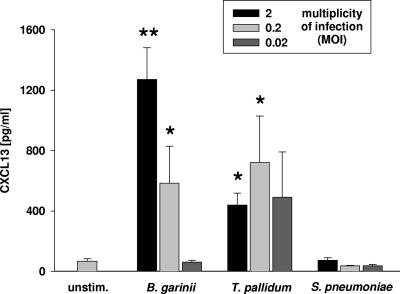
FIG. 3.
CXCL13 concentrations in the supernatants of monocytes incubated with bacteria (B. garinii, T. pallidum, or S. pneumoniae) in vitro. The bars indicate the means of three independent experiments done in duplicate, and the error bars indicate the standard errors of the means of the independent experiments. Two asterisks indicate that the P value is ≤0.01 for comparisons with the unstimulated control (unstim.) and equal concentrations of T. pallidum and S. pneumoniae; one asterisk indicates that the P value is ≤0.01 for comparisons with the unstimulated control and an equal concentration of S. pneumoniae.
RESULTS
CXCL13 levels in vivo.
First, we compared CXCL13 concentrations in paired CSF and serum samples. The level of CXCL13 was elevated not only in NB patient CSF samples but also in NS patient CSF samples. The concentrations observed for samples from patients suffering from one of these two spirochetal diseases were significantly higher than those observed for the NIND and PM group samples, in which hardly any CXCL13 was detectable (Fig. 1a). The CSF and serum CXCL13 concentrations for individual patient samples were compared by determining the CSF-to-serum ratios of the values. For NB patient samples, but not for NS patient samples, the ratio was high (Fig. 1b). The mean serum levels ± standard deviations of CXCL13 were 25.3 ± 15.2 pg/ml in NIND patients, 58.1 ± 47.5 pg/ml in NB patients, 287.5 ± 203.2 pg/ml in NS patients, and 194.3 ± 356.5 pg/ml in PM patients.
In patients infected with T. pallidum without CNS involvement, the CSF levels of CXCL13 were significantly lower than those in the NS group (Fig. 2); they were close to the detection threshold, similar to what was found for the NIND group.
Taken together, the CXCL13 levels in the CSF of patients with the two spirochetal diseases were significantly higher than the levels in both the PM and NIND groups. The predominant intrathecal production of CXCL13 in NB patients, as well as the correlation of the CSF CXCL13 values with the CSF leukocyte counts in both NS and NB patients (for NB patients r = 0.54 with a P value of <0.01 and for NS patients r = 0.57 with a P value of <0.01), may argue for a functional role for this chemokine.
Induction of CXCL13 in monocytes by different bacteria in vitro.
Human monocytes were incubated with B. garinii strain PBi (the borrelial species most frequently found in cases of NB [23]), T. pallidum, and S. pneumoniae. Incubation with the spirochetes, but not incubation with S. pneumoniae, led to significant secretion of CXCL13 (Fig. 3), showing a pattern comparable to that in vivo (Fig. 1a). While S. pneumoniae was unable to induce CXCL13, the (general) responsiveness of the human monocytes to S. pneumoniae was shown by significant secretion of another cytokine, IL-6 (there was a 4.6-fold increase in the IL-6 concentration in the supernatant after incubation with S. pneumoniae at an MOI of 2 compared to the medium control [P < 0.001]).
Incubation with another common borrelial species found in patients with NB, Borrelia afzelii (23), also led to dose-dependent secretion of CXCL13 in monocytes; 421.9, 347.4, and 70.3 pg/ml were measured in the supernatant after incubation with B. afzelii at MOIs of 2, 0.2, and 0.02, respectively.
CXCL13 secretion in monocytes depends on the lipid moiety of the outer surface proteins.
Since different spirochetes induced CXCL13 secretion from monocytes, we analyzed the response of monocytes to confrontation with the Pam3C motif N-terminally linked to peptides. This motif, consisting of three palmitoyl residues bound to the N-terminal cysteine (Pam3C), is a typical domain of the surface lipoproteins of all spirochetes. Incubation with Pam3CSK4 (SK4 serves as a short peptidic C terminus aiding in the stimulatory capability [2] and solubility of the compound) led to dose-dependent stimulation, resulting in CXCL13 release from monocytes (Fig. 4). In addition, incubation with lipidated (Pam3C-containing) OspA protein from PBi, the B. garinii strain used for the experiments described above, led to induction of CXCL13 secretion from monocytes at high levels, while application of the unlipidated form (unlipidated OspA) had no effect compared to the medium control (Fig. 4). These results suggest that the CXCL13 secretion of monocytes in response to spirochetes is, at least in part, mediated by the tripalmitoyl moiety at the N-terminal cysteine of spirochetal lipoproteins.
