Abstract
During Helicobacter pylori infection, T cells are recruited to the gastric mucosa, but the host T-cell response is not sufficient to clear the infection. Some of the recruited T cells respond in a polarized manner to a Th1 response, while others become anergic. We have previously shown that T-cell anergy may be induced during infection by the interaction of T cells with B7-H1, which is up-regulated on the gastric epithelium during H. pylori infection. Recently, regulatory T (Treg) cells with a CD4+ CD25high FoxP3+ phenotype were found at an increased frequency in the gastric mucosa of biopsy specimens from H. pylori-infected patients. While Treg cells are important in maintaining tolerance, they can also suppress immune responses during infection. In this study, we examined the induction of the Treg phenotype when naïve T cells were incubated with gastric epithelial cells exposed to H. pylori. The frequency of this phenotype was markedly decreased when B7-H1 was blocked with monoclonal antibodies or its expression was blocked with small interfering RNA. The functional role of these Treg cells was assessed in proliferation assays when the cells were cocultured with activated T cells, which effectively decreased proliferation of the cells.
Helicobacter pylori is possibly the world's second most common human pathogen. This gram-negative spiral bacterium is responsible for 90% of gastric and duodenal ulcers and has also been associated with gastric adenocarcinoma (9). Infection with the pathogen often becomes chronic and is accompanied by gastritis. Colonization of the gastric mucosa leads to an influx of host macrophages, neutrophils, B cells, and T cells. Although some of these cells are specific for H. pylori (6, 18) the responses induced are not sufficient to clear the infection. In fact, untreated infections generally persist for life, which suggests that H. pylori avoids or suppresses normal host responses. Recent studies have suggested that immune-regulatory mechanisms may be induced during infection that lead to persistent infection by limiting damage to the host but result in low-level chronic inflammation.
T cells are generally hyporesponsive during H. pylori infection, and the existent response is polarized toward a Th1 response (2, 25). These cells have been shown to produce gamma interferon, which may play an important role in the proinflammatory responses induced during infection. Additionally, studies of infected patient biopsy specimens have also shown the presence of transforming growth factor β (TGF-β) (13, 14), which has a suppressive effect on other T cells. Several studies have shown that T cells exposed to H. pylori exhibit impaired proliferation rates (11). Also, H. pylori virulence factors, such as the vacuolating toxin (VacA), have been suggested to suppress T cells. When mixed with T-cell lines, VacA was shown to inhibit interleukin 2 (IL-2) production, arrest cell cycle progression, and down-regulate surface expression of IL-2 receptors, all of which are required for T-cell proliferation and viability (4). Another study also showed that the CagA virulence factor may inhibit T-cell proliferation (19).
The inability of the host response to eradicate the infection may also be attributed to the depressed T-cell response during H. pylori infection. Although gastric epithelial cells (GEC) have been shown to express the class II major histcompatibility complex and to function as antigen-presenting cells (3, 10), we have recently shown that the coinhibitory molecule, the programmed death ligand 1, or B7-H1, is up-regulated on GEC during exposure to H. pylori both in vitro and in vivo (5). Since binding of B7-H1 to the programmed death 1 receptor on T cells causes them to undergo anergy or apoptosis (23, 27), B7-H1 may play a key role in the chronicity of those infections and associated inflammatory responses. H. pylori-exposed GEC incubated with isolated CD4+ T cells resulted in decreased proliferation and IL-2 production, but this inhibition was abrogated by anti-B7-H1 blocking antibodies (5). These findings suggest that B7-H1 plays an important role in T-cell inhibition during H. pylori infection.
Recently, CD4+ CD25high FoxP3+ regulatory T (Treg) cells have been detected in the gastric mucosa of H. pylori-infected biopsy specimens (16), but their origin is not known. Current knowledge of Treg cells suggests that they may play a crucial role in suppressing the overall T-cell response to infection and cancer. Cells of this phenotype may inhibit T-cell activation by producing IL-10 and TGF-β. In H. pylori-infected individuals, Treg cells have been shown to impair the response of memory T cells in the periphery (17). The depletion of Treg cells from the memory population led to an increase in proliferation in response to H. pylori antigens, while addition of Treg cells to the memory T cells suppressed the H. pylori-specific responses. Similarly, in infected mice, depleting the Treg cells led to increased pathology (20, 21). Since Treg cells may play an important role in the impaired T-cell responses during H. pylori infection, and we and others have shown that B7-H1 also plays a role in T-cell suppression or generation of Treg cells (5, 12, 30), we sought to determine the role of B7-H1 on GEC in the development of CD4+ CD25high FoxP3+ Treg cells during H. pylori infection. In this study, we show that coculture of naïve CD4+ T cells with H. pylori-exposed GEC generates CD4+ CD25high FoxP3+ T cells. Blocking B7-H1 on H. pylori-exposed GEC with monoclonal antibodies or specific knockdown with small interfering RNA (siRNA) prevents the formation of the CD4+ CD25high FoxP3+ phenotype within those cocultures. T cells with this phenotype were also shown to produce TGF-β and IL-10 and to have the ability to suppress T effector (Teff) cell proliferation.
MATERIALS AND METHODS
Cell lines.
