Abstract
Large, general population-based data on carriage rates of periodontal pathogens hardly exist in the current literature. The objectives of the present study were to examine the salivary detection of Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Campylobacter rectus, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Treponema denticola in a representative sample of the adult population living in southern Finland and to clarify which determinants are associated with the presence of these pathogens in saliva. 16S rRNA-based PCR methods with species-specific primers were employed to determine the presence of the six target bacteria in stimulated saliva samples, which were available from 1,294 subjects aged ≥30 years. The age group, gender, level of education, marital status, smoking history, number of teeth, and number of teeth with deepened pockets were included in the statistical analysis. In general, the carriage of periodontal pathogens was common, since at least one of the examined pathogens was found in 88.2% of the subjects. In descending order, the total detection rates were 56.9%, 38.2%, 35.4%, 31.3%, 20.0%, and 13.9% for T. forsythensis, T. denticola, P. gingivalis, C. rectus, A. actinomycetemcomitans, and P. intermedia, respectively. Age per se was strongly associated with the carriage of P. gingivalis (P = 0.000), and the level of education with that of T. denticola (P = 0.000). There was an association between the number of teeth with deepened pockets and carriage of P. gingivalis (P = 0.000), P. intermedia (P = 0.000), T. denticola (P = 0.000), and A. actinomycetemcomitans (P = 0.004). The data suggest that distinct species have a different carriage profile, depending on variables such as age, educational level, and periodontal status.
Periodontitis is characterized by local infection and inflammation of tooth-supporting tissues, leading to various degrees of periodontal attachment loss in affected teeth. Different forms of periodontitis are multifactorial diseases where microorganisms present in dental biofilms are involved. Etiologic bacteria of periodontal diseases typically include gram-negative anaerobic bacteria: among those, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis (formerly Bacteroides forsythus), and Treponema denticola are strictly anaerobic and Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans and Campylobacter rectus are facultative/microaerobic (2, 4, 13, 33). Several demographic and behavioral characteristics, such as race, age, gender, and smoking, as well as socioeconomic status, appear to be related to the prevalence of periodontitis, as shown in different populations worldwide (1). It may be expected that similar factors relate to the carriage pattern of periodontitis-associated microorganisms in similar ways as well. For example, the cumulative increase of periodontitis due to aging could be reflected by the increasing carriage of periodontal pathogen(s). As far as we know, natural carriage rates of periodontal pathogens and determinants of their carriage have not been studied in representative general population-based surveys, but mainly in relatively small, more-or-less-selected study populations available by convenience.
Saliva is an easily obtainable and noninvasively collected microbiological specimen, containing the microbes which detach from various oral surfaces (5, 8, 15, 18, 32). It is a suitable sample material for large-scale oral microbiological studies utilizing PCR-based assays, which offer a labor-minimizing technique. In oral microbiological studies, culture has been appreciated as the gold standard despite its relatively low sensitivity. Due to the development of molecular biological methods, which do not need viable cells for detection, more accurate data on the presence of target bacteria in the specimen can be expected.
In order to get information on the natural distribution of periodontal pathogens at the population level, we examined the salivary carriage of A. actinomycetemcomitans, C. rectus, P. gingivalis, P. intermedia, T. forsythensis, and T. denticola in a representative sample of the adult population living in southern Finland. The carriage was related to the age group, gender, level of education, marital status, and smoking history, as well as some clinical characteristics, including the number of teeth and number of teeth with deepened periodontal pockets, the latter being a manifestation of periodontitis.
MATERIALS AND METHODS
Study population.
The study subjects belong to a national population-based “Health 2000 Health Examination Survey” coordinated by the National Public Health Institute (KTL), where the main aim was to provide up-to-date information on the health and functional ability of the working-aged and elderly Finnish population (http://www.ktl.fi/health2000/index.uk.html). All protocols were approved by the institutional ethics committees. A nationally representative two-stage stratified cluster sample was formed by Statistics Finland, the final sample size being 8,028 subjects, aged ≥30 years and steadily living in Finland. Data on each individual were gathered by questionnaires, an interview at home or in an institute, and health examination, including laboratory tests, in the local health care center or comparable premises. The fieldwork began in September 2000, lasting until March 2001. Eighty regions all over Finland formed five districts (around the five university hospitals): southern (Helsinki), western (Turku), middle (Tampere), eastern (Kuopio), and northern (Oulu) Finland. In the southern district premises, an oral specimen collection for microbiological analyses was included in the laboratory procedures. This region covers roughly one million inhabitants, of whom a random sample of 2,616 subjects was invited to participate in the survey. The present study includes data on 1,294 subjects who participated in both the interviews and the clinical oral health examination and from whom salivary specimens were available.
