Abstract
Burkholderia pseudomallei is a facultative intracellular gram-negative bacterium that can survive and multiply inside macrophages. One of the mechanisms by which B. pseudomallei escapes macrophage killing is by interfering with the expression of inducible nitric oxide synthase (iNOS). However, the bacterial components that modulate antimicrobial activity of the macrophage have not been fully elucidated. In the present study, we demonstrated that B. pseudomallei strain SRM117, a lipopolysaccharide (LPS) mutant that lacks the O-antigenic polysaccharide moiety, was more susceptible to macrophage killing during the early phase of infection than the parental wild-type strain (1026b). Unlike the wild type, the LPS mutant could readily stimulate Y701-STAT-1 phosphorylation (pY701-STAT-1) and interferon-regulatory factor 1 (IRF-1) expression, both of which are essential transcription factors of iNOS. Neutralizing antibody against beta interferon was able to inhibit the phosphorylation of Y701-STAT-1 and the expression of IRF-1 and iNOS, all of which resulted in an increased rate of intracellular replication. These data suggest that the O-antigenic polysaccharide moiety of B. pseudomallei modulates the host cell response, which in turn controls the intracellular fate of B. pseudomallei inside macrophages.
Melioidosis is a serious disease caused by Burkholderia pseudomallei and is most commonly seen in Southeast Asia and Northern Australia (10, 33). Clinical manifestations vary from acute, lethal sepsis and chronic, localized abscess formation to latent and asymptomatic infection (10). The mortality rate in the acute cases exceeds 40%, with 10% to 15% of the survivors experiencing a relapse despite prolonged antibiotic treatment (32). The mechanisms of antibiotic resistance of B. pseudomallei are limited, and currently there is no vaccine against human melioidosis. Interest in the pathogenesis of melioidosis has increased following the classification of B. pseudomallei as a category B bioterrorism agent by the U.S. Centers for Disease Control and Prevention.
B. pseudomallei is able to survive and multiply inside both phagocytic and nonphagocytic cells (19). After internalization, the bacterium can escape from a membrane-bound phagosome into the cytoplasm (19). The internalized bacteria can induce cell-to-cell fusion, resulting in multinucleated giant cell formation (16, 22). This unique phenomenon, which has never been reported for any other bacterium, may facilitate the spreading of the bacterium from one cell to another (22).
The mechanism by which B. pseudomallei escapes the innate immune response, particularly macrophage killing, is not fully understood. It has been demonstrated that B. pseudomallei is resistant to serum bactericidal activity even though the alternative complement pathway is activated (13, 18). Furthermore, we previously demonstrated that B. pseudomallei failed to activate inducible nitric oxide synthase (iNOS) expression (30). The failure to stimulate iNOS expression may result from its inability to activate beta interferon (IFN-β) production, thus leading to reduced Y701-STAT-1 phosphorylation and IFN-regulatory factor 1 (IRF-1) and iNOS expression (14, 29). Addition of exogenous IFN-β could restore the ability of macrophages to activate iNOS expression, thus resulting in enhanced killing of intracellular B. pseudomallei (28). These results not only demonstrate that the macrophage signaling pathway for IFN-β production is essential for controlling the fate of intracellular B. pseudomallei but also suggest that the bacterium has the ability to modulate the macrophage antibacterial response.
B. pseudomallei has been reported to possess several potential surface-associated components, e.g., lipopolysaccharide (LPS), capsular polysaccharide, and flagella, that are associated with its pathogenicity (6, 7, 11, 24). Among these, LPS has been most intensively investigated. LPSs from B. pseudomallei strains isolated from a variety of sources are rather homogenous, and melioidosis patient sera contain antibody reactive to the LPSs from many different isolates (1, 8, 17). The level of antibody to LPS on admission to hospital was higher in patients with melioidosis who survived than in those who died and was higher in patients with nonsepticemic than in those with septicemic melioidosis (9). The significant role of LPS has also been demonstrated in several experimental in vitro studies (24, 31). A B. pseudomallei mutant deficient in O-antigenic polysaccharide production is more susceptible to serum bactericidal activity, particularly via the alternative complement pathway (11, 34). In the present study, we demonstrated that this LPS mutant of B. pseudomallei is more susceptible to macrophage killing than its wild-type parental strain. However, unlike the wild type, the LPS mutant can readily activate iNOS expression through the IFN-β production which could in turn determine its intracellular fate.