TLR2 mediates cell activation upon Borrelia infection, leading to CXCL13 release.
Since Pam3C peptides stimulate CXCL13 production in monocytes and are known to signal via TLR2 (2, 5, 11, 12, 17), we investigated the Borrelia-induced CXCL13 release from human monocytes with respect to its TLR2 dependence. We used a neutralizing TLR2 antibody (15) to block TLR2-mediated cell activation. While coincubation with the TLR2 monoclonal antibody significantly reduced CXCL13 production by human monocytes exposed to B. garinii (compared to cells coincubated with an isotype control), the TLR2 antibody did not interfere with TLR4-dependent cell activation by LPS (Fig. 5). In addition, a dose-dependent inhibitory effect of this TLR2 antibody was demonstrated: 12.5 μg/ml of the antibody reduced the CXCL13 secretion to 108 ± 23 pg/ml, 1.25 μg/ml of the antibody reduced the CXCL13 secretion to 218 ± 24 pg/ml, and 0.125 μg/ml of the antibody reduced the CXCL13 secretion to 1,019 ± 69 pg/ml (means ± standard deviations; experiments were done in triplicate). This finding suggests that TLR2 is critical for induction of CXCL13 upon challenge with B. garinii.
In additional experiments, we tested whether murine cells (bone marrow-derived macrophages, the murine macrophage cell line RAW 264.7, and whole blood cells) also produce CXCL13 upon challenge with B. garinii, T. pallidum, Pam3CSK4, lipidated OspA, or LPS. However, none of these microbial stimuli caused an increase in CXCL13 concentrations in murine cell culture supernatants, while detection of significant IL-6 secretion indicated the responsiveness of murine cells to all these stimuli. For example, incubation of RAW 264.7 cells with B. garinii at an MOI of 0.6 for 24 h led to a substantial increase in IL-6 production (606 ± 72 pg/ml versus <5 pg/ml after incubation with medium alone), whereas the CXCL13 concentration remained below the detection threshold. These data suggest that the lack of CXCL13 production by murine macrophages exposed to spirochetes or their products is not related to unresponsiveness of these cells to the pathogen.
DISCUSSION
The major findings of our study are that (i) high levels of CXCL13 can be found in the CSF of patients with the two most common spirochetal CNS diseases, NB and NS, (ii) incubation of monocytes with both spirochetes in vitro leads to release of CXCL13 into the supernatant at high levels, and (iii) both the common Pam3C motif of the spirochetal lipoproteins and its host receptor, TLR2, are crucial for elicitation of CXCL13 production by human monocytes.
In a recent study, we suggested that CXCL13 could be an additional diagnostic CSF marker for NB since high levels of this chemokine have been found in CSF samples from NB patients but not in pooled samples from other inflammatory CNS disease patients investigated (19). However, in this previous study, NS patients were not included, and only limited information was available on the serum levels in these patients. The analysis of CXCL13 levels in NS patients is of special importance, as it is not known whether the induction of this chemokine is specific for NB or can also be found in other spirochetal diseases. Comparison of CSF and serum values for CXCL13 concentrations for sample pairs derived from specific patients led to two important findings of this study. First, as observed for NB patient samples, the CSF levels of CXCL13 were substantially elevated in NS patients but not in PM patients. This hinted at a common property of the spirochetes for induction of CXCL13 (which was confirmed by the in vitro studies). Second, the fact that elevated CSF levels of CXCL13 were found in both spirochetal diseases does not limit the diagnostic role of CXCL13 for NB, as the serum levels were substantially elevated in NS patients but not in NB patients. Therefore, the CSF-to-serum ratio of this chemokine can differentiate between the two spirochetal diseases; while all 15 NS patients had a ratio below 4, the ratios for 17 of the 19 (89.5%) NB patients were above this value.