Saliva collection, transport, and storage.
Paraffin-stimulated whole saliva samples were collected by expectoration into calibrated medical cups. Saliva was divided into two Eppendorf tubes, which were immediately frozen in carbonic acid ice for 1 to 3 days before and during the transportation to KTL, where the specimens were stored frozen at −70°C until they were processed.
Bacterial detection.
Bacterial DNA was extracted from saliva samples with a cation-chelating resin (Chelex 100; Bio-Rad Laboratories, CA) as previously reported (19), and the supernatant was used as a template for PCR amplifications. A. actinomycetemcomitans, T. forsythensis, and P. gingivalis were detected using three species-specific forward primers (AaF, BfF, and PgF) and a conserved reverse primer (C11R) in a multiplex PCR as previously described (29). Species-specific primers for C. rectus and T. denticola (4) and for P. intermedia (24) were used in separate PCR detections as previously reported. DNA from A. actinomycetemcomitans ATCC 29523, P. gingivalis ATCC 33277, T. forsythensis ATCC 43037, C. rectus ATCC 33238, P. intermedia ATCC 25611, and T. denticola ATCC 35405 were used as positive controls and sterile water as negative controls in each series of PCRs. In cases of faint bands, the PCR was repeated. Only clear bands were interpreted as positive.
Data analysis.
The study subjects (570 men and 724 women) were divided into nine 5-year age groups (including seven 5-year groups and, from the age of 65 years, two groups) or five 10-year age groups (including four 10-year groups and one group with subjects older than 70 years). In addition to the age and gender, explanatory variables used in the present study were gathered by questionnaires and interviewing (the level of education, marital status, and smoking history) and by examining oral health status (the number of teeth and number of teeth with probing-pocket depth(s) ≥4 mm). Using information on formal schooling and vocational training, three educational categories were formed: basic (no formal vocational training or senior secondary education), secondary (completed vocational training or passed matriculation examination), and higher (degree or diploma from higher vocational institution, polytechnic, or university). The marital status classifications were married or cohabiting and single, divorced, or widowed. Smoking history was categorized into four classes to distinguish daily, occasional, and former (those who had quit ≥12 months ago) smokers and those who had never smoked. Information on oral health status was based on clinical oral examination conducted by a specially trained dentist with the assistance of a dental nurse in a standard dental unit.
The outcome variables included the salivary carriage of A. actinomycetemcomitans, C. rectus, P. gingivalis, P. intermedia, T. forsythensis, and T. denticola.
As a stratified two-stage cluster sampling design was used in the survey, weights were used for correcting the effects of oversampling people aged ≥80 years and nonresponse. Weighting of the sample was based on poststratification with age group, gender, and region. Chi-square tests were performed to study associations between the explanatory variables and the six dichotomous outcome variables. Separate logistic regression analyses were fitted for each outcome variable, and the results are presented in terms of odds ratios, together with 95% confidence intervals. A logistic model was used to estimate adjusted indicators in dentate participants. For adjustment, predictive marginals were calculated by fixing the values of confounding factors (11, 16). Data analyses were performed with SAS (version 8.0) software using the SUDAAN program (25).
RESULTS
Carriage of periodontal pathogens in this adult general population-based sample was common, since at least one of the examined pathogens was found in saliva in 88.2% of the 1,294 subjects (of these, 1,216 were dentate, i.e., they had at least one tooth). Subjects without any pathogens were typically female, young, with higher education, married, nonsmoking, and having full dentition and no pocket teeth. The mean detection rates were 56.9% for T. forsythensis, 38.2% for T. denticola, 35.4% for P. gingivalis, 31.3% for C. rectus, 20.0% for A. actinomycetemcomitans, and 13.9% for P. intermedia. Table 1 presents the detection rates in saliva by different variables.
TABLE 1.
