Abstract
The Klebsiella pneumoniae carbapenem (KPC) β-lactamase occurs in Enterobacteriaceae and can confer resistance to all β-lactam agents including carbapenems. The enzyme may confer low-level carbapenem resistance, and the failure of susceptibility methods to identify this resistance has been reported. Automated and nonautomated methods for carbapenem susceptibility were evaluated for identification of KPC-mediated resistance. Ertapenem was a more sensitive indicator of KPC resistance than meropenem and imipenem independently of the method used. Carbapenemase production could be confirmed with the modified Hodge test.
Carbapenems are commonly used to treat infections caused by multidrug-resistant Enterobacteriaceae. In the United States and other locations, an increasingly common mechanism of carbapenem resistance is the Klebsiella pneumoniae carbapenemase (KPC) (2, 10, 15, 18, 19, 24, 26, 27). The KPC β-lactamase occurs most commonly in K. pneumoniae, but it has also been reported sporadically in other species of Enterobacteriaceae (Klebsiella oxytoca, Enterobacter spp., Escherichia coli, Salmonella spp., Citrobacter freundii, and Serratia spp.) and Pseudomonas aeruginosa (4, 10-12, 17, 23, 28). The KPC enzyme confers resistance to all β-lactam agents including penicillins, cephalosporins, monobactams, and carbapenems (1, 21, 27, 28). Some isolates containing KPC demonstrate low-level carbapenem resistance, but when combined with other cellular changes, such as porin loss, the carbapenem MIC increases (21, 26). The gene encoding the KPC enzyme is usually flanked by transposon-related sequences and has been identified on conjugative plasmids; therefore, the potential for dissemination is significant (17, 26-28). Several outbreaks of KPC-producing bacteria have occurred in the northeast United States (2, 26). Isolates that acquired this enzyme are usually resistant to several other classes of antimicrobial agents used as treatment options. Laboratory identification of KPC-producing clinical isolates will be critical for limiting the spread of this resistance mechanism. The failure of automated susceptibility testing systems to detect KPC-mediated resistance was previously noted (5, 22).
We evaluated commonly used susceptibility testing methods to identify the most sensitive conditions for KPC detection with 31 KPC-producing Enterobacteriaceae isolates (25 of K. pneumoniae, 2 of K. oxytoca, 1 of E. coli, 1 of Enterobacter spp., 1 of Citrobacter freundii, and 1 of Salmonella spp.). These were isolated from different patients who were hospitalized in 13 different healthcare institutions from seven different states: Maryland (one), New Jersey (two), New York (four), Pennsylvania (two), Michigan (two), Missouri (one), and North Carolina (one). The presence of blaKPC was determined using previously described oligonucleotide primers and cycling conditions (27). Enzyme activity was demonstrated in all isolates by isoelectric focusing (6, 16).
To measure the specificity of methods to detect KPC-mediated resistance, 45 isolates (26 of K. pneumoniae, 9 of K. oxytoca, and 10 of E. coli) were chosen for testing. All 45 isolates were negative for blaKPC by PCR. These isolates were submitted to the CDC for reference susceptibility testing. Using the reference broth microdilution (BMD) method, all isolates met the CLSI extended-spectrum β-lactamase (ESBL) screening test criteria; that is, they demonstrated reduced susceptibility to at least one extended-spectrum cephalosporin (7, 8). Twenty-six isolates were positive by the CLSI ESBL broth confirmatory test, and the other isolates were presumed to have another broad-spectrum β-lactamase or other mechanism of cephalosporin resistance. Five isolates were nonsusceptible to a carbapenem (imipenem, meropenem, or ertapenem) by BMD. Since two of the isolates were ESBL producers by BMD and the other three isolates produced an AmpC-type enzyme as demonstrated by isoelectric focusing and PCR (20), it is likely that the mechanism of reduced carbapenem susceptibility is a combination of a cephaloporinase and porin loss (3, 9, 13).
