Abstract
Intestinal microsporidiosis due to Enterocytozoon bieneusi is a leading cause of chronic diarrhea in severely immunocompromised human immunodeficiency virus (HIV)-positive patients. It may be a public health problem in Africa due to the magnitude of the HIV pandemic and to poor sanitary conditions. We designed two prevalence studies of E. bieneusi in Central Africa, the first with HIV-positive patients from an urban setting in Gabon and the second with a nonselected rural population in Cameroon. Stool samples were analyzed by an immunofluorescence antibody test and PCR. Twenty-five out of 822 HIV-positive patients from Gabon and 22 out of 758 villagers from Cameroon were found to be positive for E. bieneusi. The prevalence rates of the two studies were surprisingly similar (3.0% and 2.9%). Genotypic analysis of the internal transcribed spacer region of the rRNA gene showed a high degree of diversity in samples from both countries. In Gabon, 15 isolates showed seven different genotypes: the previously reported genotypes A, D, and K along with four new genotypes, referred to as CAF1, CAF2, CAF3, and CAF4. In Cameroon, five genotypes were found in 20 isolates: the known genotypes A, B, D, and K and the new genotype CAF4. Genotypes A and CAF4 predominated in Cameroon, whereas K, CAF4, and CAF1 were more frequent in Gabon, suggesting that different genotypes present differing risks of infection associated with immune status and living conditions. Phylogenetic analysis of the new genotype CAF4, identified in both HIV-negative and HIV-positive subjects, indicates that it represents a highly divergent strain.
As many as 90% of AIDS patients experience diarrhea in Africa, where “slim disease” (prolonged diarrhea and wasting) is a pathognomonic trait of AIDS (30). While early investigation revealed that coccidian parasites were a leading cause of chronic diarrhea, the development of improved microscopic, immunological, and molecular diagnostic methods has highlighted the importance of microsporidian infections (11). Two species of microsporidia can cause gastrointestinal disease: Enterocytozoon bieneusi (10) and Encephalitozoon intestinalis (7, 18), although E. bieneusi is the more frequently reported species. Data on the prevalence of E. bieneusi in human immunodeficiency (HIV)-positive patients in Africa is scarce, and values differ greatly, from 5% to 50%, depending on the population studied (whether or not it is selected for diarrhea) and the diagnostic methods (2, 5, 17, 21, 27, 38, 43). Besides, E. bieneusi also can infect immunocompetent patients, especially children, although the disease is less serious and the infection is self-limited in the absence of immune abnormalities (42). Since mature spores are shed in the host's feces, the transmission routes of this pathogen may involve person-to-person as well as waterborne or food-borne contaminations, especially in developing countries with poor sanitation (3). E. bieneusi also has been isolated from a large number of domestic and wild mammals, as well as birds (29). The potential of zoonotic transmission is supported by phylogenetic studies showing that several genotypes can infect humans as well as animals (9, 12, 26, 37, 39-41).
To address the importance of E. bieneusi infections in Africa, we conducted two studies of prevalence in different populations, the first with HIV-infected patients from an urban setting in Gabon and the second with a rural population in Cameroon. The presence of E. bieneusi spores in stool samples was detected by an immunofluorescent antibody test (IFAT) and PCR. Genotypic analysis of E. bieneusi isolates was performed by sequencing the internal transcribed spacer (ITS) portion of the rRNA gene. On the basis of these results, a phylogenetic interpretation regarding the sources and modes of transmission of the different groups of E. bieneusi genotypes is proposed.
MATERIALS AND METHODS
Origin of E. bieneusi samples. (i) Gabon.
A cross-sectional study was conducted between September 2002 and December 2003 in Gabon to determine the prevalence of intestinal microsporidiosis in adult HIV-positive patients. This study, approved by the Ministry of Health in Gabon and the Ethics Committee of the Comité Consultatif pour la Protection des Personnes en Recherche Biomédicale in St-Germain-en-Laye (France), was conducted in three HIV reference centers in Libreville. Upon informed consent, patients aged 16 or older and with an HIV-positive test were included in the study. The CD4+ lymphocyte count was performed with a fluorescence-activated cell sorter (FACScount; Becton Dickinson Biosciences, Erembodegem, Belgium). The detection and identification of E. bieneusi spores in stool samples were performed using an IFAT with the monoclonal antibody 6E5-2D9, which is specific for the spore wall (1, 2). Positive or undetermined samples were confirmed by a species-specific PCR test as previously described (2). HIV serologic status, CD4+ cell count, and microsporidium identification were done in Libreville. One aliquot of each positive stool sample and 30% of the negative samples were diluted in 10% formalin and shipped to France as an external blinded control.
(ii) Cameroon.
In March 2003, the population of the Bongo village in the central region of Cameroon, 120 km north of Yaoundé, was asked to participate in a study on microsporidiosis, regardless of sex, age, or HIV status. The village, with a population of around 2,200 inhabitants, spreads 12 km along a main road, among mixed savannah and rain forest. The climate is tropical, with two rainy seasons running from March to May and August to November. The water supply is restricted to a single well, and sanitation facilities essentially consist of private latrines outside dwellings. After giving informed consent, 758 villagers gave stool and blood samples. Stool samples in 10% formalin and serum samples were shipped to France for analysis. Serologic HIV status was determined using the Core Diagnostics HIV 1&2 test (Birmingham, United Kingdom). In the case of a positive result, enzyme-linked immunosorbent assay, Immunocomb HIV1/HIV2 (PBS-Orgenics, Courbevoie, France), and Western blot HIV-1 analysis (Newlavblot 1 Bio-Rad, Marnes-la-Coquette, France), performed on the same serum sample, were used for confirmation. Stool samples were screened for microsporidium spores as described above.
DNA extraction and PCR amplification.
For DNA extraction, samples in phosphate-buffered saline or formalin were washed three times in CTAB buffer (10% [wt/vol] cetyltrimethylammonium bromide, 0.7 M NaCl) and centrifuged. The pellet was resuspended in 200 μl of CTAB buffer and then digested for 1 h at 56°C with proteinase K, followed by a phenol-chloroform extraction. DNA was further purified from the aqueous phase using the QIAmp DNA kit (QIAGEN, Courtaboeuf, France) according to the manufacturer's instructions.