Carriage rates of periodontal pathogens in saliva by background variables and corresponding P values in 1,294 subjects representing the adult general population in southern Finlanda
| Background variable | Total no. of subjects, or P value | % subjects positive for indicated pathogen, or P value
|
|||||
|---|---|---|---|---|---|---|---|
| Aa | Cr | Pg | Pi | Tf | Td | ||
| Gender | |||||||
| Men | 570 | 21 | 34 | 34 | 16 | 59 | 42 |
| Women | 724 | 19 | 29 | 37 | 12 | 55 | 35 |
| P value | 0.374 | 0.065 | 0.352 | 0.107 | 0.168 | .006 | |
| Age group (yrs) | |||||||
| 30-34 | 188 | 16 | 23 | 13 | 13 | 51 | 31 |
| 35-39 | 172 | 16 | 29 | 19 | 19 | 62 | 33 |
| 40-44 | 158 | 19 | 39 | 31 | 14 | 59 | 46 |
| 45-49 | 155 | 24 | 28 | 28 | 12 | 67 | 38 |
| 50-54 | 205 | 23 | 33 | 46 | 16 | 56 | 38 |
| 55-59 | 123 | 23 | 31 | 51 | 19 | 54 | 40 |
| 60-64 | 89 | 26 | 36 | 56 | 18 | 52 | 51 |
| 65-74 | 110 | 22 | 35 | 51 | 18 | 57 | 46 |
| ≥75 | 94 | 10 | 33 | 49 | 7 | 47 | 22 |
| P value | .012 | 0.078 | .000 | .028 | .015 | .003 | |
| Level of education | |||||||
| Basic | 342 | 21 | 31 | 45 | 16 | 50 | 46 |
| Secondary | 395 | 20 | 31 | 36 | 16 | 59 | 45 |
| Higher | 552 | 20 | 32 | 29 | 11 | 60 | 29 |
| Missing value | 5 | ||||||
| P value | 0.952 | 0.897 | .000 | 0.071 | 0.168 | .000 | |
| Marital status | |||||||
| Married/cohabiting | 881 | 21 | 30 | 33 | 12 | 50 | 37 |
| Single | 408 | 19 | 34 | 41 | 18 | 59 | 40 |
| Missing value | 5 | ||||||
| P value | 0.304 | 0.223 | .003 | .004 | 0.168 | 0.380 | |
| Smoking history | |||||||
| Never | 627 | 20 | 32 | 29 | 11 | 60 | 29 |
| Former | 274 | 20 | 32 | 29 | 11 | 60 | 29 |
| Occasional | 74 | 20 | 31 | 36 | 16 | 59 | 45 |
| Daily | 313 | 21 | 31 | 45 | 16 | 50 | 46 |
| Missing value | 6 | ||||||
| P value | 0.952 | 0.897 | .000 | 0.071 | 0.168 | .000 | |
| Number of teeth | |||||||
| 25-32 | 876 | 20 | 31 | 31 | 14 | 58 | 38 |
| 20-24 | 168 | 24 | 38 | 45 | 18 | 60 | 48 |
| 10-19 | 102 | 28 | 40 | 53 | 17 | 63 | 49 |
| 1-9 | 70 | 14 | 20 | 57 | 16 | 48 | 31 |
| 0 | 75 | 2 | 14 | 20 | 3 | 33 | 5 |
| Missing value | 3 | ||||||
| P value | .000 | .001 | .000 | .000 | .003 | .000 | |
| Number of teeth with pocket(s) ≥4 mmb | |||||||
| 0 | 222 | 12 | 27 | 23 | 10 | 57 | 19 |
| 1-3 | 330 | 20 | 27 | 29 | 10 | 58 | 31 |
| 4-6 | 232 | 20 | 30 | 35 | 10 | 64 | 41 |
| ≥7 | 414 | 28 | 39 | 49 | 23 | 60 | 58 |
| Missing value | 18 | ||||||
| P value | .000 | .019 | .000 | .000 | .046 | .000 | |
Aa, A. actinomycetemcomitans; Cr, C. rectus; Pg, P. gingivalis; Pi, P. intermedia; Tf, T. forsythensis; and Td, T. denticola. P values refer to chi-square tests. Significant P values are marked in bold.
Data on dentate subjects.
The adjusted associations between the detection rates of each species examined and the selected variables in the dentate subjects are presented in Table 2. After adjusting for multiple variables, being in the older age group remained as a highly significant factor for the increasing detection rates of P. gingivalis, whereas the level of education was a significant factor for detecting T. denticola in subjects with lower education and T. forsythensis in subjects with higher education. Men had slightly but not significantly higher detection rates for periodontal pathogens, except for P. gingivalis, which was more common in women. Single subjects had P. intermedia more often than did married or cohabiting subjects. Daily smokers harbored P. intermedia and T. denticola more frequently but A. actinomycetemcomitans less frequently than those who had never smoked. An increasing number of teeth with probing-pocket depths of ≥4 mm associated significantly with the detection rates of P. gingivalis, P. intermedia, T. denticola, and A. actinomycetemcomitans (Table 2).