Meropenem, imipenem, and ertapenem susceptibilities were determined by BMD using cation-adjusted Mueller-Hinton broth in panels that were prepared in-house (7), disk diffusion (Becton Dickinson, Sparks, MD) (8), Etest (AB Biodisk, Piscataway, NJ), Microscan Autoscan using the NM32 panel (Dade Behring, West Sacramento, CA), and the Vitek 2 test using the AST GN14 card (bioMérieux, Durham, NC). Susceptibility testing of meropenem and imipenem was performed with the Phoenix test using the NEG MIC 30 panel or NEG MIC 112 panel (Becton Dickinson, Sparks, MD), the Vitek Legacy test using the GNS-122 and GNS-127 panels (bioMérieux, Durham, NC), and the Sensititre Auto Reader using the GN2F panel (Trek Diagnostics, West Lake, OH). All methods were performed according to the manufacturers' recommendations. Quality control testing of susceptibility testing methods was performed with Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. Two criteria were evaluated for detection of KPC-mediated resistance: (i) an intermediate or resistant susceptibility to a carbapenem or (ii) a carbapenem MIC of >1 μg/ml (see Table 1).
TABLE 1.
Performance of susceptibility testing methods for detecting KPC-mediated resistance
| Method | Sensitivity (%)/specificity (%) of:
|
|||||
|---|---|---|---|---|---|---|
| Intermediate or resistant susceptibility resulta
|
Carbapenem MIC of >1 μg/ml
|
|||||
| Meropenem | Imipenem | Ertapenem | Meropenem | Imipenem | Ertapenem | |
| Reference BMD | 94/98 | 94/93 | 97/89 | 100/93 | 100/93 | 100/89 |
| Etest | 58/96 | 55/96 | 90/84 | 84/91 | 90/89 | 100/84 |
| Disk diffusion | 71/96 | 42/96 | 97/87 | NAb | NA | NA |
| Vitek Legacy | 52/98 | 55/96 | NAd | NAc | NAc | NAd |
| Vitek 2 | 48/96 | 71/96 | 94/93 | 71/93 | 94/89 | 94/89 |
| MicroScan | 84/98 | 74/96 | 100/89 | 100/93 | 100/93 | NAc |
| Phoenix | 61/98 | 81/96 | NAd | 74/96 | 87/93 | NAd |
| Sensititre | 42/98 | 29/96 | NAd | 81/96 | NAc | NAd |
Interpretive criteria were based upon CLSI criteria.
NA, not applicable.
Not applicable because the MIC range tested was not low enough (e.g., lowest dilution tested was either 2 μg/ml or 4 μg/ml) for the identification of a carbapenem MIC of >1 μg/ml.
Not applicable because ertapenem was not available on a panel.
Overall, reference BMD was the most sensitive method for the identification of KPC-mediated carbapenem resistance (Table 1). An interpretation of intermediacy or resistance for any of the three carbapenems tested (meropenem, imipenem, and ertapenem) demonstrated greater than 90% sensitivity. For disk diffusion, Etest, Vitek 2, and MicroScan greater than 90% sensitivity was achieved only when ertapenem was tested. When interpreting the results for the disk diffusion test and the Etest, it was important to recognize the presence of small colonies growing inside the zone of inhibition. Meropenem and imipenem susceptibility demonstrated poor sensitivity for methods other than BMD. However, the specificity of meropenem and imipenem susceptibility testing was higher than that for ertapenem susceptibility testing regardless of test method. For nearly all methods (i.e., those demonstrating a specificity of ≥89%), the specificity errors occurred with one or more of the five isolates that were nonsusceptible to carbapenem by a mechanism other than carbapenemase production. It is important that these are not specificity errors in measuring carbapenem susceptibility but rather errors in the detection of carbapenemase production.
Using the criterion of a carbapenem MIC of >1 μg/ml, the sensitivity of detecting KPC-mediated resistance increased for ertapenem, meropenem, and imipenem with only small changes in specificity. Meropenem susceptibility testing demonstrated greater than 90% sensitivity by BMD and MicroScan whereas imipenem susceptibility testing was at least 90% sensitive by BMD, Etest, Vitek 2, and MicroScan.
