Abstract
Giardia intestinalis is a significant cause of diarrheal disease worldwide. Infections in animal models have been shown to cause changes in gastrointestinal transit that depend on adaptive immune responses and are mediated, in part, through neuronal nitric oxide synthase. Nitric oxide is an inhibitory neurotransmitter, and we therefore investigated potential excitatory pathways that might be involved in the response to Giardia infection. Infected mice exhibited increased spontaneous and cholecystokinin (CCK)-induced contractions of longitudinal smooth muscle. In contrast, enhanced contractile responses were not observed in response to acetylcholine, 5-hydroxytryptamine, or the protease-activated receptor-1 agonist peptide TFFLR. Giardia-induced changes in smooth muscle function appear to be mediated primarily by mast cells, as both spontaneous and CCK-induced contractions were blocked by pretreatment with either ketotifen or compound 48/80. Together, these data support a model in which CCK release triggers mast cell degranulation, leading to increases in smooth muscle contractility. These contractions, coupled with nitric oxide-mediated muscle relaxation, promote intestinal transit and parasite elimination.
Giardia intestinalis (syn. G. lamblia, G. duodenalis) is a protozoan parasite that replicates in the lumen of the small intestines of humans and many other mammals. Infections may result in severe diarrhea, cramps, nausea, and nutrient malabsorption, although subclinical infections with only mild nutrient malabsorption appear to be common (reviewed in references 9 and 17). It is unknown whether variation in host responses to the parasite, differences among parasite genotypes, or both, are responsible for the differences seen in clinical outcomes. For example, differences in the ability to induce apoptosis in cultured epithelial cells have been noted among Giardia strains (4), while changes in brush border enzymes involved in nutrient absorption were shown to require CD8+ T-cell responses in mice (26).
We showed recently that G. intestinalis infection in mice leads to changes in intestinal motility that are important for parasite elimination (22). These changes in intestinal transit rate required an intact immune system, as they were not observed in SCID mice. In addition, we showed that the neuronal isoform of nitric oxide synthase (nNOS) was important both for parasite elimination and changes in intestinal motility. These results have been confirmed using the rodent-specific parasite species Giardia muris (2). An earlier study noted that gerbils infected with G. intestinalis also had increases in intestinal motility that were correlated with an enhanced contractile response of longitudinal muscles to the muscarinic receptor agonist bethanechol (7). However, the mechanisms regulating changes in motility during Giardia infection remain poorly defined.
Host responses to enteric infections make use of a number of physiological pathways that impact smooth muscle function (reviewed in references 23 and 28). Infections can lead to the upregulation of receptors on smooth muscle that bind agonists that alter responses, as well as to changes in enteric neuronal reflex circuits. In addition, an influx of inflammatory/immune cells amplifies the well-documented interaction between mast cells and the nerves that affect smooth muscle activity.
Significant mast cell responses during Giardia infections have been observed in several experimental systems, and these were also shown to be important for parasite elimination (8, 16, 21). Mast cell responses have been seen also in numerous intestinal helminth infection models, where they participate variably in parasite elimination, depending on the particular parasite under study (5, 24). Interestingly, many of the changes in intestinal motility during these infections do not depend on mast cells (12, 33). Recently, however, cholecystokinin (CCK) was shown to affect motility in Trichinella-infected rats through a mast cell-dependent process (27). In this study, we further examine the mechanism responsible for Giardia infection-induced changes in intestinal motility.
MATERIALS AND METHODS
Mice.
C57BL/6J female mice were obtained from Jackson Laboratories (Bar Harbor, ME). The mice were between 6 and 12 weeks of age for these studies. The mice were euthanized by the injection of ketamine (0.5 mg/kg, intramuscularly). All experiments were performed in accordance with protocols approved by the Animal Care and Use Committees of Georgetown University and the University of Maryland, Baltimore.
Infections.