TABLE 2.
Associations of the salivary carriage of periodontal pathogens with indicated variables in 1,216 dentate subjects in logistic regression models
| Variable | Odds ratio (95% confidence interval), or P value, for:a
|
|||||
|---|---|---|---|---|---|---|
| Aa | Cr | Pg | Pi | Tf | Td | |
| Gender | ||||||
| Men | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Women | 1.0 (0.7-1.3) | 0.8 (0.6-1.1) | 1.4 (1.0-1.9) | 0.8 (0.6-1.2) | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) |
| P valueb | 0.757 | 0.139 | .037 | 0.285 | 0.378 | 0.263 |
| Age group | ||||||
| 30-34 yr | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 35-39 yr | 1.0 (0.5-1.8) | 1.4 (0.9-2.3) | 1.5 (0.8-2.8) | 0.8 (0.4-1.4) | 1.5 (1.0-2.2) | 1.1 (0.7-1.7) |
| 40-44 yr | 1.2 (0.6-2.3) | 2.3 (1.4-3.7) | 2.7 (1.6-4.7) | 1.0 (0.5-1.9) | 1.4 (0.9-2.2) | 1.8 (1.1-2.9) |
| 45-49 yr | 1.5 (0.8-2.7) | 1.3 (0.8-2.1) | 2.1 (1.1-3.9) | 0.9 (0.5-1.7) | 1.8 (1.2-2.9) | 1.1 (0.7-1.8) |
| 50-54 yr | 1.3 (0.7-2.4) | 1.5 (0.9-2.5) | 4.5 (2.6-7.6) | 1.1 (0.6-2.1) | 1.2 (0.7-1.9) | 1.1 (0.7-1.7) |
| 55-59 yr | 1.5 (0.8-2.6) | 1.7 (1.0-3.0) | 5.6 (3.0-10.4) | 1.8 (0.9-3.4) | 1.3 (0.8-2.0) | 1.3 (0.8-2.2) |
| 60-64 yr | 1.6 (0.8-3.3) | 2.0 (1.1-3.8) | 7.7 (3.8-15.6) | 1.4 (0.6-3.1) | 1.1 (0.6-2.0) | 2.0 (1.1-3.6) |
| 65-74 yr | 1.4 (0.7-2.8) | 1.9 (1.0-3.6) | 5.5 (2.6-11.6) | 1.7 (0.8-3.6) | 1.5 (0.9-2.8) | 1.7 (1.0-3.2) |
| ≥75 yr | 0.8 (0.3-2.1) | 2.3 (1.1-4.6) | 8.5 (3.9-18.5) | 0.8 (0.3-2.4) | 1.5 (0.8-3.1) | 1.0 (0.5-2.2) |
| P value | 0.667 | 0.069 | .000 | 0.234 | 0.238 | 0.088 |
| Level of education | ||||||
| Basic | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Secondary | 0.9 (0.6-1.4) | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) | 0.9 (0.6-1.4) | 1.4 (1.0-1.9) | 0.8 (0.6-1.2) |
| Higher | 0.9 (0.5-1.5) | 1.1 (0.8-1.6) | 0.8 (0.6-1.2) | 0.8 (0.5-1.2) | 1.5 (1.1-2.0) | 0.5 (0.3-0.6) |
| P value | 0.876 | 0.734 | 0.321 | 0.511 | .033 | .000 |
| Marital status | ||||||
| Married/cohabiting | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Single | 0.9 (0.6-1.2) | 1.2 (0.9-1.6) | 1.1 (0.8-1.6) | 1.5 (1.1-2.1) | 0.9 (0.7-1.1) | 1.0 (0.8-1.4) |
| P value | 0.315 | 0.186 | 0.396 | .011 | 0.383 | 0.863 |
| Smoking history | ||||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Former | 0.7 (0.4-1.1) | 1.2 (0.8-1.6) | 1.2 (0.9-1.7) | 1.0 (0.6-1.6) | 0.9 (0.6-1.2) | 0.7 (0.5-0.9) |
| Occasional | 0.7 (0.4-1.5) | 0.9 (0.5-1.6) | 0.9 (0.5-1.7) | 1.7 (0.9-3.4) | 0.8 (0.5-1.3) | 1.4 (0.8-2.4) |
| Daily | 0.6 (0.4-0.8) | 0.9 (0.7-1.3) | 0.9 (0.7-1.3) | 1.9 (1.3-2.9) | 1.2 (0.8-1.8) | 1.4 (1.0-2.0) |
| P value | .022 | 0.637 | 0.579 | .005 | 0.246 | .007 |
| Number of teeth | ||||||
| 25-32 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 20-24 | 1.0 (0.6-1.5) | 1.1 (0.8-1.6) | 0.9 (0.6-1.4) | 1.0 (0.6-1.7) | 1.1 (0.7-1.7) | 1.0 (0.7-1.5) |
| 10-19 | 0.3 (0.8-2.2) | 1.4 (0.8-2.3) | 1.1 (0.6-1.9) | 0.9 (0.5-1.6) | 1.5 (0.9-2.4) | 1.1 (0.7-1.7) |
| 1-9 | 0.7 (0.3-1.7) | 0.4 (0.2-8.8) | 1.6 (0.8-3.0) | 0.8 (0.3-2.0) | 0.8 (0.4-1.3) | 0.6 (0.3-1.2) |
| P value | 0.619 | .030 | 0.396 | 0.976 | 0.191 | 0.450 |
| Number of teeth with pocket(s) ≥4 mm | ||||||
| 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-3 | 1.7 (1.0-2.9) | 0.9 (0.6-1.3) | 1.3 (0.8-2.1) | 1.0 (0.5-1.9) | 1.3 (0.9-1.8) | 1.6 (1.1-2.4) |
