Abstract
We monitored early viral response during the treatment of hepatitis C virus (HCV) infection with the aim of identifying predictors of treatment outcome. We studied 53 patients with genotype 1 infection who received 180 μg/week pegylated interferon alfa-2a and 1,000 or 1,200 mg/day ribavirin depending on body weight and serially assessed HCV RNA in serum, using the Cobas TaqMan assay. Thirty-one patients (58%) achieved sustained viral response (SVR). SVR was obtained in 100% (10/10) of patients with pretreatment viremia concentrations below 400,000 IU/ml, in 100% (14/14) of patients with more than 1.5 log reduction of HCV RNA after 4 days of treatment, and in 95% (22/23) of patients with a rate of decline in viremia higher than 0.70 log units/week during the second phase. Non-SVR was seen in all patients with a second-phase decline rate lower than 0.35 log units/week. Patients with slopes between 0.50 and 0.80 log units/week achieved SVR (4/4) unless the treatment dose was modified (3/3). We conclude that the second-phase slope appears to be an accurate and useful predictor of treatment response. On the basis of these findings, we propose a model of tailored treatment which takes into account the second-phase slope and the amount of HCV RNA after 21 days of treatment.
The current standard regimen for patients with chronic hepatitis C virus (HCV) genotype 1 infection, i.e., 48 weeks of treatment with pegylated interferon (Peg-IFN) and ribavirin (RBV), needs to be improved because of limited treatment efficacy, with only ≈50% of patients achieving sustained viral response (SVR), high costs, and significant side effects (9, 12, 19).
The shortcomings of the current antiviral treatment for chronic HCV infection have prompted the development of methods to predict treatment outcome and, hence, to identify responding and nonresponding patients. Indeed, the likelihood of a cure may be predicted with a reasonable level of accuracy as early as after the first dose of interferon or after 1 month of treatment (2, 11, 15, 22, 26, 27), but this has not been applied in clinical practice. As of today, only the absence of HCV RNA (or at least a 2 log decrease) after 12 weeks of therapy is considered clinically useful in deciding whether or not to continue therapy (21). In addition, it was recently suggested that patients with undetectable viremia after 4 weeks of treatment can be treated for 24 instead of 48 weeks (14, 24).
The response to antiviral treatment of HCV infection may be separated into two components: first, a rapid reduction of HCV RNA in serum after one dose of Peg-IFN, presumably reflecting its antiviral efficiency, and second, a slower log-linear decline of viremia (the “second slope”), considered to represent the clearance of infected hepatocytes (22, 27). We initiated this exploratory study with the aim of identifying how analysis of these events might be used in clinical practice in predicting response and, possibly, in tailoring therapy.
MATERIALS AND METHODS
Patients.
From June 2003 to May 2005, patients with chronic genotype 1 infection at four infectious disease clinics in western Sweden were invited to participate if they had clinical indications for therapy (elevated alanine aminotransferase [ALT] and/or a histologically verified inflammation or fibrosis) and had not been treated before. Fifty-three patients (33 men and 20 women) with a mean age of 46.9 years (range, 24 to 68 years) were included and treated. The weights of the patients ranged from 47 to 120 kg (median, 79.5 kg; mean, 81.3 kg). All patients were serologically negative for human immunodeficiency virus antibody and hepatitis B surface antigen. A written informed consent was obtained from each patient. The study protocol was approved by the Regional Research Ethics committee in Göteborg.
Therapy.
All patients were given 48 weeks of standard therapy with Peg-IFN and RBV, following the current recommendations. Thus, Peg-IFN alfa-2a (Pegasys; Roche, Basel, Switzerland) was given at a dosage of 180 μg/week, and RBV (Copegus, Roche) was given orally at a dosage of 1,200 mg/day or 1,000 mg/day, depending on the patient's body weight (above or below 75 kg). SVR was defined as a negative HCV RNA level according to a Cobas TaqMan assay performed 24 weeks after end of treatment.
Thirty-four patients completed 48 weeks of therapy. Treatment was discontinued prematurely (at weeks 22 to 40) in 7 patients (3 SVR, 4 non-SVR) because of side effects (and to some extent in combination with insufficient responses) and in 12 patients (after 12 to 25 weeks of treatment) because their virological responses were absent or insufficient (as determined by Cobas Amplicor assay at treatment weeks 12 and 24). The dose of Peg-IFN was reduced because of neutropenia in 14 patients; for 10 of these patients, the mean weekly dose was reduced to below 150 μg. The dose of RBV was reduced because of anemia in three patients. Two patients discontinued RBV treatment (after 1 week and 10 weeks) because of skin rash.