The GEC line AGS and the nontransformed fetal gastric cell line HS-738 were obtained from the American Tissue Culture Collection (ATCC) (Manassas, VA). AGS cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and 1 mM glutamine. The HS-738 cells were maintained in Dulbecco's minimal essential medium with high glucose, 10% fetal calf serum, and 1 mM glutamine.
Bacterial cultures.
H. pylori strain 26695 was obtained from Yoshio Yamaoka at Baylor College of Medicine. The bacteria were grown on blood agar plates (Becton Dickinson, San Jose, CA) at 37°C under microaerophilic conditions and used as previously described (8).
siRNA transfection.
GEC were transfected with siRNA for B7-H1 by using the basic nucleofection kit for epithelial cells (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer's instructions with a cocktail of 1 μg of siRNA numbers 134191, 20742, and 134192 or a negative control siRNA (Ambion, Austin, TX). For AGS cells, program B-023 was used as recommended by Amaxa's Cell Database, while program T-20 was used for HS-738 cells for optimal cell viability. Knockdown of expression of B7-H1 was verified by staining the cells with anti-B7-H1-phycoerythrin (PE)-conjugated monoclonal antibodies (eBiosciences, San Diego, CA) and analyzing them by flow cytometry compared to cells stained with isotype control.
GEC exposure to H. pylori.
AGS and HS-738 cells were incubated at a 100:1 bacteria/cell ratio with H. pylori for 24 h. The cells were then trypsinized, washed twice with phosphate-buffered saline, gamma irradiated at 14,000 rad, and plated at 1 × 105 cells per well in a 24-well plate for 24 h before the addition of T cells.
T-cell isolation and incubation with GEC.
Heparinized venous blood was obtained from a healthy adult volunteer negative for H. pylori, using an Institutional Review Board approved protocol (no. 94-102). Peripheral blood mononuclear cells (PBMC) were prepared from collected blood by density gradient centrifugation over Ficoll-Paque Plus (Amersham Bioscience, Piscataway, NJ) according to the manufacturer's instructions. Naïve CD4+ T cells were isolated from the PBMC using the naïve-T-cell isolation kit for negative selection (Miltenyi Biotech Inc., CA) with magnetic beads labeled with monoclonal antibodies for CD45RO, CD8, CD14, CD16, CD19, CD36, CD56, CD123, T-cell receptor γ/ζ, and glycophorin A. Negative selection was performed to prevent the accidental activation of signals in the cells that were used in the cocultures described below. Approximately 3 × 107 to 4 × 107 naïve T cells were isolated from each donor per experiment. The naïve CD4+ T cells were stained with anti-human CD25-PEcy5 (BD Biosciences) and anti-human FoxP3 (eBiosciences) monoclonal antibodies as negative controls for the starting population. For coculture with GEC, 1 × 106 naïve T cells were added to each well of GEC in RPMI as described above for a 10:1 T-cell/GEC ratio and incubated for 7 days at 37°C with 5% CO2. For blocking, anti-B7-H1 blocking antibody (5 μg/ml; functional grade from eBiosciences) was incubated with the cells for 1 h before the addition of T cells. As an isotype control, mouse immunoglobulin G1 antibody (5 μg/ml; functional grade from eBioscience) was used. B7-H1 expression was also blocked in other cells by siRNA.
Flow cytometry.
T cells from coculture assays were stained for CD4, CD25, and FoxP3 for analysis by flow cytometry. Mouse anti-human CD4-fluorescein isothiocyanate (FITC) or -PE (BD Biosciences) and CD25-FITC antibodies (BD Biosciences) were incubated with the cells for 1 h at 4°C. After being washed, the cells were permeabilized with a Fixation and Permeabilization Kit from eBiosciences (San Diego, CA) according to the manufacturer's instructions. The cells were then incubated with mouse anti-human FoxP3-PE-conjugated antibodies (eBiosciences) for 1 h at 4°C. Some samples were single and triple stained with isotype control antibodies, as well. After being washed two more times, the cells were analyzed by flow cytometry on a FACScan cytometer (Becton Dickinson) using Cell Quest software. Gates were set up to eliminate dead cells and cellular debris based on controls.
IL-10 and TGF-β ELISA.
Supernatants were collected from coculture wells after 7 days and used to quantitate the production of IL-10 and TGF-β by enzyme-linked immunosorbent assays (ELISAs) (BD Biosciences), according to the manufacturer's instructions.
Real-time PCR.