| 4-6 | 1.7 (1.0-3.0) | 0.9 (0.6-1.4) | 1.5 (1.0-2.4) | 0.9 (0.5-1.6) | 1.5 (1.0-2.2) | 2.5 (1.6-3.7) |
| ≥7 | 2.8 (1.6-5.0) | 1.3 (0.8-2.1) | 2.6 (1.5-4.4) | 2.0 (1.2-3.5) | 1.3 (0.9-1.9) | 4.2 (2.8-6.4) |
| P value | .004 | .160 | .000 | .000 | .193 | .000 |
Aa, A. actinomycetemcomitans; Cr, C. rectus; Pg, P. gingivalis; Pi, P. intermedia; Tf, T. forsythensis; and Td, T. denticola.
The significance of the variables is based on the Wald F test. Significant P values are marked in bold.
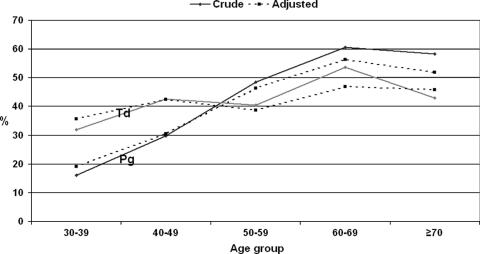
The unadjusted and adjusted detection rates of P. gingivalis and T. denticola by age are presented in Fig. 1. When adjusted with all other variables determined, there was a considerable increase in the carriage of P. gingivalis by age, from approximately 20% in the youngest age group up to over 50% in the two oldest age groups.
FIG. 1.
Crude and adjusted carriage rates of P. gingivalis (Pg) and T. denticola (Td) in dentate subjects (n = 1,216) by 10-year age cohorts. Adjustment was made by the gender, level of education, marital status, smoking history, number of teeth, and number of teeth with probing-pocket depth(s) ≥4 mm.
DISCUSSION
The present large-scale, general population-based data suggest that distinct species have a different carriage profile, depending on variables such as age group, educational level, smoking history, and periodontal status. In general, the carriage of periodontal pathogens proved to be common, and only 12% of the 1,294 subjects were free of all six target bacteria examined. More than half of the population had T. forsythensis, one-third T. denticola, P. gingivalis or C. rectus, and one-fifth P. intermedia or A. actinomycetemcomitans. Logistic regression models revealed different variables to be associated with distinct species in dentate subjects.
Periodontitis-associated organisms colonize not only subgingival sites, but also supragingival sites (20, 35), and appear in saliva (5, 7, 8, 15, 30, 32). Stimulation by masticating a piece of paraffin looses still-attached microorganisms or clumps of microorganisms from oral biofilms into salivary sediment. According to Umeda et al. (30), whole saliva was even superior to pooled subgingival samples to detect P. gingivalis, P. intermedia, and T. denticola in the oral cavity, and reasonably good detection rates were obtained also for A. actinomycetemcomitans and T. forsythensis (formerly B. forsythus). Also, in a study by Eger at al. (9), saliva proved to have a high diagnostic value for the identification of individuals positive for A. actinomycetemcomitans. Multiplex PCR for A. actinomycetemcomitans, P. gingivalis, and T. forsythensis (B. forsythus) (29) and individual PCRs for C. rectus, P. intermedia, and T. denticola (4, 24) have been developed for the detection of these bacteria in subgingival plaque. Since inhibitory agents present in saliva result in difficulties in PCR, the protocols were slightly modified by treating the saliva samples with cation-chelating resin to avoid false-negative results (19). The PCR assays showed a good reproducibility; the samples that had differences in the replicates were due to weak bands, probably containing small quantities of the target microbe, near to the detection limit.