Mice were infected with G. intestinalis strain GS(M)-H7 as previously described (29). Briefly, parasites were grown in vitro in TYI-S-33 media supplemented with bovine bile, l-cysteine, ascorbic acid, and antibiotics. Parasites were harvested by chilling on ice for 15 min and washed with phosphate-buffered saline, and mice were infected via gavage with 1 million trophozoites in 100 μl phosphate-buffered saline.
Reagents.
Krebs buffer contained 4.74 mM KCl, 2.54 mM CaCl2, 118.5 mM NaCl, 1.19 mM NaH2PO4, 1.19 mM MgSO4, 25.0 mM NaHCO3, and 11.0 mM glucose. All drugs were obtained from Sigma-Aldrich Chemicals (St. Louis, MO) unless indicated otherwise. Stock solutions were prepared as follows: sulfated CCK octapeptide, ketotifen, acetylcholine, compound 48/80, histamine, and 5-hydroxytryptamine (5-HT) were all dissolved in distilled water and stored at −20°C. The protease-activated receptor 1 (PAR-1) agonist peptide TLFFR was dissolved in 20% dimethyl sulfoxide and stored at −70°C. All drugs were diluted in distilled water to the appropriate concentrations just before use.
Contractility studies.
Longitudinal smooth muscle function was assessed as previously described (35). One-centimeter segments of jejunum were flushed of their intestinal contents, suspended longitudinally in individual 8-ml organ baths, and maintained in oxygenated Krebs solution at 37°C. One end of the tissue was attached to an isometric tension transducer (model FT03; Grass Medical Instruments) and the other to the bottom of the bath. The tissues were stretched to a load of 9.9 mN (2 g). Preliminary experiments showed that this load stretched the tissues to their optimal length for active contraction. The tissues were allowed to equilibrate for at least 30 min in Krebs buffer solution before the study, and the bath solution was replaced every 10 min. The tension was recorded by using a Grass model 79 polygraph and was expressed as force per cross-sectional area. After the equilibration, the frequency and amplitude of the spontaneous, rhythmic contractions that occur in the absence of any stimulation were measured over a 2-min period. The force of the spontaneous contractions represents the amplitude of these contractions normalized to the cross-sectional area of the longitudinal muscle layer of the tissue. The tissues were then challenged with acetylcholine (100 μM), histamine (100 μM), 5-HT (100 μM), CCK (100 μM), or the PAR-1-activating peptide TFLLR (100 μM). The responses to the agonists are expressed as the increase from the baseline tension before the addition of the agonist. Some tissue segments were treated with 100 μM ketotifen or 100 μM compound 48/80 for 20 min prior to the addition of CCK or acetylcholine. In these studies, ketotifen was also present during CCK and acetylcholine treatment, while compound 48/80 was removed prior to treatments.
RT-PCR.
RNA was isolated from jejunal segments using TRIzol reagent (Invitrogen, Carlsbad, CA), by following the manufacturer's instructions. Five micrograms of total RNA was reverse transcribed using Superscript II (Invitrogen). Real-time PCR was performed in a LightCycler (Bio-Rad, Hercules, CA) using a SYBR green master mix and primers for PAR-1, 5-HT2A, 5-HT3, Mcpt1, CCKA, and 18S rRNA as described previously (36). The threshold cycle (CT) numbers were converted to relative changes in gene expression using the ΔCT formula and normalizing the values to 18S rRNA.
Statistics.
P values were calculated using t tests and Prism software (GraphPad Software, San Diego, CA).
RESULTS
Effects of G. intestinalis infection on smooth muscle contractions evoked by acetylcholine, histamine, 5-HT, and PAR-1 agonist peptide.