Total cellular RNA was isolated from T cells with an RNeasy RNA isolation kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. The sample concentration was measured with a spectrophotometer at 260 nm, and the RNA quality was determined on a 1% agarose gel. Real-time PCR was performed according to the Applied Biosystems's two-step real-time PCR protocol (Applied Biosystems, Foster City, CA). All reagents were purchased from Applied Biosystems. The reverse transcription (RT) reaction mixture included 2.5 μM random hexamers, 500 μM deoxynucleoside triphosphates, 0.4 U/μl of the RNase inhibitors, 5.5 mM MgCl2, MultiScribe Reverse Transcriptase (3.125 U/μl) and its buffer, 1 μg of cellular RNA. The RT mixture was adjusted to a final volume of 50 μl by using RNase- and DNase-free H2O (Sigma). The RT step was performed using a GeneAmp PCR system 9700 thermocycler (Perkin-Elmer Applied Biosystems) according to the following protocol: 10 min at 25°C, 60 min at 37°C, 5 min at 95°C. The cDNA samples obtained were stored at −20°C (if necessary) and used for the PCR step. The PCR mixture was prepared using TaqMan Universal PCR Master Mix (Applied Biosystems). The assays-on-demand gene expression assay mix (Applied Biosystem) for human 18S rRNA, FoxP3, IL-10, TGF-β1, TGF-β2, or TGF-β3 (a 20× mixture of unlabeled PCR primers and TaqMan MGB probe, 6-carboxyfluorescein dye labeled) and 2 μl of cDNA were added to the PCR mixture. The reaction was carried out in a 20-μl final volume using a GeneAmp 5700 Sequence Detection System (Applied Biosystems) according to the following protocol: 2 min at 50°C, 10 min at 95°C (1 cycle), 15 s at 95°C, and 1 min at 60°C (40 cycles). The negative controls were included in the RT real-time two-step reaction. The endpoint used in real-time PCR quantification, the threshold cycle (CT), was defined as the PCR cycle number that crossed the signal threshold. CT values ranged from 0 to 40, with the latter number assumed to represent no product formation. Quantification of cytokine gene expression was performed using the comparative CT method (Sequence Detector User Bulletin 2; Applied Biosystems) and was reported as the difference relative to the human housekeeping gene, 18S rRNA. In order to calculate the change (n-fold increase or decrease), the CT value of 18S rRNA was subtracted from the CT value of the target cytokine gene to yield the ΔCT. Change in the expression of the normalized target gene as a result of experimental conditions was expressed as 2−ΔΔCT, where ΔΔCT was equal to ΔCT experimental samples minus the ΔCT biological control.
T-cell suppression assays.
CD4+ T cells recovered from the cocultures with GEC were washed twice with Hanks solution without Ca2+ and Mg2+ (Cellgro, Herndon, VA) and resuspended in RPMI at a concentration of 107 cells/ml. The population of CD4+ CD25high cells was sorted by staining them with monoclonal antibodies against CD4 and CD25 (BD Biosciences) and was sorted into CD4+ CD25bright and CD4+ CD25− cells using the BD FACSAria cell-sorting system (Becton Dickinson, Heidelberg, Germany). The purity of the CD4+ CD25high T cells after being sorted was >98% (as determined by flow cytometry). For T-cell suppression assays, 105 naïve CD4+ T cells isolated from PBMC, as described above, were activated by using a T-cell Activation/Expansion kit containing beads with anti-CD2, anti-CD3, and anti-CD28 antibodies (Miltenyi Biotec) according to the manufacturer's instructions. The activated T cells were cultured in the absence or presence of increasing numbers of newly generated CD4+ CD25bright T cells at Treg/Teff cell ratios of 1:20 to 1:120. The cells were cultured for 72 h at 37°C in 5% CO2 using 96-well flat-bottom plates. One microcurie of [3H]thymidine was added 18 h prior to cell harvesting. At the end of the cultures, cells were harvested onto glass fiber filter mats and [3H]thymidine incorporation (cpm) was determined using a liquid scintillation counter (Perkin Elmer, Wellesley, MA). The direct suppressive effect of CD4+ CD25high T cells was evaluated by measuring [3H]thymidine incorporation. The results were expressed in cpm as the mean cpm ± standard error (SE) of triplicate cultures.
Statistical analysis.
Results are expressed as the mean ± SE of the mean (SEM). Data were analyzed using one-way analysis of variance. A P value of < 0.05 was considered significant.
RESULTS
GEC exposed to H. pylori induce CD4+ CD25high FoxP3+ Treg cells.
Since Treg cells have recently been identified in the mucosa of H. pylori-infected patients (16), we sought to determine the role the gastric epithelium plays in the induction of the Treg phenotype (CD4+ CD25high FoxP3+). To this end, the AGS cell line and HS-738 normal, nontransformed GEC were treated with medium or exposed to H. pylori (100:1 H. pylori/cell ratio) for 24 h. After 24 h, the cells were washed extensively, irradiated, and incubated with isolated naïve T cells isolated from four donors negative for H. pylori. The naïve T cells were incubated with GEC at a T cell/GEC ratio of 10:1 for 7 days, which was determined to be the best time for induction of Treg cells while maintaining viability of the T-cell population in culture. At 7 days, T cells were harvested and stained for CD4, CD25, and FoxP3 with monoclonal antibodies for flow cytometry. Samples were gated to remove dead cells (Fig. 1A). Figure 1B shows a sample from the beginning naïve cell population stained for CD4-PE and CD25-FITC, indicating the purity of naïve cells and their expression of CD4 only, while Fig. 1C shows the naïve population stained for CD25 and FoxP3, indicating there were no cells staining positive for FoxP3 in the beginning population. As seen in Fig. 1D, compared to the isotype control sample, the cells staining positive for CD25 and FoxP3 harvested from cultures with HS-738 cells were minimal (5%). In contrast, cells that had been exposed to H. pylori prior to exposure to naïve T cells induced 24% of the cells with the Treg phenotype (Fig. 1 E). Similar results were seen with T cells harvested from cultures with AGS cells, as shown in Fig. 2, where the average percentage of CD4+ CD25+ FoxP3+ cells for each donor for both GEC is shown. Since H. pylori had been irradiated and the majority washed away, these results suggest that the epithelial cells can induce naïve T cells to be become Treg cells after exposure to H. pylori.