Samples positive for microsporidia were confirmed by PCR using the following primers common to the rRNA gene of E. bieneusi and Encephalitozoon intestinalis: INBI (5′-CAC CAG GTT GAT TCT GCC TGA C-3′) and PMP2 (5′-CCT CTC CGG AAC CAA ACC CTG-3′) (44). The presence of E. bieneusi was confirmed by a species-specific amplification reaction using the primer pair INBI and BIENE (5′-ACT CAG GTG TTA TAC TCA CGT C-3′) (45). PCR conditions were applied as described previously (2) using a PTC-100 thermocycler (MJ Research, Waltham, MA) in Paris and a GeneAmp PCR system 2700 (Applied Biosystems, Courtaboeuf, France) in Libreville. PCR products were separated by electrophoresis in a 2% agarose gel and visualized after ethidium bromide staining. In cases of different results between IFAT and PCR, the ambiguity was resolved by a second IFAT examination, and a modified Weber trichrome staining (20) was performed in a different laboratory (the laboratory of I. Accoceberry, CHU de Saint André, Bordeaux, France).
Nucleotide sequencing of the ITS region of the E. bieneusi rRNA gene.
E. bieneusi genotypes were analyzed by nucleotide sequencing of the ITS region of the rRNA gene. A PCR product of 508 bp, containing 122 bp of the small-subunit rRNA, 243 bp of the ITS region, and 143 bp of the large-subunit rRNA, was generated from 15 samples from Gabon and 20 samples from Cameroon, using the primers MSP-3 [5′-GGA ATT CAC ACC GCC CGT C(A/G)(C/T) TAT-3] and MSP4B (5′-CCA AGC TTA TGC TTA AGT CCA GGG AG-3′) as described previously (19). PCR products were purified using the Concert Rapid PCR kit (GIBCO-BRL) and sequenced in both directions using the ABI Big Dye Terminator kit (v1.1) and an ABI 3100 automated sequencer (Applied Biosystems). The sequence accuracy was controlled by sequencing two PCR products from the same sample.
Phylogenetic analysis.
The ITS sequences obtained were compared to those from previously published records in GenBank by using BLAST analysis. Multiple alignment of our new ITS nucleotide sequences and 62 sequences retrieved from GenBank (available by June 2006) was performed using the Multalin program (http://prodes.toulouse.inra.fr/multalin/multalin.html) (8). Only complete ITS sequences were included (241 to 243 bp, depending on the genotype). In cases of identical sequences with different type names, we retained the type name of the first published sequence in order to simplify our genotypic classification (type names of the remaining identical sequences are specified in parentheses in Table 2). On the basis of this multiple alignment, maximum-likelihood phylogenetic analysis of the E. bieneusi genotypes was carried out with the Phyml program with nonparametric bootstrapping, using the evolutionary model of Jukes and Kantor (http://bioweb.pasteur.fr/seqanal/interfaces/phyml.html) (16).
TABLE 2.
Epidemiological traits of the E. bieneusi genotypes in published records
| Genotypea | Host/Country (reference or source) | Accession no. |
|---|---|---|
| Group 1 | ||
| Subgroup 1a | ||
| CAF3 | Human/Gabon (this study) | DQ683748 |
| Peru8 | Human/Peru (39) | AY371283 |
| Peru10 | Human/Peru (39); cat/Colombia (37) | AY371285 |
| Peru11 | Human/Peru (39), Thailand (23) | AY371286 |
| T | Human/Thailand (23) | AY945810 |
| V | Human/Thailand (23) | AY945812 |
| pigEBITS6 | Swine/United States (6) | AF348474 |
| pigEBITS8 | Swine/United States (6) | AF348476 |
| L | Cat/Germany (9) | AF267142 |
| EbfelA | Cat/Switzerland (28) | AF118144 |
| 4948-FL-2 | Cattle/United States (36) | DQ154136 |
| WL7 | Beaver/United States (40) | AY237215 |
| D (pigEBITS9, WL8, Peru9) | Human/Germany (33), United Kingdom (35), Peru (39), Gabon (this study), Cameroon (this study), Thailand (23); swine/United States (6); cattle/United States (36); macaque/United States (12, 15), Germany (12); muskrat, raccoon, beaver, fox/United States (40); cat/Colombiab (37) | AF101200 |
| Subgroup 1b | ||
| Peru6 | Human/Peru (39); commensal birds/Portugalc (26) | AY371281 |
| Peru7 | Human/Peru (39) | AY371282 |
| TypeV | Human/France (25) | AF242479 |
| R | Human/Thailand (23) | AY945808 |
| S | Human/Thailand (23) | AY945809 |
| UG2145 | Human/Uganda (42) | AF502396 |
| pigEBITS5 | Swine/United States (6) | AF348473 |
| pigEBITS7 | Human/Thailand (23); swine/United States (6) | AF348475 |
| Subgroup 1c | ||
| A (Peru1) | Human/Switzerland (4), Germany (32), Peru (39), Thailand (22), Gabon (this study), Cameroon (this study) | AF101197 |
| B (TypeI) | Human/Switzerland (4), Germany (32), France (24, 25), United Kingdom (35), Cameroon (this study) | AF101198 |
| Peru3 | Human/Peru (39) | AY371278 |
| CAF2 | Human/Gabon (this study) | DQ683747 |
| TypeIII | Human/France (24, 25) | AF242477 |
| K (TypeIV, Peru2, BEB5) | Human/France (24, 25), United Kingdom (35), Peru (39), Uganda (42), Gabon (this study), Cameroon (38) | AF267141 |
| Cat/Germany (9), Colombia (37); cattle/United States, Portugal (41) | AF267146 | |
| P | Llama/Germany (9) | |
| WL9 | Beaver/United States (40) | AY237217 |
| WL10 | Muskrat/United States (40) | AY237218 |
| WL11 (Peru5) | Human/Peru (39); fox/United States (40); cat/Colombia (37) | AY237219 |
| WL12 | Beaver, otter/United States (40) | AY237220 |