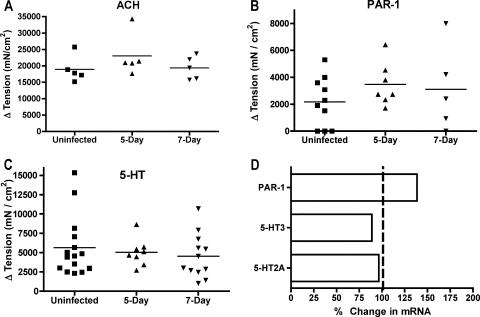
We recently reported that Giardia infection in mice induced changes in intestinal motility that required the activity of nNOS (22). Nitric oxide is a known inhibitory neurotransmitter, yet Giardia infection led to enhanced intestinal motility as measured by the transit time of a charcoal meal (22). We therefore decided to investigate if other physiological measures of smooth muscle function and enteric nervous signaling were altered in Giardia-infected mice. Segments of the jejunum were suspended in organ baths and connected to force transducers to measure the intestinal smooth muscle function. The responses to acetylcholine, 5-HT, and the peptide TFLLR, an agonist of PAR-1, were measured. Whereas responses to these agonists are typically altered during enteric infections (23, 28), no differences were seen in longitudinal muscles from Giardia-infected mice (Fig. 1A to C). Responses to histamine were also not enhanced in infected mice (data not shown). Consistent with the lack of change in neuromuscular responses to 5-HT and the PAR-1 agonist, no differences were seen in the levels of mRNA expression for two 5-HT receptors and PAR-1 (Fig. 1D).
FIG. 1.
Contractile responses to agonists. Longitudinal muscle tension was measured in response to the addition of 100 μM acetylcholine (ACH) (A), 100 μM PAR-1 agonist TFLLR (B), or 100 μM 5-HT (C). The maximum increase in tension after the addition of the agonist is shown for individual mice. Horizontal bars represent the means. No significant differences were detected between groups. (D) Real-time PCR analysis of mRNA for the receptors 5-HT2A, 5-HT3, and PAR-1. The gene expression in 5-day-infected mice is presented relative to gene expression in uninfected mice. No significant changes in expression were detected (n = 5 mice/group).
Effect of G. intestinalis infection on spontaneous contractions of smooth muscle.
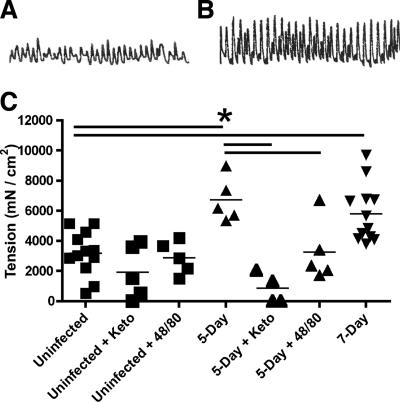
While we did not observe any increase in the contractile responses evoked by the agonists tested above, we did observe that spontaneous contractions in muscle from Giardia-infected mice were significantly greater than in muscle from uninfected controls (Fig. 2). The amplitude of spontaneous contractions, which are an inherent property of gut smooth muscle, was increased ∼75% in the infected mice at day 5 postinfection compared to the amplitude in uninfected controls (6,714 ± 659 [mean ± standard deviation] versus 3,175 ± 424 mN/cm2; P < 0.05). This difference persisted through day 7 postinfection (5,810 ± 552 mN/cm2; P = 0.001). Parasite numbers in the small intestine typically peak at 5 days postinfection and begin to decrease by day 7 in this model, indicating that expulsion is associated with the increased amplitude of spontaneous contractions during this time period. To determine if mast cells contributed to the observed change in spontaneous contractions, muscle strips were pretreated with either ketotifen or compound 48/80. Ketotifen is a mast cell-stabilizing agent, as well as a histamine H1 receptor antagonist, and is often used to block degranulation of mast cells. Compound 48/80 induces mast cell degranulation, and pretreatment with this drug therefore reduces the effect of subsequent mast cell activation. Following twenty minutes of treatment with either drug, spontaneous contractions in 5-day-infected mice were decreased significantly (Fig. 2C), indicating a role for mast cells in the increased amplitude of contractions. No significant differences in spontaneous contractions were observed in uninfected mice after treatment with ketotifen.
FIG. 2.