MATERIALS AND METHODS
Cell line and culture conditions.
The mouse macrophage cell line RAW 264.7 was originally obtained from the American Type Culture Collection (Manassas, VA). If not indicated otherwise, the cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco Laboratories, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT) at 37°C in a 5% CO2 atmosphere.
Bacterial strains.
The B. pseudomallei LPS mutant SRM117 used in this study was created from its wild-type parental strain 1026b as described previously (11). This mutant strain contains a Tn5-OT182 integration in the wbiI gene which does not have a polar effect on orf1, the gene immediately downstream of wbiI, because it is transcribed convergently with respect to wbiI, and orf1 is not required for LPS biosynthesis (11). The expression of capsular polysaccharide and LPS of the bacteria was determined by immunoblotting with antibody specific for capsular polysaccharide and LPS of B. pseudomallei, respectively (1, 2). The antibody against the 200-kDa surface polysaccharide was able to react with whole-cell lysates of both the LPS mutant and the wild-type B. pseudomallei. However, only the whole-cell lysate of the wild-type B. pseudomallei reacted with the antibody against LPS. These data indicated that the LPS mutant of B. pseudomallei used in this study, while lacking O-antigenic polysaccharide, still possesses capsular polysaccharide.
Infection of mouse macrophage cell line RAW 264.7.
An overnight culture of mouse macrophages (1 × 106 cells) in a six-well plate was cocultured with bacteria at a multiplicity of infection (MOI) of 2:1 for 1 h. To remove extracellular bacteria, the infected cell monolayers were washed three times with 2 ml of phosphate-buffered saline and residual bacteria were killed by incubation in Dulbecco's modified Eagle's medium containing 250 μg/ml kanamycin (Gibco Laboratories) for 2 h. Thereafter, the infected cells were incubated in medium containing 20 μg/ml of kanamycin until the experiment was terminated. Viability of the infected macrophages was determined by trypan blue dye staining and was found to be higher than 90% throughout the time course employed in this study.
To determine intracellular survival and multiplication of the bacteria, a standard antibiotic protection assay was performed as previously described (22). The intracellular bacteria were liberated by lysing the macrophages with 0.1% Triton X-100, and the released bacteria were serially plated on tryptic soy agar. The number of intracellular bacteria, expressed as CFU, was determined by bacterial colony counting.
Immunoblotting.
Mouse macrophage preparations were lysed in buffer containing 20 mM Tris, 100 mM NaCl, and 1% NP-40. The lysates containing 30 μg of protein were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrotransferred to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Nonspecific binding sites on the membrane were blocked with 5% milk for 1 h before incubation overnight with appropriate specific polyclonal rabbit antibodies to mouse STAT-1, phosphorylated Y701-STAT-1, iNOS, IRF-1, or actin (Santa Cruz, Santa Cruz, CA). The blots were then allowed to react with horseradish peroxidase-conjugated swine anti-rabbit immunoglobulin G (Pierce, Rockford, IL). Protein bands were detected by enhanced chemiluminescence as recommended by the manufacturer (Roche Diagnostics, Mannheim, Germany).
RT-PCR.
Total RNA was extracted from infected cells according to the manufacturer's instructions (Eppendorf, Hamburg, Germany) before being used for cDNA synthesis by cMaster reverse transcriptase (RT) enzyme (Eppendorf). The PCR was performed using cDNA as the template and primer pairs specific for IFN-β and actin in amplification reactions with Taq DNA polymerase (Invitrogen, Carlsbad, CA). The primers used to amplify each gene were as follows: IFN-β sense, 5′-TCC AAG AAA GGA CGA ACA TTC G-3′; IFN-β antisense, 5′-TGA GGA CAT CTC CCA CGT CAA-3′; actin sense, 5′-CCA GAG CAA GAG AGG TAT CC-3′; and actin antisense, 5′-CTG TGG TGG TGA AGC TGT AG-3′. The amplified products were electrophoresed on a 2% agarose gel and stained with ethidium bromide before being visualized under a UV lamp.