FIG. 1.
CD4+ CD25high FoxP3+ T cells are induced from naïve T cells after incubation with H. pylori-exposed GEC. Shown are (A) naïve T cells incubated with HS-738 cells for forward and side scatter gates, (B) CD4 and CD25 staining of freshly isolated naïve T cells, (C) FITC and PE isotype controls, (D) naïve T cells incubated with HS-738 cells plus isoptype control for 7 days and stained with anti-CD25 FITC and FoxP3 PE monoclonal antibodies, and (E) naïve T cells incubated with H. pylori-exposed HS-738 cells plus isotype control for 7 days and stained with anti-CD25 FITC and FoxP3 PE monoclonal antibodies. Representative results of five experiments are shown.
FIG. 2.
CD4+ CD25high FoxP3+ T cells induced from naïve T cells from four donors after incubation with H. pylori-exposed GEC. The average percentages of cells of the Treg phenotype induced from naïve T cells isolated from four donors negative for H. pylori from all flow cytometry experiments are shown. Naïve T cells from each donor were incubated with both AGS and HS-738 cells. n = 4 for each donor. The error bars indicate SEM.
B7-H1 expressed by GEC after H. pylori exposure induces the development of CD4+ CD25high FoxP3+ T cells.
We have previously shown that B7-H1 expression is up-regulated on GEC after H. pylori exposure and that it may play a role in decreasing overall T-cell responses (5). A separate study suggested that B7-H1 could influence the induction of Treg cells (12), and multiple studies of cancer suggest that B7-H1 expression and the presence of Treg cells in the tissue are increased. Hence, we sought to determine if the expression of B7-H1 on GEC affects the induction of the Treg phenotype, which has been shown to be present in the gastric mucosa of H. pylori-infected individuals (16). In order to investigate this, B7-H1 was blocked with monoclonal antibodies on H. pylori-exposed GEC before the addition of naïve T cells for 7 days of incubation with GEC. As seen in Fig. 3A, HS-738 cells blocked with anti-B7-H1 after H. pylori exposure reduced the numbers of Treg cells present to only 8% compared to 24% for GEC incubated with isotype control, as seen in Fig. 1.
FIG. 3.
B7-H1 on GEC induces the development of T cells to the Treg cell phenotype after H. pylori exposure. (A) Naïve T cells incubated with HS-738 cells in the presence of anti-B7-H1 are decreased in staining for CD25 and FoxP3 compared to those incubated with isotype control (Fig. 1E). Representative results of five experiments are shown. (B) Real-time PCR for FoxP3 mRNA isolated from T cells incubated with GEC exposed to H. pylori results in less FoxP3 mRNA when GEC are preincubated with anti-B7-H1 than with isotype control. The results indicate the change over the isotype control relative to the 18S housekeeping gene. The means and SEM are shown as the results of duplicates in four experiments; n = 8.
The induction of the Treg phenotype and the role B7-H1 plays in this mechanism was verified by real-time PCR. RNA was isolated from T cells in coculture assays with GEC incubated with isotype control alone, isotype control and H. pylori, or anti-B7-H1 and H. pylori and analyzed for FoxP3 message. The results indicated that naïve T cells incubated with HS-738 or AGS cells previously exposed to H. pylori yielded an approximately four- to sixfold increase in FoxP3 mRNA compared to T cells cocultured with untreated GEC in the presence of isotype control (Fig. 3B). However, when H. pylori-exposed cells were incubated with anti-B7-H1 prior to the addition of naïve T cells, the level of FoxP3 mRNA was minimally increased over untreated cells.