Spontaneous contractions of longitudinal smooth muscle are increased after Giardia infection. Tracings of contractions from representative uninfected (A) and 5-day-infected (B) mice are shown. The amplitude of the curve corresponds to the force of the contraction. (C) The force of spontaneous contractions was determined as described in Materials and Methods for uninfected (n = 12), 5-day-infected (n = 5), and 7-day-infected (n = 12) mice. Tissues were pretreated with 100 μM ketotifen to block mast cell degranulation or with 100 μM compound 48/80 to fully degranulate mast cells, followed by remeasurement of spontaneous contractions as indicated. Each point represents an individual mouse, and thin bars indicate means. *, P < 0.05 by two-tailed t test comparing groups connected by thick horizontal bars.
Effects of G. intestinalis infection on smooth muscle contractions evoked by CCK.
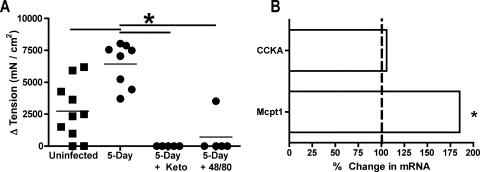
While contractile responses to acetylcholine, 5-HT, and PAR-1 agonist were not significantly increased following infection, CCK induced significantly greater muscle contractions in tissues from 5-day-infected mice than in tissues from uninfected mice (Fig. 3). These data suggest that CCK is a major mediator of the enhanced motility seen during Giardia infection. CCK is known to activate smooth muscle contractions directly, in the gall bladder, for example; however, CCK can also trigger mast cell degranulation. To determine if mast cells were involved in mediating the effects of CCK in Giardia-infected mice, tissues were pretreated with ketotifen or compound 48/80 prior to treatment with CCK. Treatment with either compound significantly reduced the contractile response to CCK (Fig. 3). CCK responses were undetectable in tissues from all five infected mice following ketotifen treatment and in tissues from four of five mice treated with compound 48/80. Importantly, while ketotifen pretreatment reduced the responses to acetylcholine (34% ± 22% in uninfected mice and 24% ± 5% in infected mice), these changes were not statistically different and verified the integrity of the muscle preparations after ketotifen exposure. The continued spontaneous contractions in tissues from uninfected mice following ketotifen and compound 48/80 treatment further confirm the specificities of these drugs. Finally, real-time reverse transcription-PCR indicated an 85% increase in gene expression for the mucosal mast cell protease Mcpt1 following infection (P < 0.01), although no change was seen in the expression of the CCK receptor CCKA (Fig. 3B).
FIG. 3.
CCK and mast cells mediate contractile responses. (A) The contractile responses of longitudinal muscle to 100 μM CCK were compared in uninfected and 5-day-infected mice. Prior to measuring responses to CCK, tissues were pretreated as indicated with 100 μM ketotifen to block mast cell degranulation or with 100 μM compound 48/80 to fully degranulate mast cells. The maximum increase in tension after the addition of the agonist is shown for individual mice. Horizontal bars represent the means. *, P < 0.05 by two-tailed t test comparing groups connected by thick horizontal bars. (B) Real-time PCR analysis of mRNA for CCKA receptor and mast cell protease 1 (Mcpt1). The gene expression in 5-day-infected mice is presented relative to gene expression in uninfected mice. *, P < 0.05 (n = 5 mice/group).
DISCUSSION
We recently showed that Giardia infection led to an increase in gastrointestinal transit rates that depended on an intact immune system (22). We now show that changes in intestinal motility during Giardia infection do not involve the stereotypic responses seen in other parasitic infections, such as heightened responses to 5-HT, acetylcholine, and PAR-1 (28). Instead, changes are mediated through a combination of enhanced activation of nNOS (22) and heightened responses to CCK via mast cells.