RESULTS
Internalization and replication of the LPS mutant in mouse macrophage cell line RAW 264.7.
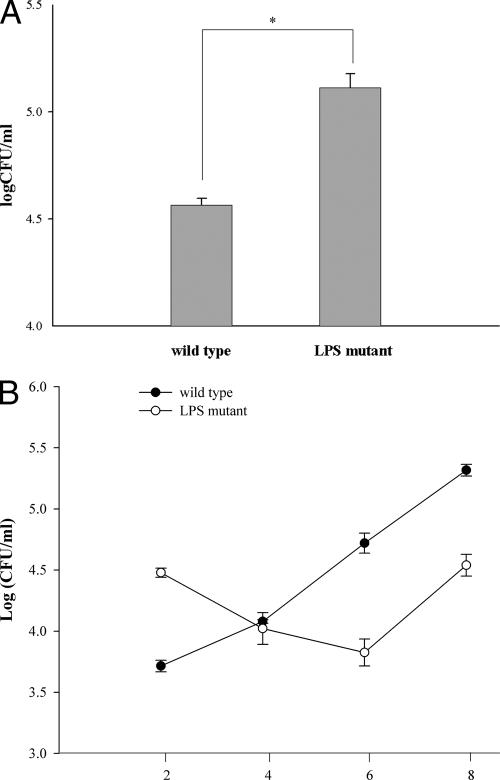
The macrophages were infected with the LPS mutant or wild-type B. pseudomallei at an MOI of 2:1 for 1 h. Internalization was determined after 2 h of infection using a standard antibiotic protection assay. The LPS mutant was found to be internalized at a rate significantly higher than that of its wild-type counterpart (Fig. 1A). However, compared to the wild type, the internalized LPS mutant appeared not to be able to withstand the hostile intracellular environment of the macrophages, as the numbers of mutant organisms continued to decline during the next 4 h while the wild type continued to replicate at a logarithmic rate (Fig. 1B). These results showed that the wild-type bacteria not only can withstand the hostile environment inside the macrophages but also can readily replicate intracellularly. It should be noted that while the replication of the LPS mutant was suppressed during the early phase of the infection, it could somehow adapt itself at a later stage, as its doubling time calculated at a later period of infection was not different from that of the wild type.
FIG. 1.
Internalization and intracellular replication of the LPS mutant. Mouse macrophages (106 cells/well) were infected with the LPS mutant or the wild-type B. pseudomallei at an MOI of 2:1 for 1 h. To determine the magnitude of internalization, the infected macrophages were lysed and the number of intracellular bacteria was analyzed by standard antibiotic protection assay after 2 h of infection (A). The intracellular survival and replication were also determined at 2, 4, 6, and 8 h after the infection (B). The data represent the means and standard deviations from three separate experiments, each carried out in duplicate. *, P < 0.01 by Student's t test.
Activation of iNOS and IRF-1 expression and phosphorylation of Y701-STAT-1 in mouse macrophages infected with the LPS mutant.