| Subgroup 1d | ||
| E (EbpC, WL13, Peru4) | Human/Peru (39), Thailand (23); swine/Switzerland (4); muskrat, raccoon, beaver, fox, otter/United States (40) | AF135832 |
| WL14 | Muskrat/United States (40) | AY237222 |
| WL15 | Muskrat, raccoon, beaver, fox/United States (40) | AY237223 |
| WL16 | Muskrat, raccoon/United States (40) | AY237224 |
| WL17 | Raccoon/United States (40) | AY237225 |
| Subgroup 1e | ||
| EbpB | Swine/Switzerland (4) | AF076041 |
| EbpD | Swine/Switzerland (4) | AF076043 |
| F (EbpA) | Swine/Switzerland (4), Germany (9, 34); cattle/Germany (9) | AF135833 |
| Gd | Swine/Germany (34) | AF135834 |
| Hd | Swine/Germany (34) | AF135835 |
| O | Swine/Germany (9); human/Thailand (23) | AF267145 |
| M | Cattle/Germany (9) | AF267143 |
| pigEBITS1 | Swine/United States (6) | AF348469 |
| pigEBITS2 | Swine/United States (6) | AF348470 |
| pigEBITS3 | Swine/United States (6) | AF348471 |
| pigEBITS4 | Swine/United States (6) | AF348472 |
| U | Human/Thailand (23) | AY945811 |
| W | Human/Thailand (23) | AY945813 |
| Subgroup 1f | ||
| C (TypeII) | Human/France (24, 25), Switzerland (4), Germany (9) | AF101199 |
| Q | Human/Germany (9) | AF267147 |
| Subgroup 1g | ||
| CAF1 | Human/Gabon (this study) | DQ683746 |
| Group 2 | ||
| I | Cattle/Germany (9, 34) | AF135836 |
| J (BEB1) | Cattle/Germany (9, 34), United States (36, 41); chicken/Germany (31) | AF135837 |
| N (BEB2) | Cattle/Germany (9), United States (36, 41) | AF267144 |
| BEB3 | Cattle/United States (36, 41) | AY331007 |
| BEB4 | Cattle/United States (36, 41) | AY331008 |
| Group 3 | ||
| WL4 | Muskrat/United States (40) | AY237212 |
| WL5 | Muskrat/United States (40) | AY237213 |
| WL6 | Muskrat/United States (40) | AY237214 |
| Group 4 | ||
| WL1 | Raccoon/United States (40) | AY237209 |
| WL2 | Raccoon/United States (40) | AY237210 |
| WL3 | Raccoon/United States (40) | AY237211 |
| Outliers | ||
| CAF4 | Human/Gabon, Cameroon (this study) | DQ683749 |
| EntCanA | Dog/Switzerland (28) | AF059610 |
Abbreviations in parentheses refer to the other genotype names assigned to identical sequences by various research teams. Accession numbers refer to the first genotype name.
Reported as genotype D-like.
Reported as genotype Peru6 and genotype Peru6-var.
Isolated from the same animal.
Nucleotide sequence accession numbers.
Nucleotide sequences of the ITS region of the rRNA gene of one isolate for each genotype from Gabon and Cameroon were deposited in the GenBank database. The accession numbers for the Gabon isolates are DQ683746 to DQ683752 for the genotypes CAF1, CAF2, CAF3, CAF4, A, D, and K, respectively, and the accession numbers for the Cameroon isolates are DQ683753 to DQ683757 for the genotypes A, B, D, K, and CAF4, respectively.
RESULTS
The Libreville study (Gabon).
A total of 822 patients were included in the study; the mean age was 39.1 years (range, 16 to 75 years), and the sex ratio was 0.63 (male/female). Of these patients, 99% were infected with HIV-1, while only 0.5% were HIV-2 positive, and 0.5% were coinfected with HIV-1 and HIV-2. The mean lymphocyte CD4+ cell count was 218/μl (range, 1 to 1,164; median, 180). About one-third of the patients (30%) suffered from diarrhea, and 24.2% were under antiretroviral therapy. Initial treatment regimens included a combination of one protease inhibitor (usually Indinavir) or a nonnucleoside reverse transcriptase inhibitor (Efavirenz) and two nucleoside reverse transcriptase inhibitors (NRTIs) (d4T, 3TC, AZT, or ddI). The majority (85%) lived in the urban area of Libreville and originated from Gabon (86.5%), whereas the remaining patients were migrant workers from West Africa.
By IFAT, 25 out of the 822 stool samples were positive for E. bieneusi (prevalence, 3%). Of the positive stools, all except three were confirmed to be positive by the species-specific PCR. Negative PCR samples contained very few spores in microscopic examination and remained negative despite repeated internal and external PCR controls. All 25 E. bieneusi-positive patients were HIV-1 positive, and one was coinfected with HIV-2. The mean age was 35.4 years (range, 21 to 57 years), and the sex ratio was 0.47 (male/female). Only four patients (16%; class B2) were not at the AIDS stage of the infection. The average CD4+ cell count of 23 patients was 98.5/μl (range, 1 to 384/μl; values were not available for two patients). A cell count below 100/μl (mean, 29.3/μl) was scored for 15 (65%) out of 23 patients. All except three patients from the class B2 HIV group suffered from digestive symptoms. These symptoms corresponded to diarrhea (14 cases), weight loss (15 cases), anorexia (6 cases), abdominal pain (5 cases), and nausea (5 cases). Six patients were undergoing antiretroviral therapy (three patients were receiving a combination of a nonnucleoside reverse transcriptase inhibitor and two NRTIs, and three patients were receiving a combination of a protease inhibitor and two NRTIs) at least 3 months before the diagnosis of intestinal microsporidiosis. Twenty of these patients (80%) resided in Libreville, while the other five came from distant provinces of the country.
The Bongo study (Cameroon).