Genotyping and quantification of HCV RNA.
Genotyping was done by real-time PCR as described previously (17). During the treatment period, HCV RNA was analyzed by Cobas Amplicor (detection limit, 50 IU/ml; Roche Diagnostics, Branchburg, NJ) after 12 and 24 weeks, as part of the clinical routine, and by Cobas Amplicor Monitor (lower limit of quantification, 600 IU/ml) when the qualitative test was positive. Stored serum samples drawn at the initiation of therapy and at days 4, 7, 14, 21, and 28 and at weeks 8, 12, 16, 20, and 24 during treatment and at 24 weeks after the termination of treatment were analyzed retrospectively by Cobas TaqMan real-time PCR assay (lower limit of quantification, 15 IU/ml; Roche Diagnostics). Log values for HCV RNA quantifications refer to log10 units/ml.
Viral kinetics.
The treatment efficiency was evaluated by comparing log HCV RNA levels at day zero with those at day 4. The second-phase decline in viremia was recorded for each patient as the slope (in log units/week) for the regression line of a plot of log HCV RNA values against the time of treatment, including values from day 7 to the last positive HCV RNA value between days 14 and 56.
Models.
We evaluated two models for tailored treatment. One model was adapted from calculations by Drusano and Preston, who postulated that 36 weeks of therapy after achieving undetectable HCV RNA (<100 copies/ml) levels were required for a 90% probability of SVR (7). Since SVR can often be obtained by treatment periods as short as 24 weeks (12, 14, 24), we also designed and evaluated an alternative model. Both models calculate a time point when HCV RNA in serum approaches zero (set to 3 IU/ml in the Drusano and Preston model and to 0.3 IU/ml in our model). This time point was calculated from the log HCV RNA level after 3 weeks of treatment in combination with the log rate of decline (i.e., the slope). The models both add a time for further treatment after achieving undetectable HCV RNA for consolidation of the effect: the Drusano and Preston model simply adds 36 weeks of treatment, while our model was designed to fit the data by using an algorithm that multiplies the time from day 21 until the loss of viremia (<0.3 IU/ml) by a factor of 2 and adds a further 24 weeks. Thus, if HCV RNA is already negative after 3 weeks, then our model produces a required treatment time of 24 weeks compared to 39 weeks with the Drusano and Preston model.
Statistics.
Statistical comparison was done by Mann-Whitney's rank sum test for nonparametric comparison of HCV RNA levels, ALT, or age or by Fischer's exact test for comparing count distribution between groups.
RESULTS
Response and baseline parameters.
In all, 31 of the 53 patients (58%) obtained SVR. The response rates of males (57.6% [19/33]) and females (60% [12/20]) were not significantly different and were not influenced by body weight (mean 81.5 kg for SVR versus 81.0 kg for non-SVR). The pretreatment HCV RNA levels were equal for those with SVR and those with non-SVR, when all patients were compared (log median 6.30 versus 6.63 IU/ml; P = 0.32), but all 10 of the patients with pretreatment levels of viremia below 400,000 IU/ml achieved SVR, compared to 21 of the 43 patients (48.8%) with levels above 400,000 IU/ml (P = 0.003). The baseline-normalized ALT (ALT/upper limit of normal) was not significantly associated with outcome (the mean ALT/upper limit of normal was 2.9 for patients with SVR versus 2.7 for non-SVR patients).
Viral kinetics during the first 12 weeks.