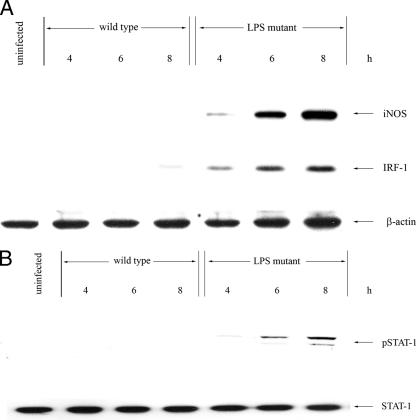
The inhibition of the intracellular replication of the LPS mutant during the early phase of infection (Fig. 1B) in mouse macrophages may imply that the mutant might be able to activate antimicrobial activity, particularly iNOS, more effectively than the wild type. In order to explore this possibility, the macrophages were first infected with the bacteria at an MOI of 2:1 for 1 h, and the expression of iNOS at 4, 6, and 8 h was determined by immunoblotting. High levels of iNOS were expressed in the LPS mutant-infected macrophage culture (Fig. 2). This was in marked contrast to infection with the wild-type strain which failed to activate the macrophages. The expression of iNOS also directly correlated with the activation of IRF-1 expression (Fig. 2A) and phosphorylation of Y701-STAT-1 (Fig. 2B), both of which are transcription factors required for iNOS expression. The wild-type B. pseudomallei, on the other hand, failed to stimulate iNOS, IRF-1, and phosphorylation of Y701-STAT-1.
FIG. 2.
Activation of iNOS and IRF-1 expression and phosphorylation of Y701-STAT-1 in mouse macrophages infected with the LPS mutant. Mouse macrophages were infected with the LPS mutant or the wild-type B. pseudomallei at an MOI of 2:1 for 1 h. After 4, 6, and 8 h of infection, the levels of iNOS, IRF-1, and actin expression (A) and of phosphorylated Y701-STAT-1 and STAT-1 (B) were determined by immunoblotting. The data showed all of these components to be upregulated in the macrophages infected with the mutant compared with those infected with its wild-type counterpart.
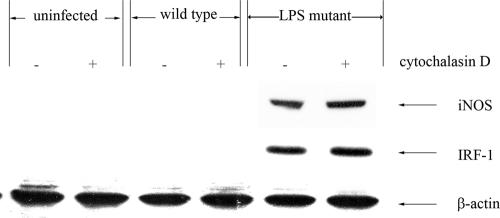
It is possible that the higher capacity of the LPS mutant to activate macrophages might be related to differences in the levels of bacterial internalization. Therefore, an experiment was performed whereby macrophages were cocultured with the mutant but internalization was blocked. In this experiment, the macrophages were pretreated with cytochalasin D prior to the infection, and the expression of iNOS and IRF-1 was analyzed by immunoblotting as described above. The levels of both iNOS and IRF-1 proteins in the LPS mutant infected-macrophages that were pretreated with cytochalasin D were not altered (Fig. 3). It should be mentioned that, at the concentration used, the cytochalasin D was able to significantly reduce the number of intracellular B. pseudomallei organisms in mouse macrophages, from 7.5 × 102 to 1 × 105 CFU.
FIG. 3.
Effect of cytochalasin D pretreatment on iNOS and IRF-1 expression by LPS mutant- or wild-type-infected macrophages. To inhibit bacterial internalization, the macrophages were pretreated with cytochalasin D (2 μg/ml) for 2 h prior to the infection, and then the macrophages were infected with the bacteria at an MOI of 2:1 for 1 h. After 8 h of infection, the infected cells were lysed and the levels of iNOS, IRF-1, and actin were determined by immunoblotting. The results showed cytochalasin D to have no effect on the macrophage response.
Activation of IFN-β expression in LPS mutant-infected macrophages.
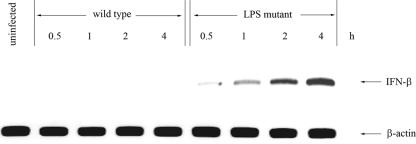
Macrophages were infected with the LPS mutant and the wild type at an MOI of 2:1 for 1 h. At 0.5, 1, 2, and 4 h after infection, the level of IFN-β gene expression was determined by RT-PCR. A trace amount of IFN-β could be detected in the LPS mutant-infected macrophages within 0.5 h of infection (Fig. 4), and the level of gene expression gradually increased. As to be expected from our previous report (29), this was different from the case for wild-type B. pseudomallei, which was unable to stimulate IFN-β gene expression. It should be mentioned that in the cytochalasin D-pretreated cells, the level of IFN-β mRNA expression induced by the LPS mutant was similar to that in the untreated macrophages that were infected with the LPS mutant (data not shown), indicating that internalization is not required for IFN-β gene expression.