So far, there have been hardly any data on carriage rates of periodontal pathogens at the general population level. In a culture-based study on subgingival bacteria among a random sample of employees in an industrial company in Sweden (22), a quite similar prevalence, i.e., 25%, as seen in the present study, was recorded for A. actinomycetemcomitans. In contrast to the present population, only 14% of these 171 randomly selected adults harbored P. gingivalis in subgingival plaque, whereas C. rectus and P. intermedia sensu lato were common, being found in 81% and 58% of the subjects, respectively (22). In an Australian study population, which was comprised of 504 adult subjects recruited from the staff of the University of Queensland, the prevalences of A. actinomycetemcomitans, P. gingivalis, and P. intermedia were 23%, 15%, and 10%, respectively, when multiple subgingival sites were collected and the pooled samples examined using monoclonal antibodies (14). Both above-mentioned studies presented surprisingly low recovery rates for P. gingivalis from subgingival samples compared to those from saliva in the present study, which, in some cases, may be due to different techniques used for the detection of target bacteria. Indeed, culture, immunologic assays, and DNA-based methods can reveal quite different results (4, 17, 23, 26). Not only various techniques but also ethnic and/or geographical factors may partly explain different observations. For example, the present and above-mentioned Western carriage rates of A. actinomycetemcomitans are seemingly low compared to much higher rates reported for rural populations in Far Eastern countries (6, 21, 28).
In the present southern Finnish population, 13% of the subjects in the youngest age group (30 to 34 years of age) harbored P. gingivalis, and then detection rates increased up to 56% in the age group of 60 to 64 years. After adjusting its carriage with other variables measured in the present study, the increasing age per se remained significantly associated with the increasing detection rates of P. gingivalis. Factors which may influence this age-dependent carriage pattern could not be explained here. In a previous PCR-based study on 263 Finnish subjects representing different age groups (19), the organism was detected in 5% of the subjects between 5 and 10 years of age, in 14% of the subjects between 11 and 30 years of age, and in 63% of the subjects between 31 and 80 years (mean 43 years) of age. Among an ethnically heterogeneous study population in Los Angeles (n = 202), an association between the age and prevalence of salivary P. gingivalis was observed (31).
In the present study, gender proved to have very little impact on the carriage of periodontal pathogens. Women were more likely to have P. gingivalis than men, whereas other pathogens were found more frequently in men than women but, after adjustment for confounding factors, any difference did not reach statistical significance. Subjects with basic and secondary educational levels had more T. denticola in their saliva than subjects with higher education. Detecting T. denticola was also associated with daily smoking. These observations may partly be explained by better oral hygiene levels, which have been related to the female gender, higher education, and nonsmoking status. In a study by Umeda et al. (31), current smoking brought a 5-fold risk for having T. denticola in saliva, whereas Mager et al. (18) failed to demonstrate any significant differences in the microbial composition of saliva samples collected from current smokers compared with those of nonsmokers for more than one year. Eggert et al. (10) suggested that smoking favors the growth of periodontal pathogens, such as A. actinomycetemcomitans, P. gingivalis, and P. intermedia, in shallow periodontal sites, i.e., supragingival plaque as being the reservoir of pathogens in smokers. If so, stimulated saliva should reflect this event. In the present study, daily smokers had not only T. denticola but also P. gingivalis and P. intermedia in saliva more frequently than nonsmokers. In contrast, A. actinomycetemcomitans proved to be most common in those who had never smoked.
There is abundant evidence that the prevalence of major periodontal pathogens in oral specimens varies between individuals due to differences in their periodontal health status (3, 4, 12, 19, 20, 27, 30). In the present study, where the detection of selected pathogens was examined in a large, true population-based study population, this seemed to be the case for P. gingivalis, P. intermedia, T. denticola, and A. actinomycetemcomitans but, surprisingly, not for T. forsythensis. In pathogen carriers, the proportion of pathogens increases in saliva due to deteriorating periodontal status (34), indicating that a subject with advanced periodontitis can serve as a potential source of pathogens to his/her close contacts. Monitoring the carriage pattern of periodontal pathogens at the general population level may help in designing preventive strategies to attempt to control the acquisition of less beneficial members of the human oral microbiota.