It is tempting to speculate that the observed difference in the CSF-to-serum ratio in the two spirochetal diseases can be explained by the predominant hematogenous spread of T. pallidum and therefore a comparable systemic and intrathecal inflammatory reaction, while there are two alternatives to explain high CSF but low serum levels in NB patient samples. First, immune evasion of Borrelia by both temporal and spatial regulation of outer surface proteins or binding of immunoinhibitory proteins to its surface is apparent during bacterial dissemination (for an overview, see reference 4). Second, migration of Borrelia using structures other than the blood or lymph vessels and less contact with the systemic circulation might be another explanation for low blood but high CSF CXCL13 levels in NB patients. In particular, in cases of polyradiculitis (Bannwarth's syndrome), the maximal radicular involvement was often linked to the location of the tick bite. However, additional studies are to be undertaken for clarification. The prominent gradient of CXCL13 between the CSF and the serum in NB patients could play an important role in the attraction of B lymphocytes to the infected brain as, in contrast to other infectious diseases of the CNS, B lymphocytes are a major subtype of the white blood cell infiltrate in the CSF of NB patients (6). Further functional studies are warranted to determine the role of this chemokine in attraction of B lymphocytes to the CSF in vivo.
Parallel to the elevated CSF CXCL13 levels in both spirochetal diseases in vivo, we observed secretion of CXCL13 by monocytes in response to spirochetes but not in response to S. pneumoniae in vitro. This is in line with the finding of Narayan et al. (16) that B. burgdorferi sensu stricto can induce this chemokine in monocytes. Since different strains of Borrelia (B. burgdorferi sensu stricto and, in our case, B. garinii and B. afzelii) and another spirochete, T. pallidum, but not S. pneumoniae, induce CXCL13 release, this further supports the hypothesis that a common spirochetal component is the stimulating agent. While spirochetes lack LPS (21), the membranes of all spirochetes contain various lipoproteins that share a common, immunodominant membrane anchor moiety, namely, three palmitoyl residues bound to cysteine (Pam3C). It has been shown in various studies that nonlipidated variants of the original lipoproteins lack stimulating activity (7, 8, 22). Accordingly, both Pam3CSK4 and the lipidated form of OspA type 4, the most frequently detected OspA type in neuroinfectious diseases in Europe (23), but not its nonlipidated counterpart, lead to secretion of large amounts of CXCL13 from monocytes. Taken together, the results show that the lipid moiety of lipoproteins is critical for CXCL13 induction in monocytes.
As lipoproteins and their Pam3C motif are known ligands of TLR2 (2, 5, 11, 12, 17), we further analyzed the role of this cellular lipopeptide detector for secretion of CXCL13. Using a neutralizing TLR2 antibody (15), we could substantially reduce the CXCL13 release upon exposure of monocytes to B. garinii. This is in line with several studies that demonstrated that Pam3CSK4 and B. burgdorferi sensu stricto induced leukocyte activation predominantly via TLR2 (1, 5, 12, 25).
However, it is of note that, in contrast to their human counterparts, murine monocytes and macrophages failed to produce CXCL13 upon challenge with various bacterial stimuli. Substantial CXCL13 secretion in these cells could not be induced by different concentrations of B. garinii, T. pallidum, lipidated OspA, Pam3CSK4, or LPS (even with concentrations of the stimuli up to 100-fold higher than the concentrations in the experiments with human monocytes), while all these stimuli induced IL-6 production by murine cells, thus indicating the responsiveness of the cells to the stimuli.
This suggests that the immune reaction of murine blood cells in response to spirochetes with respect to CXCL13 secretion appears to be different from that observed in human monocytes. Although human and mouse TLR2 share 70% amino acid identity as determined by comparative amino acid sequence analysis, murine TLR2 recognizes specific peptides in a species-specific manner (10). The fact that Borrelia specifically activates human cells but not mouse cells may be due to differences in the TLR2 molecules themselves or due to a contribution of a human non-TLR cofactor.
It is well known that TLR2 also recognizes diverse microbial products other than spirochetal lipoproteins. For example, lipoteichoic acid of S. pneumoniae was reported to activate immune cells via TLR2 (20). Surprisingly, we did not observe any CXCL13 secretion after incubation of human monocytes with S. pneumoniae, and the level of this chemokine was not elevated in the CSF of patients with pneumococcal meningitis either. Therefore, although our results suggest that activation of TLR2 is required for the induction of CXCL13 by spirochetal lipoproteins, it appears that additional, as-yet-unidentified pathways (that are activated by B. garinii and its lipoproteins but not by S. pneumoniae) are necessary to induce this chemokine. This would have to be clarified in further studies.
In conclusion, our results suggest that the activation of TLR2 on human monocytes through B. garinii, the most frequent neuroinvasive Borrelia species, is an important step in the induction of CXCL13 secretion. Since both T. pallidum and B. garinii produce surface proteins containing the Pam3C motif, these lipoproteins might be the central inducers of CXCL13 expression, and their presence in the CSF could explain the high CSF CXCL13 levels in both NS and NB patients.