FIG. 4.
LPS mutant B. pseudomallei induces IFN-β gene expression. Mouse macrophages were infected with either the LPS mutant or the wild-type B. pseudomallei at an MOI of 2:1. IFN-β gene expression was determined by RT-PCR at 0.5, 1, 2, and 4 h after infection. The LPS mutant, unlike the wild type, could readily activate IFN-β expression.
Neutralizing antibody to IFN-β inhibits iNOS and IRF-1 expression and phosphorylation of Y701-STAT-1 but enhances survival of the LPS mutant in macrophages.
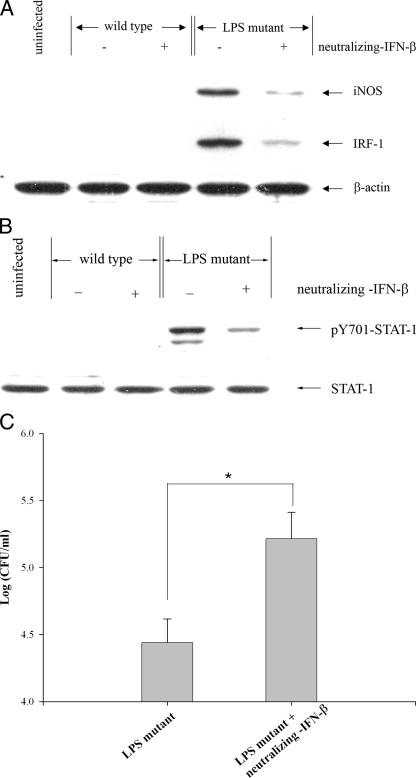
In order to determine if the above observations might be related to iNOS expression which in turn controls the intracellular fate of the LPS mutant, macrophages were pretreated with neutralizing antibody to IFN-β 1 h prior to the initiation of infection, and the presence of antibody was maintained throughout the course of the experiment. At 8 h postinfection, the expression of iNOS and IRF-1 and phosphorylation of Y701-STAT-1 were determined. In the presence of neutralizing antibody to IFN-β, the levels of iNOS and IRF-1 (Fig. 5A) and phosphorylated Y701-STAT-1 (Fig. 5B) appeared to be depressed. These results suggest that the expression of iNOS and IRF-1 and phosphorylation of Y701-STAT-1 induced by the LPS mutant are mediated through IFN-β production and signaling. To determine the relevance of this phenomenon, a similar experiment was carried out, and the number of intracellular viable bacteria was determined 8 h after the infection was initiated. In the presence of neutralizing antibody to IFN-β, the number of intracellular bacteria was significantly higher than that in the untreated macrophages (Fig. 5C). These results suggest that the ability of infected macrophages to suppress intracellular viability of the LPS mutant is related at least in part to the ability of the mutant to induce IFN-β expression and secretion.
FIG. 5.
IFN-β-neutralizing antibody attenuates the levels of iNOS and IRF-1 protein expression and of phosphorylated Y701-STAT-1 induced by the LPS mutant, resulting in enhancing intracellular survival in LPS mutant-infected macrophages. Mouse macrophages were infected with the LPS mutant B. pseudomallei at an MOI of 2:1 in the presence or absence of anti-IFN-β (10 μg/ml). After 1 h, the cells were washed with PBS before being cultured in medium containing 250 μg/ml of kanamycin. In this experiment, the antibody was kept in the culture medium throughout the entire period. At 8 h after infection, the levels of iNOS, IRF-1, and actin (A) and of phosphorylated Y701-STAT-1 and STAT-1 (B) from the infected cells were determined by immunoblotting. The number of intracellular bacteria was determined by standard antibiotic protection assay (C). Data represent the means and standard deviations from three separate experiments, each carried out in duplicate. *, P < 0.01 by Student's t test.