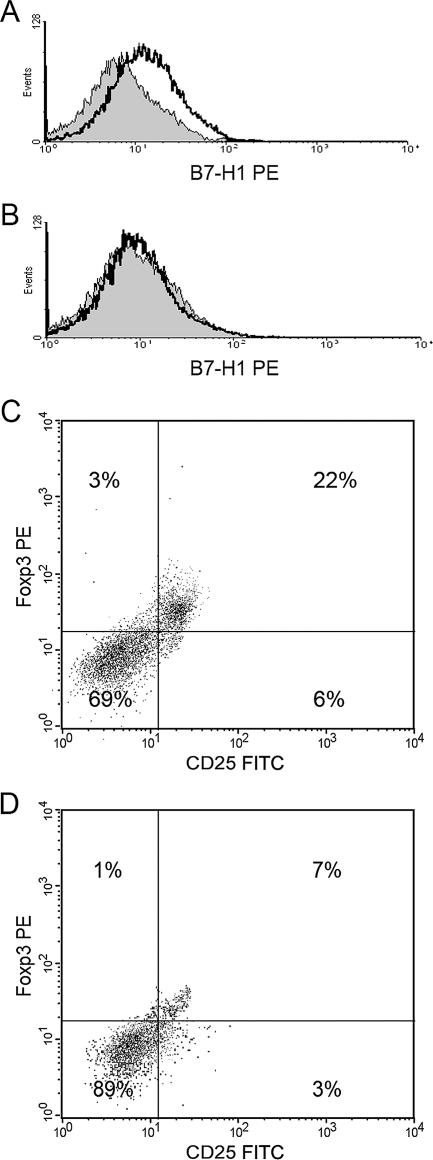
In order to further confirm that B7-H1 expressed by GEC plays a role in the induction of the Treg phenotype, a cocktail of siRNAs for B7-H1 or negative control siRNAs were incorporated into GEC previously infected with H. pylori, and the cells were rested in culture for 48 h. In order to verify that the siRNA was able to block the expression of B7-H1, samples of the transfected cells were then stained for B7-H1 with monoclonal antibodies and analyzed for expression by flow cytometry. Figure 4A and B shows that expression of B7-H1 was almost completely abolished by this procedure compared to cells transfected with siRNA control. When these cells were exposed to H. pylori and then put into coculture with naïve T cells, flow cytometric analysis for CD25 and FoxP3 revealed results similar to those of antibody blocking of B7-H1 (Fig. 3) compared to cells with control siRNA (Fig. 4C and D). Both blocking B7-H1 with monoclonal antibodies and blocking expression by utilizing siRNA to knock down B7-H1 expression in cells exposed to H. pylori clearly demonstrate the importance of this receptor in the induction of Treg cells. Since the cells treated with blocking anti-B7-H1 or siRNA were also exposed to H. pylori but failed to induce the putative Treg phenotype in the cocultured naïve T cells, the potential role of residual bacteria or bacterial components in this process was negated.
FIG. 4.
B7-H1 siRNA decreases the development of T cells to the Treg cell phenotype. AGS cells previously exposed to H. pylori were transfected with (A) control siRNA or (B) a cocktail of B7-H1 siRNA and stained for B7-H1, indicating that B7-H1 expression is blocked compared to the control. Naïve T cells incubated for 7 days with AGS cells previously exposed to H. pylori transfected with (C) control siRNA or (D) a cocktail of B7-H1 siRNA were stained with anti-CD25-FITC, and anti-FoxP3-PE antibodies. Representative results of four experiments are shown.
IL-10 and TGF-β are produced during CD4+ T-cell coculture with H. pylori-exposed GEC but are decreased by blocking B7-H1.
Treg cells have been shown to produce high levels of IL-10 and TGF-β (24). In order to investigate the role of the Treg cells that develop during H. pylori infection and to assess their potential for inhibition of other T cells, levels of these cytokines were measured in supernatants of coculture experiments. GEC were irradiated prior to the addition of naïve T cells to ensure that only T cells were producing cytokines. ELISAs indicated that IL-10 and TGF-β were produced minimally by T cells in culture with GEC for 7 days or by H. pylori-exposed GEC alone, but when naïve T cells were in cultures with GEC that had been previously exposed to H. pylori, production of both IL-10 and TGF-β was detected (Fig. 5A and B). However, when B7-H1 was blocked by monoclonal antibodies or its expression was blocked by siRNA, the levels of IL-10 and TGF-β were decreased to minimal levels, similar to those observed when naïve T cells were cocultured with GEC that were not exposed to H. pylori. These results provide further evidence for the presence of Treg cell development in cocultures of naïve T cells with H. pylori-exposed GEC. Furthermore, the presence of these cytokines suggests these Treg cells play a role in inhibiting the T-cell response during H. pylori infection.
FIG. 5.
T cells cocultured with H. pylori-exposed AGS cells produce IL-10 and TGF-β. Naïve T cells incubated with H. pylori-exposed GEC produce (A) IL-10 and (B) TGF-β, as shown after 7 days in coculture by ELISA with supernatants. Cytokine levels are decreased in the presence of anti-B7-H1 or B7-H1 siRNA. The means and SEM are shown as the results of duplicates in four experiments; n = 8 (*, P < 0.05).
CD4+ CD25high FoxP3+ T cells induced by H. pylori-exposed GEC inhibit activated-T-cell proliferation.
Since Treg cells are known to inhibit Teff cell responses, T cells expressing the Treg phenotype from coculture with H. pylori-exposed GEC were assessed for the ability to inhibit activated T cells. After 7 days of incubation with GEC or GEC preincubated with H. pylori, T cells from these cultures were sorted into CD4+ CD25high and CD4+ CD25− populations. These populations were incubated for 4 days with naïve T cells from the same donor activated with anti-CD2, anti-CD3, and anti-CD28 in various ratios of Treg to Teff cells, and [3H]thymidine uptake was measured for T-cell proliferation. Figure 6A illustrates that CD4+ CD25high T cells from H. pylori-infected AGS cell cocultures incubated with activated Teff cells significantly reduced their thymidine incorporation. In contrast, CD4+ CD25− T cells did not affect the proliferation of activated Teff cells. Thymidine incorporation decreased as the number of CD4+ CD25high cells increased. The suppressive effects of Treg cells generated from H. pylori-exposed cells is further shown in Fig. 6B, which shows the mean percent suppression of cells isolated from several donors in Treg/Teff cell ratios of 1:20. The mean percent suppression for Treg cells isolated from H. pylori-exposed GEC was approximately 40%, based on three experiments (n = 9). These results were similar to the level (percentage) of suppression seen with CD4+ CD25high T cells isolated from PBMC from the same donors. The ability of the Treg cells induced from naïve-T-cell incubation with the H. pylori-infected epithelial cells to inhibit activated T cells suggests a crucial role for these cells in the hyporesponsiveness of T cells during H. pylori infection.