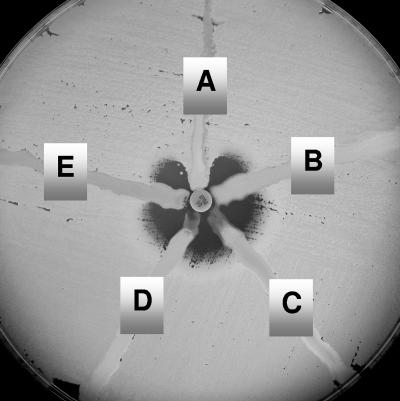
The modified Hodge test (Fig. 1) was also evaluated for detection of KPC-mediated resistance (14). This is a phenotypic test which could be used to determine if reduced susceptibility to carbapenems is mediated by a carbapenemase. The test was performed as described by Lee et al. (14), and it demonstrated 100% sensitivity and specificity for detection of KPC activity.
FIG. 1.
The modified Hodge test Mueller-Hinton agar plate was inoculated with a 1:10 dilution of a 0.5 McFarland suspension of E. coli ATCC 25922 and streaked for confluent growth using a swab. A 10-μg imipenem disk was placed in the center, and each test isolate was streaked from the disk to the edge of the plate. Isolate A is a KPC producer and positive by the modified Hodge test. Isolates B, C, D, and E do not produce a carbapenemase and are negative by the test.
We tested a limited number of isolates in this study. The actual sensitivity and specificity of these methods in any given laboratory will depend upon the prevalent mechanisms of β-lactam resistance in the laboratory's patient population. Considering the potential clinical impact of carbapenemase-producing isolates, laboratories should consider testing for ertapenem susceptibility since it was the most sensitive indicator of KPC activity regardless of method. Ertapenem is not available on panels from all manufacturers; laboratories that cannot test ertapenem on their current system may consider using the ertapenem disk diffusion test, which was a sensitive indicator for the presence of the KPC enzyme in this study and a study by Bratu et al. (5). The KPC enzyme also hydrolyzes extended-spectrum cephalosporins; therefore, screening for reduced susceptibility to carbapenems could be limited to isolates that are resistant to these cephalosporins (e.g., ceftazidime, ceftriaxone, and cefotaxime). Alternatively, meropenem and imipenem susceptibility could be used to detect KPC-mediated resistance if an elevated but susceptible MIC (MIC of >1 μg/ml) is applied. However, it should be noted that this MIC cutoff was applied only to Klebsiella spp. and E. coli in this study. Other species of Enterobacteriaceae have higher wild-type carbapenem MICs (www.eucast.org), and so this strategy may not be appropriate.
No method demonstrated 100% specificity for carbapenemase activity. In the United States, where metallo-β-lactamases are uncommon, the most likely alternative mechanism of carbapenem resistance is the combination of an ESBL or AmpC-type enzyme with porin loss (3, 9, 13). A report by Woodford et al. (25) noted that nonsusceptibility to ertapenem is not specific for carbapenemase production, especially when carbapenemase production is uncommon. Also, the authors noted that for isolates with noncarbapenemase mechanisms, ertapenem resistance does not necessarily predict resistance to other carbapenems. In this study, the modified Hodge test was a useful method for confirming carbapenemase production. Alternatively, a laboratory could confirm the carbapenemase with PCR for the blaKPC gene, which has the added benefit of confirming which enzyme is present.