Acknowledgments
We thank Beatrice Grabein for supplying bacteria and Barbara Angele for her assistance with and performance of additional experiments.
This study was supported by a grant from the Foerderprogramm Forschung und Lehre of Ludwig-Maximilians University, Munich.
We do not have a commercial or other association that might pose a conflict of interest.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A., R.-B. Yang, M. Mark, S. Suggett, B. Devaux, J. Radolf, G. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Brandes, M., D. F. Legler, B. Spoerri, P. Schaerli, and B. Moser. 2000. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 12:1285-1292. [DOI] [PubMed] [Google Scholar]
- 4.Bubeck-Martinez, S. 2005. Immune evasion of the Lyme disease spirochetes. Front. Biosci. 10:873-878. [DOI] [PubMed] [Google Scholar]
- 5.Cabral, E. S., H. Gelderblom, R. L. Hornung, P. J. Munson, R. Martin, and A. R. Marques. 2006. Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J. Infect. Dis. 193:849-859. [DOI] [PubMed] [Google Scholar]
- 6.Cepok, S., B. Rosche, V. Grummel, F. Vogel, D. Zhou, J. Sayn, N. Sommer, H. P. Hartung, and B. Hemmer. 2005. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 128:1667-1676. [DOI] [PubMed] [Google Scholar]
- 7.Erdile, L. F., M. A. Brandt, D. J. Warakomski, G. J. Westrack, A. Sadziene, A. G. Barbour, and J. P. Mays. 1993. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden, M. R., C. M. Marra, and K. K. Holmes. 2003. Update on syphilis: resurgence of an old problem. JAMA 290:1510-1514. [DOI] [PubMed] [Google Scholar]
- 10.Grabiec, A., G. Meng, S. Fichte, W. Bessler, H. Wagner, and C. J. Kirschning. 2004. Human but not murine Toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J. Biol. Chem. 279:48004-48012. [DOI] [PubMed] [Google Scholar]
- 11.Hertz, C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. Weis, R. Wooten, and J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 13.Koedel, U., B. Kohleisen, B. Sporer, F. Lahrtz, V. Ovod, A. Fontana, V. Erfle, and H. W. Pfister. 1999. HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system. J. Immunol. 163:1237-1245. [PubMed] [Google Scholar]
- 14.Marra, C. M., C. L. Maxwell, S. L. Smith, S. A. Lukehart, A. M. Rompalo, M. Eaton, B. P. Stoner, M. Augenbraun, D. E. Barker, J. J. Corbett, M. Zajackowski, C. Raines, J. Nerad, R. Kee, and S. H. Barnett. 2004. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J. Infect. Dis. 189:369-376. [DOI] [PubMed] [Google Scholar]
- 15.Meng, G., M. Rutz, M. Schiemann, J. Metzger, A. Grabiec, R. Schwandner, P. B. Luppa, F. Ebel, D. H. Busch, S. Bauer, H. Wagner, and C. J. Kirschning. 2004. Antagonistic antibody prevents Toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Investig. 113:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayan, K., D. Dail, L. Li, D. Cadavid, S. Amrute, P. Fitzgerald-Bocarsly, and A. R. Pachner. 2005. The nervous system as ectopic germinal center: CXCL13 and IgG in Lyme neuroborreliosis. Ann. Neurol. 57:813-823. [DOI] [PubMed] [Google Scholar]
- 17.Parker, L. C., M. K. Whyte, S. N. Vogel, S. K. Dower, and I. Sabroe. 2004. Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J. Immunol. 172:4977-4986. [DOI] [PubMed] [Google Scholar]
- 18.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 19.Rupprecht, T. A., H. W. Pfister, B. Angele, S. Kastenbauer, B. Wilske, and U. Koedel. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65:448-450. [DOI] [PubMed] [Google Scholar]
- 20.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 21.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 22.Weis, J. J., Y. Ma, and L. F. Erdile. 1994. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 62:4632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilske, B., U. Busch, H. Eiffert, V. Fingerle, H. W. Pfister, D. Rossler, and V. Preac-Mursic. 1996. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med. Microbiol. Immunol. 184:195-201. [DOI] [PubMed] [Google Scholar]
- 24.Wilske, B., V. Preac-Mursic, and G. Schierz. 1985. Antigenic heterogeneity of European Borrelia burgdorferi strains isolated from patients and ticks. Lancet i:1099. [DOI] [PubMed] [Google Scholar]
- 25.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]