FIG. 6.
CD4+ CD25high T cells induced by coculture with H. pylori-exposed GEC suppress the proliferation of activated CD4+ T cells. CD4+ CD25high T cells induced by coculturing of naïve T cells with AGS cells previously exposed to H. pylori for 7 days were sorted by FACSAria sorter, and CD4+ CD25high or CD4+ CD25− T cells were cocultured with CD2/CD3/CD28-activated CD45RA+ CD4+ CD25− Teff cells for 4 days in various Treg/Teff cell ratios. Eighteen hours prior to the end of coculture, the cells were pulsed with [methyl-3H]thymidine. (A) Mean cpm + SE of triplicate cultures of CD4+ T cells isolated from one donor are shown in a representative of three experiments in which CD4+ CD25high cells suppressed the proliferation of activated CD4+ T cells while CD4+ CD25− cells did not. (B) The percent suppression of Teff cells is shown, which represents the decrease in the proliferative response (cpm) of CD4+ Teff cells compared to activated Teff cells alone. The values represent the mean percent suppression for three independent healthy donors. The means are shown as the results of triplicates in three experiments; n = 9 (*, P < 0.05).
CD4+ CD25high T cells produce increased FoxP3, IL-10, and TGF-β mRNA levels.
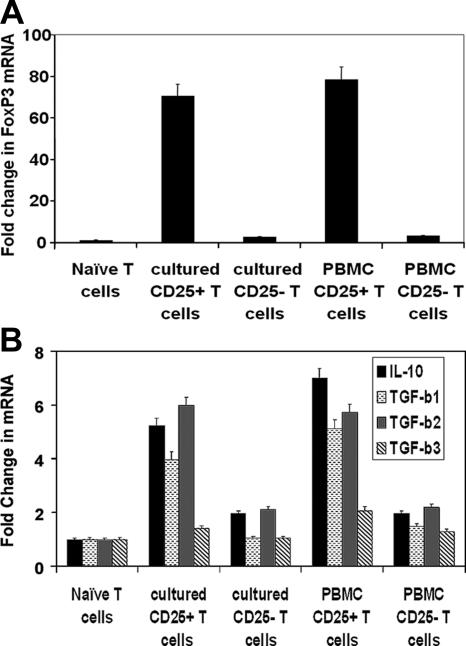
Sorted CD25high and CD25− T cells from H. pylori-infected coculture assays with GEC were further investigated for levels of FoxP3, IL-10, and TGF-β mRNA by real-time PCR to assess if differences in the suppressive abilities of these populations were correlated with the expression of these Treg markers. CD25high T cells produced high levels of FoxP3 mRNA compared to CD25− T cells, suggesting that CD25high Treg cells are the main FoxP3 expressers (Fig. 7A). However, T cells negative for CD25 had approximately twofold induction of FoxP3 mRNA, suggesting the presence of a small population of CD25− FoxP3+ T cells. Similar results were seen with Treg cells sorted from PBMC.
FIG. 7.
CD4+ CD25high T cells express high FoxP3, IL-10, and TGF-β mRNA levels. Real-time PCR for (A) FoxP3 and (B) IL-10 and TGF-β isoforms with RNA isolated from CD4+ CD25high or CD4+ CD25− T cells sorted from populations incubated with H. pylori-infected cells and compared to that of naïve T cells from the same donor illustrates that the CD25high population of T cells produced more RNA for these genes than the CD25− population. The mean (+SEM) increase over naïve T cells is shown for triplicates.
The mRNA levels of T-cell-inhibitory cytokines were also assessed by real-time PCR for CD25high and CD25− T-cell populations and compared to naïve T cells. For IL-10, T cells expressing high levels of CD25 exhibited approximately fivefold-higher mRNA levels, while CD25-negative cells produced levels only twofold higher than those in naïve T cells (Fig. 7B). The mRNA levels for the three isoforms of human TGF-β were also investigated, and the cells expressing high levels of CD25 expressed higher levels of all three than the CD25-negative cells. TGF-β1 mRNA was present in high expressers of CD25 at levels three- to fourfold higher than in naïve T cells from the same donors (Fig. 7C), while CD25-negative cells did not exhibit an increase in TGF-β1 mRNA. TGF-β2 mRNA was increased by sixfold for high expressers of CD25, while the CD25-negative cells exhibited a twofold increase. TGF-β3 mRNA resulted in only minimal increases. These results indicate that Treg cells expressing high levels of CD25 also express dramatically higher levels of FoxP3, IL-10, TGF-β1, and TGF-β2 than CD25-negative cells in this system. Although CD25− cells do not produce dramatically increased levels of IL-10 and TGF-β2 mRNA, they do have twofold-higher levels than naïve T cells, which may suggest that they could exert suppressive effects in this system.