DISCUSSION
B. pseudomallei possesses several surface-associated molecules that could play a role in innate immune evasion. For example, capsular polysaccharide, previously characterized as a type I O polysaccharide, is required for B. pseudomallei virulence in experimental animal models (3, 25). In the presence of normal human serum, phagocytosis is greater for a capsule-deficient mutant than for the wild type (26). The deposition of the complement factor C3b on the bacterial cell surface is less in the presence of capsule, suggesting that the latter may act as a barrier and block the access of complement receptor 1 on the phagocytes to the C3b deposited on the bacterial surface (26). However, using our in vitro macrophage model, we have observed that the LPS mutant SRM1015, possessing LPS that lacks the O-antigenic polysaccharide moiety (type II O polysaccharide), could still be internalized, survive, and replicate at a rate not significantly different from that of its wild-type counterpart (unpublished data).
Among the surface components of gram-negative bacteria, LPS is also known to play an essential role in innate immune evasion. The LPS isolated from B. pseudomallei has characteristics different from those of LPSs from other gram-negative bacteria. For instance, B. pseudomallei LPS has been reported to possess weaker pyrogenic activity in rodents than enterobacterial LPS but stronger mitogenic activity in murine splenocytes (21, 34). A previous study by our group showed that B. pseudomallei LPS exhibits weaker and slower activation kinetics for mouse macrophage cell lines (31). This unusual characteristic may be due to the unique structure of the B. pseudomallei LPS. For example, it has been reported that LPS isolated from B. pseudomallei exhibits an unusual chemical structure in the acid-stable inner core region attached to the lipid A moiety, which may influence its macrophage activation activity (21). It is also possible that its O-antigen side chain might be interfering with these biological activities. By using bacteria that were mutated in the genes encoding LPS synthesis, we were able to investigate the possible role of O-antigenic polysaccharide of B. pseudomallei LPS in modulation of the mouse macrophages. The results from the present study demonstrate that the O-polysaccharide moiety of B. pseudomallei LPS is also involved in invasion and survival in macrophages. Internalization of the LPS mutant was significantly increased compared to that of its wild-type counterpart (Fig. 1A). However, we also found that intracellular survival of this LPS mutant was suppressed during the first 6 h of infection (Fig. 1B). The ability of the LPS mutant-infected macrophages to express antimicrobial activity and inhibit intracellular replication of this LPS mutant may be due to the fact that the LPS mutant-infected macrophages are able to upregulate IFN-β production (Fig. 4), a property not observed when the macrophages were infected with the wild type. IFN-β produced by the infected macrophages can act as a paracrine regulating the activation of several transcription factors, including IRF-1 and phosphorylated Y701-STAT-1, required for iNOS gene expression (15, 20, 23). Failure to activate IFN-β production may therefore facilitate the ability of wild-type B. pseudomallei to survive macrophage killing. These results are also in accord with the data presented in this study; i.e., in the presence of neutralizing antibody to IFN-β, the LPS mutant significantly reduced the levels of IRF-1 and iNOS expression and phosphorylated Y701-STAT-1, which also correlated with its intracellular survival (Fig. 5). Moreover, when not internalized, the LPS mutant was still able to activate iNOS and IRF-1 expression at similar levels (Fig. 3). This result indirectly implies that the signaling pathways of iNOS and IRF-1 expression can be initiated by the mere interaction of the LPS mutant with the surface of the macrophages rather than be internalized in the cytosol. In order to argue against the possibility that the reduced number of intracellular LPS mutant organisms at the initial stage of infection was due to increased susceptibility to NO killing compared to its wild-type counterpart, an additional experiment was performed whereby the LPS mutant was cultured in a cell-free system in medium containing various concentrations of NaNO2. The results showed that the mutant was not more susceptible to NO killing than the wild type (data not shown). Therefore, a decline in the number of intracellular mutant bacteria at the early stage of infection is most likely related to the modulation of the host cell response. Although activation of iNOS expression has been demonstrated to play an essential role in controlling the intracellular fate of B. pseudomallei (28-30), the results presented in this study demonstrated that the suppression of intracellular replication of the mutant was observed only during the early stage (2 to 6 h) of the infection (Fig. 1B). Thereafter the intracellular bacteria were able to replicate normally, as indicated by the increase in the number of intracellular bacteria (Fig. 1B). This result suggests that the activation of iNOS may be important in killing only during the early stage of the infection. However, it was demonstrated very recently that NADPH oxidase was probably more important for the control of infection of B. pseudomallei in the macrophages (5). Therefore, it is possible that NADPH oxidase may be involved in regulating the intracellular fate of B. pseudomallei during the later stage of infection in our mutant model. These various possibilities are now being investigated in our laboratory.