The mean age of the Bongo study population was 28.2 years (758 individuals; range, <1 to 80 years), and the sex ratio was 0.81 (male/female). The combination of IFAT and PCR detection of microsporidia showed 22 inhabitants positive for E. bieneusi (prevalence, 2.9%). The Core Diagnostics HIV 1&2 screening showed that only four subjects were HIV-1 positive. Sera of the latter patients were confirmed to be HIV-1 positive by enzyme-linked immunosorbent assay and Western blot analysis, resulting in a prevalence rate of 0.5% in the Bongo population. None of the microsporidium-positive individuals were found to be positive for HIV. Data on the clinical status (diarrhea) of these individuals were not available.
Analysis of genotypes.
Thirty-five (15 from Gabon and 20 from Cameroon) E. bieneusi isolates yielded sufficient PCR product for the sequencing of the ITS region of the rRNA gene. Both series showed a high degree of diversity of genotypes, as shown in Table 1.
TABLE 1.
Distribution of E. bieneusi genotypes in two distinct human cohorts in Central Africaa
| Genotype and country of origin | No. (%) of isolates | Isolate code(s)g |
|---|---|---|
| Gabon | ||
| Ab | 1 (6.6) | G841♂* |
| Dd | 1 (6.6) | G100♀* |
| Ke | 4 (26.6) | G093♀, G107♀, G370♂, G624♂ |
| CAF1f | 3 (19.9) | G084♀*, G704♀, G995♀* |
| CAF2f | 1 (6.6) | G484♀* |
| CAF3f | 1 (6.6) | G577♀ |
| CAF4f | 4 (26.6) | G139♀, G293♀*, G334♂*, G475♀ |
| All genotypes | 15 (100) | |
| Cameroon | ||
| A | 8 (40) | 108B♀, 113B♀, 113H♀, 117A♂, 117B♀, 135A♀, 142G♂, 142H♀ |
| Bc | 3 (15) | 155C♀, 194A♀, 194C♀ |
| D | 3 (15) | 149D♂, 179D♂, 179E♂ |
| K | 1 (5) | 181A♂ |
| CAF4 | 5 (25) | 22K♀, 91A♂, 98A♂, 102B♂, 103A♂ |
| All genotypes | 20 (100) |
The cohort from Gabon was HIV positive and was mainly urban, while the cohort from Cameroon was rural and had a low prevalence of HIV (0.5%; none of the E. bieneusi-positive individuals had HIV).
Previously reported as Human/A/Germany/AF10197 and Human/Peru1/ Peru/AY371276.
Previously reported as Human/B/Germany/AF10198 and Human/TypeI/France/AF242475.
Previously reported as Human/D/Germany/AF101200, Human/Peru9/Peru/AY371284, Pig/PigEBITS9/USA/AF348477, and Fox/WL8/USA/AY237216.
Previously reported as Human/TypeIV/France/AF242478, Human/Peru2/ Peru/AY371277, Cat/K/Germany/AF267141, and Cattle/BEB5/USA/AY331009.
Isolated in this study.
Patients suffering from chronic diarrhea are indicated by an asterisk. Isolates with the same number code refer to members of the same household; the letter following the number indicates the family member (A represents the family chief, and B to F represent other family members). ♀, Female patient; ♂, male patient.
In Gabon, seven different genotypes were identified: the previously reported genotypes A, D, and K as well as four new genotypes. The four new genotypes are referred to as CAF1, CAF2, CAF3, and CAF4 (CAF being short for Central Africa). The genotypes K, CAF4, and CAF1 seemed to be more common among the isolates from Gabon, with four, four, and three isolates each, respectively, whereas genotypes A, D, CAF2, and CAF3 were found only once each.
In Cameroon, five genotypes were identified: the known genotypes A, B, D, and K and the new genotype CAF4. However, genotype A was the most common, with eight isolates, followed by CAF4, which was detected in five cases. Types B and D were scored three times each, and genotype K was found only once.
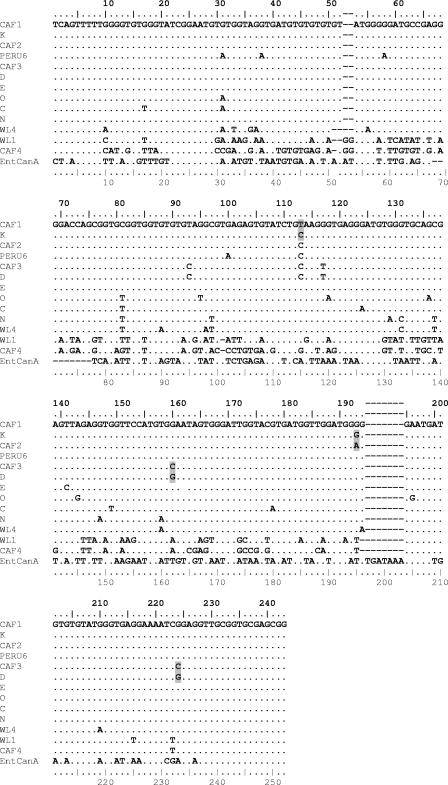
Out of the four new genotypes, three consisted of sequences displaying very high identity to sequences of the known genotypes K and D. Types CAF1 and CAF2 differed from K by only one position (CAF1, position 113 [C→T]; CAF2, position 193 [G→A]), and type CAF3 differed from D at positions 160 and 224 (G→C at both positions) (Fig. 1). The last genotype, CAF4, is 1 bp shorter (242 bp) than all of the others. It was also very divergent from previously reported sequences; the best homology, i.e., 76% identity, was found with the set of genotypes WL1 to WL3, isolated from raccoons in the United States (40). Indeed, the identities of the new sequences to all other previously published sequences were between 61 and 64% and as low as 46% with genotype EntCanA.
FIG. 1.