It is important for laboratories to be vigilant about the identification of emerging KPC resistance in their institution. Strategies for laboratory identification of this resistance will likely have to be reviewed and adjusted as this mechanism is further investigated.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Alba, J., Y. Ishii, K. Thomson, E. S. Moland, and K. Yamaguchi. 2005. Kinetics study of KPC-3, a plasmid-encoded class A carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 49:4760-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratu, S., D. Landman, M. Alam, E. Tolentino, and J. Quale. 2005. Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob. Agents Chemother. 49:776-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratu, S., M. Mooty, S. Nichani, D. Landman, C. Gullans, B. Pettinato, U. Karumudi, P. Tolaney, and J. Quale. 2005. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob. Agents Chemother. 49:3018-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., and S. B. Singer. 1989. Effective cooling allows sonication to be used for liberation of β-lactamases from gram-negative bacteria. J. Antimicrob. Chemother. 24:82-84. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests, 9th ed. Approved standard M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Crowley, B., V. J. Benedi, and A. Domenech-Sanchez. 2002. Expression of SHV-2 β-lactamase and of reduced amounts of OmpK36 porin in Klebsiella pneumoniae results in increased resistance to cephalosporins and carbapenems. Antimicrob. Agents Chemother. 46:3679-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande, L. M., P. R. Rhomberg, H. S. Sader, and R. N. Jones. 2006. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States medical centers: report from the MYSTIC program (1999-2005). Diagn. Microbiol. Infect. Dis. 56:367-372. [DOI] [PubMed] [Google Scholar]
- 11.Hong, T., E. S. Moland, B. Abdalhamid, N. D. Hanson, J. Wang, C. Sloan, D. Fabian, A. Farajallah, J. Levine, and K. S. Thomson. 2005. Escherichia coli: development of carbapenem resistance during therapy. Clin. Infect. Dis. 40:e84-e86. [DOI] [PubMed] [Google Scholar]
- 12.Hossain, A., M. J. Ferraro, R. M. Pino, R. B. Dew III, E. S. Moland, T. J. Lockhart, K. S. Thomson, R. V. Goering, and N. D. Hanson. 2004. Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 in an Enterobacter sp. Antimicrob. Agents Chemother. 48:4438-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaczmarek, F. M., F. Dib-Hajj, W. Shang, and T. D. Gootz. 2006. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of blaACT-1 β-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin PhoE. Antimicrob. Agents Chemother. 50:3396-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, K., Y. Chong, H. B. Shin, Y. A. Kim, D. Yong, and J. H. Yum. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88-91. [DOI] [PubMed] [Google Scholar]
- 15.Lomaestro, B. M., E. H. Tobin, W. Shang, and T. Gootz. 2006. The spread of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae to upstate New York. Clin. Infect. Dis. 43:e26-e28. [DOI] [PubMed] [Google Scholar]
- 16.Matthew, M., and A. M. Harris. 1976. Identification of β-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J. Gen. Microbiol. 94:55-67. [DOI] [PubMed] [Google Scholar]
- 17.Miriagou, V., L. S. Tzouvelekis, S. Rossiter, E. Tzelepi, F. J. Angulo, and J. M. Whichard. 2003. Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob. Agents Chemother. 47:1297-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naas, T., P. Nordmann, G. Vedel, and C. Poyart. 2005. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob. Agents Chemother. 49:4423-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navon-Venezia, S., I. Chmelnitsky, A. Leavitt, M. J. Schwaber, D. Schwartz, and Y. Carmeli. 2006. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob. Agents Chemother. 50:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Perez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith Moland, E., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 22.Tenover, F. C., R. K. Kalsi, P. P. Williams, R. B. Carey, S. Stocker, D. Lonsway, J. K. Rasheed, J. W. Biddle, J. E. McGowan, Jr., and B. Hanna. 2006. Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg. Infect. Dis. 12:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villegas, M. V., K. Lolans, A. Correa, J. N. Kattan, J. A. Lopez, and J. P. Quinn. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 51:1553-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villegas, M. V., K. Lolans, A. Correa, C. J. Suarez, J. A. Lopez, M. Vallejo, and J. P. Quinn. 2006. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob. Agents Chemother. 50:2880-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodford, N., J. W. Dallow, R. L. Hill, M. F. Palepou, R. Pike, M. E. Ward, M. Warner, and D. M. Livermore. 2007. Ertapenem resistance among Klebsiella and Enterobacter submitted in the UK to a reference laboratory. Int. J. Antimicrob. Agents 29:456-459. [DOI] [PubMed] [Google Scholar]
- 26.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M. F. Palepou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yigit, H., A. M. Queenan, J. K. Rasheed, J. W. Biddle, A. Domenech-Sanchez, S. Alberti, K. Bush, and F. C. Tenover. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing β-lactamase KPC-2. Antimicrob. Agents Chemother. 47:3881-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]