Most bacterial and viral enteric pathogens cause diarrhea through the production of enterotoxins or invasion of host tissue, resulting in inflammation (11). The mechanisms responsible for diarrhea in giardiasis are less well understood: no toxins have been clearly identified, and the diarrhea results from malabsorption, not secretion (3, 9, 11). Enteric infections typically affect intestinal motility patterns by altering neuronal reflex pathways and smooth muscle responses to stimulation (2, 23). For example, infectious irritable bowel syndrome is thought to result from enhanced 5-HT responses following bacterial enteritis (23, 30). Many of the neuromuscular changes are dependent on immune responses, particularly Th2 cytokines and/or mast cells (28). The typical intestinal response to helminth infection results in enhanced responses to transmitters that can act on enteric nerves and/or intestinal muscles. For example, intestinal tissue from wild-type, but not STAT6-deficient, mice infected with Nippostrongylus brasiliensis, Heligmosomoides polygyrus, and Trichinella spiralis exhibited increased contractions after stimulation with acetylcholine or the cholinergic agent carbachol (1, 19, 34). Similarly, STAT6-dependent changes in responses to 5-HT and an agonist of PAR-1 have been observed in tissue from N. brasiliensis-infected mice (35, 36). In contrast, responses to 5-HT, acetylcholine, and an agonist of PAR-1 were not increased during Giardia infection (Fig. 2). The response to Giardia infection differs from the response to N. brasiliensis infection in other ways. For example, the expulsion of N. brasiliensis is dependent on STAT6 but not on mast cells (5, 31). The expulsion of T. spiralis is also dependent on STAT6, although this dependence involves both mast cell-dependent and -independent effects (19, 32). The elimination of G. intestinalis is dependent on mast cells but is independent of STAT6 (21, 29). Thus, the mechanisms of Giardia-induced diarrhea appear to be different than those associated with other enteric pathogens. Importantly, the changes in motility observed in giardiasis do not originate from the Th2-dominant responses typical of enteric helminth infections.
While we saw no increase in contractility after treatment with histamine, 5-HT, or PAR-1 agonist, we observed a strong response to the addition of CCK. The increased muscle contractions after exposure to CCK were completely blocked by ketotifen. In addition, pretreatment with compound 48/80 in order to deplete mast cell granule contents prior to treatment with CCK also completely blocked the CCK response in tissue from four out of five mice tested. CCK has previously been shown to increase intestinal motility in vivo in T. spiralis-infected rats (27) and to decrease feeding in T. spiralis-infected mice (25). As in Giardia-infected mice, the effect of CCK on motility changes in rats was blocked by pretreatment with ketotifen, suggesting a role for mast cells (27). In contrast, the effects on feeding were not blocked by anti-c-kit treatment to reduce mast cell responses, although they were blocked by anti-CD4 treatment (25). Motility responses to CCK were also augmented in N. brasiliensis-infected rats (12), but ketotifen did not block this response, suggesting a mast cell-independent pathway. Interestingly, while the expulsion of both Giardia and T. spiralis requires mast cell responses (14, 21, 24), the expulsion of N. brasiliensis is mast cell independent (5, 6).
Changes in intestinal motility and mast cell responses are essential components of the immune-mediated elimination of Giardia infections in mice (2, 21, 22). However, it was not clear if mast cell responses contributed to changes in motility during giardiasis or how these responses might be activated. In this study, we have shown that nitric oxide, CCK, and mast cells are all involved in the host response to this intestinal infection. The identification of CCK as an important mediator of motility responses in the mouse model of Giardia infection has several implications for human disease. Importantly, elevated CCK levels have been reported in humans with symptomatic giardiasis (20). It remains to be determined if CCK levels are also elevated in asymptomatic infections, which are quite common. The major effect of CCK in the gastrointestinal tract is to cause gall bladder contraction and delivery of bile into the small intestine. Bile is a required growth factor for Giardia trophozoites and is readily consumed by the parasite (10, 15, 18). Bile is also an important regulator for the development of the cyst form of this parasite (13). Thus, the production of CCK and the release of bile are central both to the parasite life cycle and to host response to infection.
Acknowledgments
We thank Rex Sun, Justin Elfrey, and Jennifer Stiltz for technical assistance.