Sequence alignment of the ITS region of the rRNA gene of E. bieneusi genotypes using the Multalin software. New sequences from Gabon and Cameroon (CAF1 to CAF4) are compared to sequences representative of each group. (Representative sequences are the following: group 1, subgroup 1a, genotype D; group 1, subgroup 1b, genotype Peru6; group 1, subgroup 1c, genotype K; group 1, subgroup 1d, genotype E; group 1, subgroup 1e, genotype O; group 1, subgroup 1f, genotype C; group 2, genotype N; group 3, genotype WL4; group 4, genotype WL1; and the outlier sequence EntCanA). Dots indicate identity to CAF1. Point mutations of the new sequences CAF1, CAF2, and CAF3 compared to their closest homologues, K and D, are shaded gray (for accession numbers, see Table 2) (note that the upper ruler indicates the true sequence position for group 1 genotypes [243 bp], and the lower ruler indicates the Multalin alignment position number, with gaps counted).
The ITS flanking regions (122 bp of the small-subunit rRNA and 143 bp of the large-subunit rRNA) of the nine CAF4 isolates from both studies showed 100% identity to the corresponding regions of the E. bieneusi rRNA sequences from other genotypes.
Phylogenetic analysis.
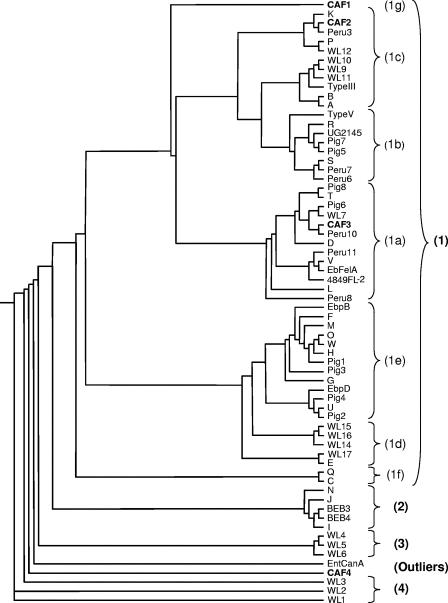
An unrooted phylogram and a phylogenetic unrooted tree inferred by the maximum-likelihood analysis of the ITS region were constructed using the Phyml software (Fig. 2 and 3). As seen in previous studies (12, 40), four main groups, numbered 1 to 4, are segregated from the most divergent sequence, EntCanA, which was isolated from dogs (28). Group 1 contains most of the sequences previously published as well as the new sequences CAF1, CAF2, and CAF3 (53 out of 66 sequence) (Fig. 2). These sequences have been isolated from a wide diversity of hosts worldwide, including humans and animals (Table 2). The other three groups contain sequences isolated from one host species. Group 2 contains five sequences from cattle (N to I) (9, 31, 34, 36, 41). Group 3 is made up of three sequences from muskrat (WL4 to WL6), and group 4 is made up of three divergent sequences from raccoon (WL1 to WL3) (40). Our new human sequence CAF4 is placed, along with EntCanA, between group 3 and group 4, far away from group 1, which contains all the genotypes isolated from humans so far.
FIG. 2.
Unrooted phylogram inferred by the maximum-likelihood analysis of the ITS of E. bieneusi genotypes. The phylogram was constructed using the Phyml software. Groups and subgroups are indicated in parentheses. New genotypes from this study are in boldface (for accession numbers, see Table 2).
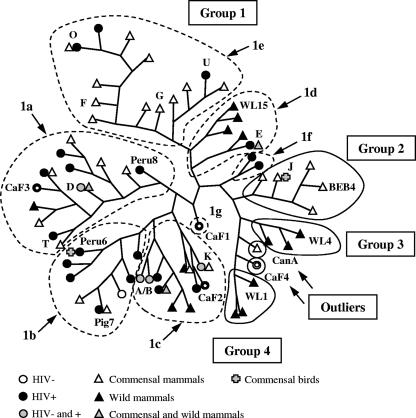
FIG. 3.
Representation of group and host associations of the phylogenetic tree inferred by the maximum-likelihood analysis of the ITS of E. bieneusi genotypes. The tree was constructed using the Phyml software. Groups are circled with a solid line; subgroups composing group 1 are delineated by a dotted line. New genotypes from this study are denoted by an open circle. Only a few genotype names are indicated for clarity and to permit comparison with the positions in the phylogram shown in Fig. 2. Abbreviations: CanA, EntCanA; Pig7, EBITSPig7. For accession numbers, see Table 2. Hosts were classified as HIV-positive or HIV-negative humans, commensal mammals (cat, dog, cattle, swine, and zoo animals), commensal birds (chicken, urban park pigeon, pet, and zoo birds), and wild mammals (beaver, otter, muskrat, fox, and raccoon).
Maximum-likelihood analysis shows that group 1 is further subdivided into at least seven clades (Fig. 2). To provide a more comprehensive classification of group 1 E. bieneusi genotypes, the simplified nomenclature of Drosten et al. (12) was used, i.e., subgroups 1a to 1f, and subgroup 1g was added. The identities within this group are high: 79.4% of the nucleotides (193/243) are conserved among the 53 sequences.
There are apparent differences in the range of host specificity within the group 1 clades, which are divided into two main branches and one isolated sequence (CAF1). The right branch contains subgroups with more restricted host specificity than those of the left branch. Subgroup 1f is human specific (mainly genotype C cases), subgroup 1d is found in wild mammals (apart from genotype E, which has a wide host range), and subgroup 1e is found mainly in farm animals (11 out of 13 sequences were isolated from swine), although some genotypes of this group (O, U, and W) recently have been reported in Thailand from HIV-positive patients (23). The left branch supporting subgroups 1a, 1b, and 1c shows the greatest host diversity, including commensal birds, commensal and wild mammals, and HIV-negative and HIV-positive individuals. Genotypes from humans (including those also found in animal hosts) predominate, with 21 out of 32 sequences in subgroups 1a, 1b, and 1c. These sequences from the left-branch subgroups represent 72% of a total of 29 genotypes reported to be found in humans so far.
The new genotype CAF1 is found at the base of the two main branches. It was identified in three isolates from Gabon collected over a period of 12 months and is proposed to constitute the new subgroup 1g. CAF1's position on the tree is intermediate between the subgroups 1c and 1e, containing types K and E, respectively. It differs by one point mutation each from K (position 113) and E (position 141) (Fig. 1).