DISCUSSION
The host immune response during H. pylori infection is not sufficient to clear infection. Interestingly, T cells from infected individuals are hyporesponsive and are polarized toward a Th1 phenotype. H. pylori has been shown to directly affect the T-cell response by multiple mechanisms. For example, H. pylori can induce T-cell apoptosis by up-regulating Fas ligand on Fas-expressing T cells (29). Also, the VacA virulence factor has been shown to down-regulate IL-2 receptors on T cells, inhibit production of IL-2, and decrease cell viability (4). We have shown that H. pylori may inhibit T-cell responses in an indirect way by up-regulating B7-H1 expression on GEC, both transformed and nontransformed, which in turn inhibited T-cell proliferation in coculture experiments (5).
Recently, yet another mechanism of T-cell inhibition during infection has been discovered. The presence of Treg cells has been revealed in the gastric mucosa of H. pylori-infected patients (16). In another recent study, CD4+ FoxP3+ T cells were detected in H. pylori-infected patients with gastric adenocarcinoma (7). More specifically, higher levels of these cells were present in tumor tissue than in tumor-free gastric tissue. Together, these observations could contribute not only to the chronicity of H. pylori infection, but also to the development of associated neoplasia. Although recent studies have reported the presence of Treg cells in H. pylori-infected gastric mucosa, the recruitment or development of these T cells has not been characterized. Here, we have shown that naïve T cells cultured with H. pylori-infected GEC can develop into cells with the Treg phenotype. The formation of these cells was dependent upon B7-H1 expressed on GEC, which we previously showed was dramatically up-regulated by exposure both in vivo and in vitro to multiple strains of H. pylori (5) and which has been similarly shown in other studies to be induced by microbial products or Th1 cells (15). However, it is unlikely that residual H. pylori-derived products influenced the expansion of cells with a Treg phenotype, since cocultures of GEC were extensively washed and the presence of anti-B7-H1 antibodies prevented their expansion. The increased expression of B7-H1 on epithelium appears to be complex and can be induced by gamma interferon and tumor necrosis factor alpha to some extent. The induction of cells of the Treg phenotype by B7-H1 may very well play an important role in the T-cell suppression associated with H. pylori infection, as well as other infections that result in an up-regulation of B7-H1. These cells have already been shown to play crucial inhibitory roles in cancer and parasitic infections (1, 26).
Since IL-10 and TGF-β are produced by some types of Treg cells and have essential T-cell-inhibitory functions, their expression during H. pylori infection by induced Treg cells may play an important role in the inhibition of Teff cells. In this study, the T-cell population expressing high levels of CD25 also expressed high levels of FoxP3 mRNA, along with IL-10 and TGF-β, particularly TGF-β2. In contrast, the CD25− sorted population expressed very little FoxP3 mRNA but also exhibited small increases in IL-10 and TGF-β levels, suggesting the presence of a small population of T-regulatory type 1 (Tr1) cells. Although several studies have suggested that Tr1 cells are more important in inhibitory cytokine production than CD25-expressing Treg cells (22, 28), we found that both cell types produced increased mRNA for IL-10 and TGF-β, with CD25-expressing Treg cells producing much higher levels.
As for functionality, the cells expressing high levels of CD25 were able to decrease the proliferation of activated T cells from the same donors at much higher levels than the CD25− cells. The high expressers of CD25 were also sorted from the PBMC of the same donors to compare functionality with those induced by H. pylori-infected GEC. The results from this population indicated that the induced Treg cells had functionality similar to that of the cells from PBMC. It should be noted that the numbers of Treg cells used here in functional assays were similar to or below the numbers reported to be present in patient samples of H. pylori-infected gastric mucosa (16) and still had a suppressive effect on the proliferation of CD4+ Teff cells. The studies presented here show how H. pylori may use the epithelium to promote the development of Treg cells. These Treg cells, in turn, play a key role in the suppression of CD4+ Teff cells that are recruited to the gastric mucosa during infection. While this may represent an important mechanism that facilitates the persistence of H. pylori in the host, perhaps these cells may also facilitate immune avoidance of any cancer cells that develop within the infected stomach. Thus, the presence of CD4+ CD25high FoxP3+ Treg cells during H. pylori infection may be crucial in down-regulating the T-cell response and failure to clear infection. These Treg cells may play a crucial role in promoting chronic infections, and perhaps eventual carcinogenesis.