Acknowledgments
We would like to acknowledge the Health 2000 organization, especially the working group on laboratory tests and the working group for oral health, for data collection.
The bacterial work has received financial support from the Academy of Finland (grant 78443 to E.K., grant 209152 to S.P., and grant 211129 to P.J.P.). The oral health examination was partly supported by the Finnish Dental Society Apollonia and the Finnish Dental Association.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Albandar, J. M. 2002. Global risk factors and risk indicators for periodontal diseases. Periodontol. 2000 29:177-206. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Periodontology. 1996. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann. Periodontol. 1:926-932. [DOI] [PubMed] [Google Scholar]
- 3.Asai, Y., T. Jinno, H. Igarashi, Y. Ohyama, and T. Ogawa. 2002. Detection and quantification of oral treponemes in subgingival plaque by real-time PCR. J. Clin. Microbiol. 40:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashimoto, A., C. Chen, I. Bakker, and J. Slots. 1996. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 11:266-273. [DOI] [PubMed] [Google Scholar]
- 5.Asikainen, S., S. Alaluusua, and L. Saxén. 1991. Recovery of A. actinomycetemcomitans from teeth, tongue, and saliva. J. Periodontol. 62:203-206. [DOI] [PubMed] [Google Scholar]
- 6.Dahlén, G., F. Widar, R. Teanpaisan, P. N. Papapanou, V. Baelum, and O. Fejerskov. 2002. Actinobacillus actinomycetemcomitans in a rural adult population in southern Thailand. Oral Microbiol. Immunol. 17:137-142. [DOI] [PubMed] [Google Scholar]
- 7.Darout, I. A., J. M. Albandar, and N. Skaug. 2002. Correlations between bacterial levels in autologous subgingival plaque and saliva of adult Sudanese. Clin. Oral Investig. 6:210-216. [DOI] [PubMed] [Google Scholar]
- 8.Denepitiya, L., and I. Kleinberg. 1982. A comparison of the microbial compositions of pooled human dental plaque and salivary sediment. Arch. Oral Biol. 27:739-745. [DOI] [PubMed] [Google Scholar]
- 9.Eger, T., L. Zöller, H.-P. Müller, S. Hoffmann, and D. Lobinsky. 1996. Potential diagnostic value of sampling oral mucosal surfaces for Actinobacillus actinomycetemcomitans in young adults. Eur. J. Oral Sci. 104:112-117. [DOI] [PubMed] [Google Scholar]
- 10.Eggert, F. M., M. H. McLeod, and G. Flowerdew. 2001. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J. Periodontol. 72:1210-1220. [DOI] [PubMed] [Google Scholar]
- 11.Graubard, B. I., and E. L. Korn. 1999. Predictive margins with survey data. Biometrics 55:652-659. [DOI] [PubMed] [Google Scholar]
- 12.Griffen, A. L., M. R. Becker, S. R. Lyons, M. L. Moeschberger, and E. J. Leys. 1998. Prevalence of Porphyromonas gingivalis and periodontal health status. J. Clin. Microbiol. 36:3239-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 14.Hamlet, S. M., M. P. Cullinan, B. Westerman, M. Lindeman, P. S. Bird, J. Palmer, and G. J. Seymour. 2001. Distribution of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in an Australian population. J. Clin. Periodontol. 28:1163-1171. [DOI] [PubMed] [Google Scholar]
- 15.Könönen, E., H. Jousimies-Somer, and S. Asikainen. 1994. The most frequently isolated gram-negative anaerobes in saliva and subgingival samples taken from young women. Oral Microbiol. Immunol. 9:126-128. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. 1981. Covariance adjustment of rates based on the multiple logistic regression model. J. Chronic Dis. 34:415-426. [DOI] [PubMed] [Google Scholar]
- 17.Loesche, W. J., D. E. Lopatin, J. Stoll, N. van Poperin, and P. P. Hujoel. 1992. Comparison of various detection methods for periodontopathic bacteria: can culture be considered the primary reference standard? J. Clin. Microbiol. 30:418-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mager, D. L., A. D. Haffajee, and S. S. Socransky. 2003. Effects of periodontitis and smoking on the microbiota of oral mucous membranes and saliva in systemically healthy subjects. J. Clin. Periodontol. 30:1031-1037. [DOI] [PubMed] [Google Scholar]