DISCUSSION
Results of both studies showed (i) that prevalence rates of E. bieneusi were similar between immunocompetent people in the Bongo village and HIV-positive patients from Libreville; (ii) that despite the limited number of isolates analyzed, a substantial amount of genotypic diversity was observed in both rural and urban contexts; and (iii) that new genotypes that differed from known types by one or more nucleotides were identified, in particular, type CAF4, which is the most divergent genotype reported from humans to date.
Prevalence of E. bieneusi in Africa.
In previous studies conducted in Africa, the prevalence of E. bieneusi in HIV-infected adults with diarrhea was around 10% (2, 5, 13, 14, 21, 43), but values as high as 32% and 51% have been reported (17, 27). Compared to these rates, the prevalence rates of E. bieneusi infections we report for Cameroon and Gabon (2.9% and 3.0%) seem to be low and, considering the difference of HIV status in the populations studied, are surprisingly similar. However, the prevalence rate we observed in the Gabonese HIV-infected population is comparable to those reported for the urban areas of Yaoundé, Cameroon (5.2%) (38), and Lima, Peru (3.9%) (39). These studies were also conducted with HIV-infected adults not selected for diarrhea. On the contrary, the E. bieneusi prevalence rate of 2.9% we observed in Cameroon may be surprising, since it could be expected to be lower in a population with low HIV prevalence. This is the first report of E. bieneusi prevalence in an African population not selected for age, diarrhea status, or HIV status, since only two previous studies addressed this issue on the continent. Bretagne et al. reported a prevalence for E. bieneusi of 0.8% in children in Niger, for whom HIV status could not be assessed (5), whereas Tumwine et al. found a high prevalence in Ugandan children with or without diarrhea (17.4% or 16.%, respectively); the rate of positive HIV status was estimated to be 18 to 20% (42). Therefore, intestinal infection of immunocompetent subjects with E. bieneusi might be common in Africa, especially among children.
Genotypic diversity and host specificity.
Although a high degree of diversity of genotypes was found in both populations studied, differences in their relative distribution may be relevant for the epidemiology of E. bieneusi.
Our studies revealed for the first time the presence of genotypes A and B in Africa. These subgroup 1c anthroponotic genotypes previously have been reported from HIV-positive and HIV-negative populations in Europe (4, 24, 25, 32, 35), Peru (39), and Thailand (22). Moreover, we found quantitative differences between these types A and B and the previously described types A and B, depending on the study cohort, as types A and B were identified in 40% and 15%, respectively, of the isolates from Cameroon, whereas only one type A isolate was retrieved in the Gabonese study. These genotypes appear unequally present in other parts of the world: type B is the dominant strain in France (24), Germany (32), Switzerland (4), and the United Kingdom (35), making up 50 to 85% of the isolates, whereas type A was the most frequent strain in Peru (35% of isolates) and type B was absent (39). Type A also has been reported from Thailand, but not type B (22).
Genotype K is another member of subgroup 1c that we identified several times: four isolates (26%) in Gabon and one in Cameroon. Unlike types A and B, type K may be common in developing countries but is rare in Europe (25, 35), being found in 6 out of the 10 isolates from children in Uganda (42), all 4 isolates from HIV-positive adults in Cameroon (38), and 18 out of 89 patients in Peru (39). However, type K, which initially was identified from HIV-positive individuals as type IV in France (24), is not strictly anthroponotic, since it also has been identified from cattle in the United States and Portugal (41) and from cats in Germany (9) and Colombia (37).
Interestingly, we identified type D, a subgroup 1a genotype that has not been found in immunocompetent humans yet, in one HIV-positive patient in Gabon and in three HIV-negative individuals in Cameroon. Type D has a large host and geographic range. It is now commonly reported for HIV-positive humans (22 of 33 isolates in Thailand [23] and 9 of 89 patients in Peru [39]), and it was previously reported for two isolated cases in Europe (33, 35). It has been found in a wide variety of domestic and wild mammals (6, 12, 15, 36, 37, 40) and represented 15% of isolates from four species of wildlife animals in North America (40) and 26% of isolates found in cats in Colombia (37), supporting a zoonotic route of transmission for this strain.
Like type K, type E, which was not found in our studies, has been reported from HIV-positive humans: one patient in Peru (39) and 5 of 33 cases in Thailand (23). Previously reported as genotype EbpC from swine from Switzerland (4), type E was the most common strain from wild mammals in the United States (22% of isolates from five species [40]).
Our new sequences CAF1, CAF2, and CAF3 are close relatives of types E, K, and D, respectively. These families of genotypes dominate the isolates found in the HIV-positive patients from Gabon, with 10 out of 15 isolates, whereas in the Cameroon study types K and D represented only 4 out of 20 isolates.
Finally, the new genotype CAF4, the most highly divergent genotype reported from humans to date, is equally represented in HIV-positive patients in Gabon and HIV-negative individuals in Cameroon. Its high frequency (≈25%) in both countries may indicate that this genotype is common in Central Africa. The relative proximity of the CAF4 genotype to genotypes found in wild aquatic mammals could indicate that similar hosts exist in Central Africa.
Epidemiological implications.
The analysis of E. bieneusi genotypes highlights different risks of infection in the two human populations studied, possibly due to differences in sources of contamination and routes of transmission. In the rural community of Cameroon, anthroponotic genotypes A and B predominate and may indicate person-to-person transmission, possibly involving contact with contaminated water stored for household use. This is supported by the observation that carriers from the same household have parasites with the same genotype (genotype A for cases 113B, 113H, 117A/117B, and 142G/142H, genotype B for cases 194A/194C, and genotype CAF4 for cases 179D/179E) (Table 1). In the study of HIV-positive individuals from Gabon, the wider diversity of genotypes and the higher proportion of mixed-host genotypes D and K (and the close relatives CAF1 to CAF3) suggest that immunocompromised patients acquire infection from additional reservoirs (probably of zoonotic origin).