Acknowledgments
This work was supported by National Institutes of Health grant DK050669.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Antony, P. A., and N. P. Restifo. 2005. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J. Immunother. 28:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 3.Barrera, C., G. Ye, R. Espejo, S. Gunasena, R. Almanza, J. Leary, S. Crowe, P. Ernst, and V. E. Reyes. 2001. Expression of cathepsins B, L, S, and D by gastric epithelial cells implicates them as antigen presenting cells in local immune responses. Hum. Immunol. 62:1081-1091. [DOI] [PubMed] [Google Scholar]
- 4.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3:320-332. [DOI] [PubMed] [Google Scholar]
- 5.Das, S., G. Suarez, E. J. Beswick, J. C. Sierra, D. Y. Graham, and V. E. Reyes. 2006. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J. Immunol. 176:3000-3009. [DOI] [PubMed] [Google Scholar]
- 6.D'Elios, M. M., M. Manghetti, F. Almerigogna, A. Amedei, F. Costa, D. Burroni, C. T. Baldari, S. Romagnani, J. L. Telford, and P. G. Del. 1997. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 27:1751-1755. [DOI] [PubMed] [Google Scholar]
- 7.Enarsson, K., A. Lundgren, B. Kindlund, M. Hermansson, G. Roncador, A. H. Banham, B. S. Lundin, and M. Quiding-Jarbrink. 2006. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin. Immunol. 121:358-368. [DOI] [PubMed] [Google Scholar]
- 8.Fan, X., S. E. Crowe, S. Behar, H. Gunasena, G. Ye, H. Haeberle, N. Van Houten, W. K. Gourley, P. B. Ernst, and V. E. Reyes. 1998. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J. Exp. Med. 187:1659-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham, D. Y., R. E. Rakel, A. M. Fendrick, M. F. Go, B. J. Marshall, D. A. Peura, and J. E. Scherger. 1999. Scope and consequences of peptic ulcer disease. How important is asymptomatic Helicobacter pylori infection? Postgrad. Med. 105:100-110. [DOI] [PubMed] [Google Scholar]
- 10.Ishii, N., M. Chiba, M. Iizuka, H. Watanabe, T. Ishioka, and O. Masamune. 1992. Expression of MHC class II antigens (HLA-DR, -DP, and -DQ) on human gastric epithelium. Gastroenterol. Jpn. 27:23-28. [DOI] [PubMed] [Google Scholar]
- 11.Knipp, U., S. Birkholz, W. Kaup, K. Mahnke, and W. Opferkuch. 1994. Suppression of human mononuclear cell response by Helicobacter pylori: effects on isolated monocytes and lymphocytes. FEMS Immunol. Med. Microbiol. 8:157-166. [DOI] [PubMed] [Google Scholar]
- 12.Krupnick, A. S., A. E. Gelman, W. Barchet, S. Richardson, F. H. Kreisel, L. A. Turka, M. Colonna, G. A. Patterson, and D. Kreisel. 2005. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J. Immunol. 175:6265-6270. [DOI] [PubMed] [Google Scholar]
- 13.Li, Z., and J. Li. 2006. Local expressions of TGF-β1, TGF-β1RI, CTGF, and Smad-7 in Helicobacter pylori-associated gastritis. Scand. J. Gastroenterol. 41:1007-1012. [DOI] [PubMed] [Google Scholar]
- 14.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loke, P., and J. P. Allison. 2003. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA 100:5336-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundgren, A., E. Stromberg, A. Sjoling, C. Lindholm, K. Enarsson, A. Edebo, E. Johnsson, E. Suri-Payer, P. Larsson, A. Rudin, A. M. Svennerholm, and B. S. Lundin. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattsson, A., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, I. Ahlstedt, and A. Svennerholm. 1998. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect. Immun. 66:2705-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paziak-Domanska, B., M. Chmiela, A. Jarosinska, and W. Rudnicka. 2000. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol. 202:136-139. [DOI] [PubMed] [Google Scholar]
- 20.Rad, R., L. Brenner, S. Bauer, S. Schwendy, L. Layland, C. P. da Costa, W. Reindl, A. Dossumbekova, M. Friedrich, D. Saur, H. Wagner, R. M. Schmid, and C. Prinz. 2006. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 131:525-537. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roncarolo, M. G., S. Gregori, and M. Levings. 2003. Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells. Novartis Found. Symp. 252:115-127. [PubMed] [Google Scholar]
- 23.Selenko-Gebauer, N., O. Majdic, A. Szekeres, G. Hofler, E. Guthann, U. Korthauer, G. Zlabinger, P. Steinberger, W. F. Pickl, H. Stockinger, W. Knapp, and J. Stockl. 2003. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J. Immunol. 170:3637-3644. [DOI] [PubMed] [Google Scholar]
- 24.Shevach, E. M. 2006. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25:195-201. [DOI] [PubMed] [Google Scholar]
- 25.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suvas, S., and B. T. Rouse. 2006. Treg control of antimicrobial T cell responses. Curr. Opin. Immunol. 18:344-348. [DOI] [PubMed] [Google Scholar]
- 27.Tamura, H., K. Ogata, H. Dong, and L. Chen. 2003. Immunology of B7-H1 and its roles in human diseases. Int. J. Hematol. 78:321-328. [DOI] [PubMed] [Google Scholar]
- 28.Vieira, P. L., J. R. Christensen, S. Minaee, E. J. O'Neill, F. J. Barrat, A. Boonstra, T. Barthlott, B. Stockinger, D. C. Wraith, and A. O'Garra. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172:5986-5993. [DOI] [PubMed] [Google Scholar]
- 29.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 30.Yao, S., and L. Chen. 2006. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol. Med. 12:244-246. [DOI] [PubMed] [Google Scholar]