This work was supported by Public Health Service awards AI045965 to S.M.S. and AI/DK049316 to T.S.-D.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Akiho, H., P. Blennerhassett, Y. Deng, and S. M. Collins. 2002. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G226-G232. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, Y. S., F. D. Gillin, and L. Eckmann. 2006. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect. Immun. 74:2473-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buret, A. G. 2005. Immunopathology of giardiasis: the role of lymphocytes in intestinal epithelial injury and malfunction. Mem. Inst. Oswaldo Cruz 100(Suppl. 1):185-190. [DOI] [PubMed] [Google Scholar]
- 4.Chin, A. C., D. A. Teoh, K. G. Scott, J. B. Meddings, W. K. Macnaughton, and A. G. Buret. 2002. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect. Immun. 70:3673-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowle, P. K. 1983. Mucosal mast cell reconstitution and Nippostrongylus brasiliensis rejection by W/Wv mice. J. Parasitol. 69:66-69. [PubMed] [Google Scholar]
- 6.Crowle, P. K., and N. D. Reed. 1981. Rejection of the intestinal parasite Nippostrongylus brasiliensis by mast cell-deficient W/Wv anemic mice. Infect. Immun. 33:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deselliers, L. P., D. T. Tan, R. B. Scott, and M. E. Olson. 1997. Effects of Giardia lamblia infection on gastrointestinal transit and contractility in Mongolian gerbils. Dig. Dis. Sci. 42:2411-2419. [DOI] [PubMed] [Google Scholar]
- 8.Erlich, J. H., R. F. Anders, I. C. Roberts-Thomson, J. W. Schrader, and G. F. Mitchell. 1983. An examination of differences in serum antibody specificities and hypersensitivity reactions as contributing factors to chronic infection with the intestinal protozoan parasite, Giardia muris, in mice. Aust. J. Exp. Biol. Med. Sci. 61:599-615. [DOI] [PubMed] [Google Scholar]
- 9.Farthing, M. J. 1997. The molecular pathogenesis of giardiasis. J. Pediatr. Gastroenterol. Nutr. 24:79-88. [DOI] [PubMed] [Google Scholar]
- 10.Farthing, M. J., S. R. Varon, and G. T. Keusch. 1983. Mammalian bile promotes growth of Giardia lamblia in axenic culture. Trans. R. Soc. Trop. Med. Hyg. 77:467-469. [DOI] [PubMed] [Google Scholar]
- 11.Gascon, J. 2006. Epidemiology, etiology and pathophysiology of traveler's diarrhea. Digestion 73(Suppl. 1):102-108. [DOI] [PubMed] [Google Scholar]
- 12.Gay, J., J. Fioramonti, R. Garcia-Villar, and L. Bueno. 2001. Enhanced intestinal motor response to cholecystokinin in post-Nippostrongylus brasiliensis-infected rats: modulation by CCK receptors and the vagus nerve. Neurogastroenterol. Motil. 13:155-162. [DOI] [PubMed] [Google Scholar]
- 13.Gillin, F. D., D. S. Reiner, M. J. Gault, H. Douglas, S. Das, A. Wunderlich, and J. F. Sauch. 1987. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science 235:1040-1043. [DOI] [PubMed] [Google Scholar]
- 14.Grencis, R. K., K. J. Else, J. F. Huntley, and S. I. Nishikawa. 1993. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 15:55-59. [DOI] [PubMed] [Google Scholar]
- 15.Halliday, C. E., P. M. Inge, and M. J. Farthing. 1995. Characterization of bile salt uptake by Giardia lamblia. Int. J. Parasitol. 25:1089-1097. [DOI] [PubMed] [Google Scholar]
- 16.Hardin, J. A., A. G. Buret, M. E. Olson, M. H. Kimm, and D. G. Gall. 1997. Mast cell hyperplasia and increased macromolecular uptake in an animal model of giardiasis. J. Parasitol. 83:908-912. [PubMed] [Google Scholar]
- 17.Ish-Horowicz, M., S. H. Korman, M. Shapiro, U. Har-Even, I. Tamir, N. Strauss, and R. J. Deckelbaum. 1989. Asymptomatic giardiasis in children. Pediatr. Infect. Dis. J. 8:773-779. [DOI] [PubMed] [Google Scholar]