Overall, the epidemiology of E. bieneusi is still unclear, probably due to the fact that at present different species or subspecies of this parasite are not differentiated by classical microscopy and immunological methods, stressing the need for further molecular studies.
Acknowledgments
We thank the population of the village Bongo and the patients of Libreville for participation in this study, M. Owono-Medang and E. Mozogho for excellent technical assistance, C. Djengue and G. Obame-Mebale for sample collection in Libreville, and A. Arnaud for sample collection in Cameroon. We also thank H. Rinder (University of Munich) for initial help with ITS sequencing and I. Desportes-Livage (MNHN, Paris, France) for helpful discussions.
This work was supported by a grant from the French National AIDS Research Agency (PED ANRS-1264/MAE).
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa Cisse, O., A. Ouattara, M. Thellier, I. Accoceberry, S. Biligui, D. Minta, O. Doumbo, I. Desportes-Livage, M. A. Thera, M. Danis, and A. Datry. 2002. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali). J. Clin. Microbiol. 40:1715-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bern, C., V. Kawai, D. Vargas, J. Rabke-Verani, J. Williamson, R. Chavez-Valdez, L. Xiao, I. Sulaiman, A. Vivar, E. Ticona, M. Navincopa, V. Cama, H. Moura, W. E. Secor, G. Visvesvara, and R. H. Gilman. 2005. The epidemiology of intestinal microsporidiosis in patients with HIV/AIDS in Lima, Peru. J. Infect. Dis. 191:1658-1664. [DOI] [PubMed] [Google Scholar]
- 4.Breitenmoser, A. C., A. Mathis, E. Burgi, R. Weber, and P. Deplazes. 1999. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology 118:447-453. [DOI] [PubMed] [Google Scholar]
- 5.Bretagne, S., F. Foulet, W. Alkassoum, J. Fleury-Feith, and M. Develoux. 1993. Prévalence des spores d'Enterocytozoon bieneusi dans les selles de patients sidéens et d'enfants Africains non infectés par le VIH. Bull. Soc. Pathol. Exot. 86:351-357. [PubMed] [Google Scholar]
- 6.Buckholt, M. A., J. H. Lee, and S. Tzipori. 2002. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 68:2595-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cali, A., D. P. Kotler, and J. M. Orenstein. 1993. Septata intestinalis n. g., n. sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J. Eukaryot. Microbiol. 40:101-112. [DOI] [PubMed] [Google Scholar]
- 8.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dengjel, B., M. Zahler, W. Hermanns, K. Heinritzi, T. Spillmann, A. Thomschke, T. Loscher, R. Gothe, and H. Rinder. 2001. Zoonotic potential of Enterocytozoon bieneusi. J. Clin. Microbiol. 39:4495-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desportes, I., Y. Le Charpentier, A. Galian, F. Bernard, B. Cochand-Priollet, A. Lavergne, P. Ravisse, and R. Modigliani. 1985. Occurrence of a new microsporidan: Enterocytozoon bieneusi n. g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 32:250-254. [DOI] [PubMed] [Google Scholar]
- 11.Didier, E. S., and L. M. Weiss. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosten, C., J. Laabs, E. M. Kuhn, and J. Schottelius. 2005. Interspecies transmission of Enterocytozoon bieneusi supported by observations in laboratory animals and phylogeny. Med. Microbiol. Immunol. (Berlin) 194:207-209. [DOI] [PubMed] [Google Scholar]
- 13.Endeshaw, T., A. Kebede, J. J. Verweij, D. Wolday, A. Zewide, K. Tsige, Y. Abraham, T. Messele, A. M. Polderman, and B. Petros. 2005. Detection of intestinal microsporidiosis in diarrheal patients infected with the human immunodeficiency virus (HIV-1) using PCR and Uvitex-2B stain. Ethiop. Med. J. 43:97-101. [PubMed] [Google Scholar]
- 14.Endeshaw, T., A. Kebede, J. J. Verweij, A. Zewide, K. Tsige, Y. Abraham, D. Wolday, T. Woldemichael, T. Messele, A. M. Polderman, and B. Petros. 2006. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn. J. Infect. Dis. 59:306-310. [PubMed] [Google Scholar]
- 15.Green, L. C., P. J. Didier, L. C. Bowers, and E. S. Didier. 2004. Natural and experimental infection of immunocompromised rhesus macaques (Macaca mulatta) with the microsporidian Enterocytozoon bieneusi genotype D. Microbes Infect. 6:996-1002. [DOI] [PubMed] [Google Scholar]
- 16.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 17.Gumbo, T., S. Sarbah, I. T. Gangaidzo, Y. Ortega, C. R. Sterling, A. Carville, S. Tzipori, and P. M. Wiest. 1999. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS 13:819-821. [DOI] [PubMed] [Google Scholar]
- 18.Hartskeerl, R. A., T. Van Gool, A. R. Schuitema, E. S. Didier, and W. J. Terpstra. 1995. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology 110:277-285. [DOI] [PubMed] [Google Scholar]
- 19.Katzwinkel-Wladarsch, S., M. Lieb, W. Helse, T. Loscher, and H. Rinder. 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1:373-378. [DOI] [PubMed] [Google Scholar]
- 20.Kokoskin, E., T. W. Gyorkos, A. Camus, L. Cedilotte, T. Purtill, and B. Ward. 1994. Modified technique for efficient detection of microsporidia. J. Clin. Microbiol. 32:1074-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebbad, M., H. Norrgren, A. Naucler, F. Dias, S. Andersson, and E. Linder. 2001. Intestinal parasites in HIV-2 associated AIDS cases with chronic diarrhea in Guinea-Bissau. Acta Trop. 80:45-49. [DOI] [PubMed] [Google Scholar]