- 19.Mättö, J., M. Saarela, S. Alaluusua, V. Oja, H. Jousimies-Somer, and S. Asikainen. 1998. Detection of Porphyromonas gingivalis from saliva by PCR by using a simple sample-processing method. J. Clin. Microbiol. 36:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayanagi, G., T. Sato, H. Simauchi, and N. Takahashi. 2004. Detection frequency of periodontitis-associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjects. Oral Microbiol. Immunol. 19:379-385. [DOI] [PubMed] [Google Scholar]
- 21.Mombelli, A., R. Gmür, N. P. Lang, E. Corbert, and J. Frey. 1999. Actinobacillus actinomycetemcomitans in Chinese adults. Serotype distribution and analysis of the leukotoxin gene promoter locus. J. Clin. Periodontol. 26:505-510. [DOI] [PubMed] [Google Scholar]
- 22.Papapanou, P. N., A. Sellén, J. L. Wennström, and G. Dahlén. 1993. An analysis of the subgingival microflora in randomly selected subjects. Oral Microbiol. Immunol. 8:24-29. [DOI] [PubMed] [Google Scholar]
- 23.Papapanou, P. N., P. N. Madianos, G. Dahlén, and J. Sandros. 1997. “Checkerboard” versus culture: a comparison between two methods for identification of subgingival microbiota. Eur. J. Oral Sci. 105:389-396. [DOI] [PubMed] [Google Scholar]
- 24.Premaraj, T., N. Kato, K. Fukui, H. Kato, and K. Watanabe. 1999. Use of PCR and sodium dodecyl sulfate-polyacrylamide gel electrophoresis techniques for differentiation of Prevotella intermedia sensu stricto and Prevotella nigrescens. J. Clin. Microbiol. 37:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Research Triangle Institute. 2001. SUDAAN user's manual, release 8.0. Research Triangle Institute, Research Triangle Park, NC.
- 26.Riggio, M. P., T. W. MacFarlane, D. MacKenzie, A. Lennon, A. J. Smith, and D. Kinane. 1996. Comparison of polymerase chain reaction and culture methods for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in subgingival plaque samples. J. Periodont. Res. 31:496-501. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, Y., M. Umeda, M. Sakamoto, Y. Benno, Y. Huang, and I. Ishikawa. 2001. Treponema socranskii, Treponema denticola, and Porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J. Periodontol. 72:1354-1363. [DOI] [PubMed] [Google Scholar]
- 28.Timmerman, M. F., G. A. Van der Weijden, S. Armand, F. Abbas, E. G. Winkel, A. J. Winkelhoff, and U. van der Velden. 1998. Untreated periodontal disease in Indonesian adolescents. Clinical and microbiological baseline data. J. Clin. Periodontol. 25:215-224. [DOI] [PubMed] [Google Scholar]
- 29.Tran, S. D., and J. D. Rudney. 1999. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J. Clin. Microbiol. 37:3504-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umeda, M., A. Contreras, C. Chen, I. Bakker, and J. Slots. 1998. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J. Periodontol. 69:828-833. [DOI] [PubMed] [Google Scholar]
- 31.Umeda, M., C. Chen, I. Bakker, A. Contreras, J. L. Morrison, and J. Slots. 1998. Risk indicators for harboring periodontal pathogens. J. Periodontol. 69:1111-1118. [DOI] [PubMed] [Google Scholar]
- 32.Van Winkelhoff, A. J., U. van der Velden, M. Clement, and J. de Graaff. 1988. Intra-oral distribution of black-pigmented Bacteroides species in periodontitis patients. Oral Microbiol. Immunol. 3:83-85. [DOI] [PubMed] [Google Scholar]
- 33.Van Winkelhoff, A. J., B. G. Loos, W. A. van der Reijden, and U. van der Velden. 2002. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 29:1023-1028. [DOI] [PubMed] [Google Scholar]
- 34.Von Troil-Lindén, B., H. Torkko, S. Alaluusua, H. Jousimies-Somer, and S. Asikainen. 1995. Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. J. Dent. Res. 74:1789-1793. [DOI] [PubMed] [Google Scholar]
- 35.Ximénez-Fyvie, L. A., A. D. Haffajee, and S. S. Socransky. 2000. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J. Clin. Periodontol. 27:722-732. [DOI] [PubMed] [Google Scholar]