- 18.Keister, D. B. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 19.Khan, W. I., B. A. Vallance, P. A. Blennerhassett, Y. Deng, E. F. Verdu, K. I. Matthaei, and S. M. Collins. 2001. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect. Immun. 69:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie, F. C., D. G. Thompson, J. T. McLaughlin, A. Varro, G. J. Dockray, and B. K. Mandal. 2003. Plasma cholecystokinin concentrations are elevated in acute upper gastrointestinal infections. QJM 96:870-871. [DOI] [PubMed] [Google Scholar]
- 21.Li, E., P. Zhou, Z. Petrin, and S. M. Singer. 2004. Mast cell-dependent control of Giardia lamblia infections in mice. Infect. Immun. 72:6642-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, E., P. Zhou, and S. M. Singer. 2006. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J. Immunol. 176:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mawe, G. M., S. M. Collins, and T. Shea-Donohue. 2004. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol. Motil. 16(Suppl. 1):133-136. [DOI] [PubMed] [Google Scholar]
- 24.McDermott, J. R., R. E. Bartram, P. A. Knight, H. R. Miller, D. R. Garrod, and R. K. Grencis. 2003. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 100:7761-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott, J. R., F. C. Leslie, M. D'Amato, D. G. Thompson, R. K. Grencis, and J. T. McLaughlin. 2006. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut 55:492-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott, K. G., L. C. Yu, and A. G. Buret. 2004. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect. Immun. 72:3536-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serna, H., M. Porras, and P. Vergara. 2006. Mast cell stabilizer ketotifen [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9 H)-one fumarate] prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental Trichinella spiralis inflammation in the rat. J. Pharmacol. Exp. Ther. 319:1104-1111. [DOI] [PubMed] [Google Scholar]
- 28.Shea-Donohue, T., and J. F. Urban, Jr. 2004. Gastrointestinal parasite and host interactions. Curr. Opin. Gastroenterol. 20:3-9. [DOI] [PubMed] [Google Scholar]
- 29.Singer, S. M., and T. E. Nash. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect. Immun. 68:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiller, R. C. 2007. Role of infection in irritable bowel syndrome. J. Gastroenterol. 42(Suppl. 17):41-47. [DOI] [PubMed] [Google Scholar]
- 31.Urban, J. F., Jr., N. Noben-Trauth, D. D. Donaldson, K. B. Madden, S. C. Morris, M. Collins, and F. D. Finkelman. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8:255-264. [DOI] [PubMed] [Google Scholar]
- 32.Urban, J. F., Jr., L. Schopf, S. C. Morris, T. Orekhova, K. B. Madden, C. J. Betts, H. R. Gamble, C. Byrd, D. Donaldson, K. Else, and F. D. Finkelman. 2000. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J. Immunol. 164:2046-2052. [DOI] [PubMed] [Google Scholar]
- 33.Vallance, B. A., P. A. Blennerhassett, J. D. Huizinga, and S. M. Collins. 2001. Mast cell-independent impairment of host defense and muscle contraction in T. spiralis-infected W/W(V) mice. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G640-G648. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, A., J. McDermott, J. F. Urban, Jr., W. Gause, K. B. Madden, K. A. Yeung, S. C. Morris, F. D. Finkelman, and T. Shea-Donohue. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171:948-954. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, A., M. Morimoto, H. Dawson, J. E. Elfrey, K. B. Madden, W. C. Gause, B. Min, F. D. Finkelman, J. F. Urban, Jr., and T. Shea-Donohue. 2005. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J. Immunol. 175:2563-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, A., J. F. Urban, Jr., M. Morimoto, J. E. Elfrey, K. B. Madden, F. D. Finkelman, and T. Shea-Donohue. 2006. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology 131:568-578. [DOI] [PubMed] [Google Scholar]