- 22.Leelayoova, S., I. Subrungruang, R. Rangsin, P. Chavalitshewinkoon- Petmitr, J. Worapong, T. Naaglor, and M. Mungthin. 2005. Transmission of Enterocytozoon bieneusi genotype a in a Thai orphanage. Am. J. Trop. Med. Hyg. 73:104-107. [PubMed] [Google Scholar]
- 23.Leelayoova, S., I. Subrungruang, Y. Suputtamongkol, J. Worapong, P. C. Petmitr, and M. Mungthin. 2006. Identification of genotypes of Enterocytozoon bieneusi from stool samples from human immunodeficiency virus-infected patients in Thailand. J. Clin. Microbiol. 44:3001-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liguory, O., F. David, C. Sarfati, F. Derouin, and J. M. Molina. 1998. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J. Clin. Microbiol. 36:1882-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liguory, O., C. Sarfati, F. Derouin, and J. M. Molina. 2001. Evidence of different Enterocytozoon bieneusi genotypes in patients with and without human immunodeficiency virus infection. J. Clin. Microbiol. 39:2672-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo, M. L., L. Xiao, V. Cama, N. Magalhaes, F. Antunes, and O. Matos. 2006. Identification of potentially human-pathogenic Enterocytozoon bieneusi genotypes in various birds. Appl. Environ. Microbiol. 11:7380-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiga, I., O. Doumbo, M. Dembele, H. Traore, I. Desportes-Livage, I. Hilmarsdottir, E. Giboyau, L. Maiga, L. Kassambara, Y. el Fakhry, A. Datry, M. Gentilini, and E. Pichard. 1997. Microsporidiose intestinale humaine à Bamako (Mali): présence d'Enterocytozoon bieneusi chez les patients séropositifs pour le VIH. Santé 7:257-262. [PubMed] [Google Scholar]
- 28.Mathis, A., A. C. Breitenmoser, and P. Deplazes. 1999. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite 6:189-193. [DOI] [PubMed] [Google Scholar]
- 29.Mathis, A., R. Weber, and P. Deplazes. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Keefe, E. A., and R. Wood. 1996. AIDS in Africa. Scand. J. Gastroenterol. 220:147-152. [DOI] [PubMed] [Google Scholar]
- 31.Reetz, J., H. Rinder, A. Thomschke, H. Manke, M. Schwebs, and A. Bruderek. 2002. First detection of the microsporidium Enterocytozoon bieneusi in non-mammalian hosts (chickens). Int. J. Parasitol. 32:785-787. [DOI] [PubMed] [Google Scholar]
- 32.Rinder, H., S. Katzwinkel-Wladarsch, and T. Loscher. 1997. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol. Res. 83:670-672. [DOI] [PubMed] [Google Scholar]
- 33.Rinder, H., S. Katzwinkel-Wladarsch, A. Thomschke, and T. Loscher. 1998. Strain differentiation in microsporidia. Tokai J. Exp. Clin. Med. 23:433-437. [PubMed] [Google Scholar]
- 34.Rinder, H., A. Thomschke, B. Dengjel, R. Gothe, T. Loscher, and M. Zahler. 2000. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J. Parasitol. 86:185-188. [DOI] [PubMed] [Google Scholar]
- 35.Sadler, F., N. Peake, R. Borrow, P. L. Rowl, E. G. Wilkins, and A. Curry. 2002. Genotyping of Enterocytozoon bieneusi in AIDS patients from the north west of England. J. Infect. 44:39-42. [DOI] [PubMed] [Google Scholar]
- 36.Santin, M., J. M. Trout, and R. Fayer. 2005. Enterocytozoon bieneusi genotypes in dairy cattle in the eastern United States. Parasitol. Res. 97:535-538. [DOI] [PubMed] [Google Scholar]
- 37.Santin, M., J. M. Trout, J. A. Vecino, J. P. Dubey, and R. Fayer. 2006. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet. Parasitol. 141:334-339. [DOI] [PubMed] [Google Scholar]
- 38.Sarfati, C., A. Bourgeois, J. Menotti, F. Liegeois, R. Moyou-Somo, E. Delaporte, F. Derouin, E. M. Ngole, and J. M. Molina. 2006. Prevalence of intestinal parasites including microsporidia in human immunodeficiency virus-infected adults in Cameroon: a cross-sectional study. Am. J. Trop. Med. Hyg. 74:162-164. [PubMed] [Google Scholar]
- 39.Sulaiman, I. M., C. Bern, R. Gilman, V. Cama, V. Kawai, D. Vargas, E. Ticona, A. Vivar, and L. Xiao. 2003. A molecular biologic study of Enterocytozoon bieneusi in HIV-infected patients in Lima, Peru. J. Eukaryot. Microbiol. 50:591-596. [DOI] [PubMed] [Google Scholar]
- 40.Sulaiman, I. M., R. Fayer, A. A. Lal, J. M. Trout, F. W. Schaefer III, and L. Xiao. 2003. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 69:4495-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sulaiman, I. M., R. Fayer, C. Yang, M. Santin, O. Matos, and L. Xiao. 2004. Molecular characterization of Enterocytozoon bieneusi in cattle indicates that only some isolates have zoonotic potential. Parasitol. Res. 92:328-334. [DOI] [PubMed] [Google Scholar]
- 42.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, M. A. Buckholt, and S. Tzipori. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299-303. [DOI] [PubMed] [Google Scholar]
- 43.van Gool, T., E. Luderhoff, K. J. Nathoo, C. F. Kiire, J. Dankert, and P. R. Mason. 1995. High prevalence of Enterocytozoon bieneusi infections among HIV-positive individuals with persistent diarrhoea in Harare, Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 89:478-480. [DOI] [PubMed] [Google Scholar]
- 44.Weiss, L. M., X. Zhu, A. Cali, H. B. Tanowitz, and M. Wittner. 1994. Utility of microsporidian rRNA in diagnosis and phylogeny: a review. Folia Parasitol. (Praha) 41:81-90. [PubMed] [Google Scholar]
- 45.Zhu, X., M. Wittner, H. B. Tanowitz, D. Kotler, A. Cali, and L. M. Weiss. 1993. Small subunit rRNA sequence of Enterocytozoon bieneusi and its potential diagnostic role with use of the polymerase chain reaction. J. Infect. Dis. 168:1570-1575. [DOI] [PubMed] [Google Scholar]