The LPS of B. pseudomallei, which exhibits unique structural and functional characteristics compared to the LPSs of other gram-negative bacteria (21, 31), has been demonstrated to play an essential role in the pathogenesis of melioidosis (10, 33). In the present study, we demonstrated that the O polysaccharide of B. pseudomallei LPS plays an essential role in innate immune evasion by the bacteria, particularly in modulation of macrophage activation. For example the O-polysaccharide moiety of B. pseudomallei LPS could interfere with bacterial uptake by decreasing the phagocytic ability of macrophages. Moreover, the O-polysaccharide moiety of B. pseudomallei LPS could also be involved in the evasion of macrophage killing by interfering with the stimulation of macrophage bactericidal activities, including iNOS expression. In the presence of purified B. pseudomallei LPS, the mutant was still able to activate iNOS expression and replicated inside the macrophages at the same magnitude as when the experiment was performed with the mutant alone (data not shown). These results suggested that the exogenous O-polysaccharide moiety could not interfere with the signaling that was triggered by the bacteria. Our results are consistent with those reported earlier for Neisseria meningitides showing that exogenously added LPS could not reconstitute cytokine induction in an LPS mutant (12). It has been demonstrated that in Pseudomonas aeruginosa lacking LPS O antigen, the type III secretion system is affected (4). The Bsa type III secretion system of B. pseudomallei is considered to be one of the key virulence determinant controlling the intracellular fate of the pathogen (27). However, whether the absence of the O-polysaccharide moiety in our B. pseudomallei mutant would interfere with the type III secretion system remained to be investigated. The information that we have reported here may provide important insights for the future development of vaccines against B. pseudomallei and/or B. mallei infections, which is being pursued by several groups of investigators.
Acknowledgments
This work was supported by a research grant from the National Science and Technology Developmental Agency of Thailand (BT-B-01-MG-12-4818).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Anuntagool, N., and S. Sirisinha. 2002. Antigenic relatedness between Burkholderia pseudomallei and Burkholderia mallei. Microbiol. Immunol. 46:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Anuntagool, N., P. Intachote, V. Wuthiekanun, N. J. White, and S. Sirisinha. 1998. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara− clinical isolate. Clin. Diagn. Lab. Immunol. 5:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins, T., R. G. Prior, K. Mack, P. Russell, M. Nelson, P. C. Oyston, G. Dougan, and R. W. Titball. 2002. A mutant of Burkholderia pseudomallei, auxotropic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 70:5290-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustin, D. K., Y. Song, M. S. Baek, Y. Sawa, G. Singh, B. Taylor, A. Rubio-Mills, J. L. Flanagan, J. P. Wiener-Kronish, and S. V. Lynch. 2007. Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. J. Bacteriol. 189:2203-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitbach, K., S. Klocke, T. Tschernig, N. van Rooijen, U. Baumann, and I. Steinmetz. 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect. Immun. 74:6300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brett, P. J., and D. E. Woods. 1996. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect. Immun. 64:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brett, P. J., D. C. Mah, and D. E. Woods. 1994. Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect. Immun. 62:1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryan, L. E., S. Wong, D. E. Woods, D. A. B. Dance, and W. Chaowagul. 1994. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide from Pseudomomas pseudomallei. Can. J. Infect. Dis. 5:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charuchaimontri, C., Y. Suputtamongkol, C. Nilakul, W. Chaowakul, P. Chetchotisakd, N. Lertpatanasuwun, S. Intraranongpai, P. J. Brett, and D. E. Woods. 1999. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin. Infect. Dis. 29:813-818. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, A. C., and B. J. Currie. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeShazer, D., P. J. Brett, and D. E. Woods. 1998. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol. Microbiol. 30:1081-1100. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, G. L. J., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. vander Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitides: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekchariyawat, P., S. Pudla, K. Limposuwan, S. Arjcharoen, S. Sirisinha, and P. Utaisincharoen. 2005. Burkholderia pseudomallei-induced expression of suppressor of cytokine signaling 3 (SOCS3) and cytokine-inducible Src homology 2-containing protein (CIS) in mouse macrophages: a possible mechanism for suppression of the response to gamma interferon stimulation. Infect. Immun. 73:7332-7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, J., D. C. Morrison, T. J. Parmely, S. W. Russell, and W. J. Murphy. 1997. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J. Biol. Chem. 272:1226-1230. [DOI] [PubMed] [Google Scholar]
- 16.Harley, V. S., D. A. B. Dance, B. J. Drasar, and G. Tovey. 1998. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios 96:71-93. [PubMed] [Google Scholar]
- 17.Ho, M., T. Schollaardt, M. D. Smith, M. B. Perry, P. J. Brett, W. Chaowagul, and L. E. Bryan. 1997. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect. Immun. 65:3648-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail, G., N. Razak, R. Mohamed, N. Embi, and O. Omar. 1988. Resistance of Pseudomonas pseudomallei to normal human serum bactericidal action. Microbiol. Immunol. 32:645-652. [DOI] [PubMed] [Google Scholar]
- 19.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamijo, R., H. Harda, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, T. W. Mak, T. Taniguchi, and J. Vilcek. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrohages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara, K., S. Dejsirilert, H. Danbara, and T. Ezaki. 1992. Extraction and characterization of lipopolysaccharide from Pseudomonas pseudomallei. FEMS Microbiol. Lett. 96:129-134. [DOI] [PubMed] [Google Scholar]
- 22.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuura, M., K. Kawahara, T. Ezaki, and M. Nakano. 1996. Biological activities of lipopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. FEMS Microbiol. Lett. 137:79-83. [DOI] [PubMed] [Google Scholar]
- 25.Reckseidler, S. L., D. DeShazer, P. A. Sokol, and D. E. Woods. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reckseidler-Zenteno, S. L., R. DeVinney, and D. E. Woods. 2005. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect. Immun. 73:1106-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens, M. P., M. W. Wood, L. A. Taylor, P. Monaghan, P. Hawes, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2002. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol. Microbiol. 46:649-659. [DOI] [PubMed] [Google Scholar]
- 28.Utaisincharoen, P., N. Anuntagool, S. Arjcharoen, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2004. Induction of iNOS expression and antimicrobial activity by interferon (IFN)-β is distinct from IFN-γ in Burkholderia pseudomallei-infected mouse macrophages. Clin. Exp. Immunol. 136:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utaisincharoen, P., N. Anuntagool, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2003. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect. Immun. 71:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 31.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, N. Anuntagool, P. Chaisuriya, and S. Sirisinha. 2000. Kinetic studies of the production of nitric oxide (NO) and tumor necrosis factor-alpha (TNF-α) in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin. Exp. Immunol. 122:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White, N. J. 2003. Melioidosis. Lancet 361:1715-1722. [DOI] [PubMed] [Google Scholar]
- 33.Wiersinga, W. J., T. vander Poll, N. J. White, N. P. Day, and S. J. Peacock. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Immunol. 4:272-282. [DOI] [PubMed] [Google Scholar]
- 34.Woods, D. E., D. DeShazer, R. A. Moore, P. J. Brett, M. N. Burtnick, S. L. Reckseidler, and M. D. Senkiw. 1999. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1:157-162. [DOI] [PubMed] [Google Scholar]