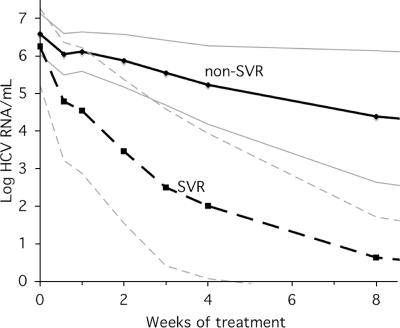
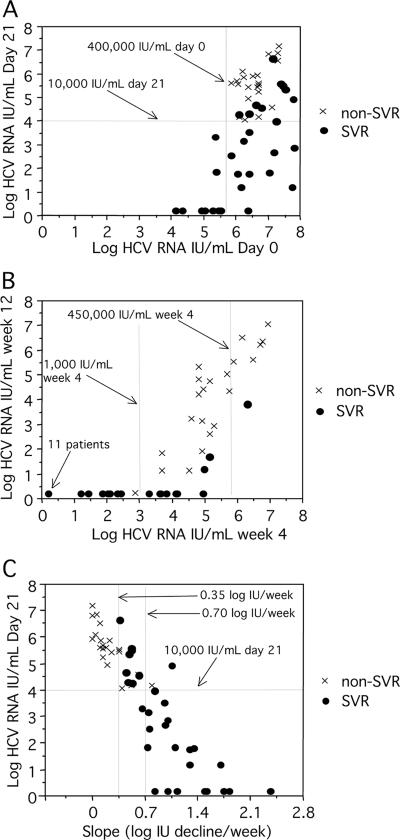
The virological responses for all patients are described in Fig. 1, and the corresponding predictive values are summarized in Table 1. Differences in the virological response were already apparent after 4 days of therapy, when HCV RNA levels had decreased by a median of 1.50 log units in patients achieving SVR, compared to 0.54 log unit in those with non-SVR (P < 0.0001). All 14 patients with a reduction of more than 1.5 log units at day 4 achieved SVR, while 91% (10/11) of those with a reduction of less than 0.5 log unit were classified as non-SVR. All (22/22) patients with HCV RNA levels below 10,000 IU/ml after 3 weeks of treatment, compared to 20% (4/20) of those with HCV RNA levels above 100,000 IU/ml, achieved SVR. Figure 2A shows that most (7/8) of the patients who were HCV RNA negative on day 21 had low baseline levels but also that several of the patients with HCV RNA below 10,000 IU/ml on day 21 had high pretreatment levels. At week 4, 11 patients (21% of all patients) were HCV RNA negative; all 11 showed an SVR (Fig. 2B). In comparison, 48% (20/42) of patients who were HCV RNA positive at week 4 achieved SVR. At week 12, 29 patients were HCV RNA negative by Cobas TaqMan (all but one achieved SVR, and 24 were HCV RNA positive), and 3 achieved SVR (Fig. 2B). Discrepant results by Cobas Amplicor and Cobas TaqMan were seen in three samples from week 12, all from SVR patients (two samples were positive by TaqMan only, and one was positive by Amplicor only). A >2-log reduction of viremia at week 12 was observed for all SVR patients (range, 3.4 to 7.6 log units) and for 50% (11/22) of non-SVR patients (range, 0.2 to 6.5 log units).
FIG. 1.
The mean log HCV RNA levels (thin lines, standard deviations) for non-SVR and SVR during the initial 12 weeks of treatment.
TABLE 1.
Predictive values of HCV RNA levels and changes at different time points
| Time point | HCV RNA level | Sensitivity (%)a | Specificity (%)b | PPV (%)c | NPV (%)d |
|---|---|---|---|---|---|
| Baseline | <400,000 IU/ml | 32 | 100 | 100 | 51 |
| Day 21 | <10,000 IU/ml | 71 | 100 | 100 | 70 |
| Day 21 | <100,000 IU/ml | 87 | 76 | 84 | 80 |
| Wk 4 | <1,000 IU/ml | 68 | 95 | 95 | 68 |
| Wk 4 | <15 IU/ml (negative) | 48 | 100 | 100 | 58 |
| Wk 4 | <450,000 IU/ml | 97 | 36 | 68 | 89 |
| Wk 12 | <50 IU/ml (negative) | 93 | 95 | 97 | 91 |
| Wk 12 | <15 IU/ml (negative) | 90 | 95 | 97 | 88 |
| Second slopee | >0.70 log IU/wk | 74 | 91 | 92 | 71 |
| Second slopee | >0.35 log IU/wk | 97 | 73 | 83 | 94 |
| Second slopef | >0.70 log IU/wk | 71 | 95 | 96 | 70 |
| Second slopef | >0.35 log IU/wk | 100 | 73 | 84 | 100 |
| Decrease, day 4 | >1.5 log IU | 45 | 100 | 100 | 53 |
| Decrease, wk 12 | >2 log IU | 100 | 50 | 74 | 100 |
Sensitivity, proportion of patients with SVR who had HCV RNA levels below, a slope value above, or HCV RNA reduction above the given limit.
Specificity, proportion of patients with non-SVR who did not have HCV RNA below, a slope value above, or HCV RNA reduction above the given limit.
PPV, proportion of patients with HCV RNA below, a slope value above, or HCV RNA reduction above the given limit who achieved SVR.
NPV, proportion of patients without HCV RNA below, a slope value above, or HCV RNA reduction above the given limit who did not achieve SVR.
Based on HCV RNA levels at days 7, 14, and 21.
Based on all HCV RNA values.
FIG. 2.
HCV RNA levels at baseline and on day 21 (A) after 4 and 12 weeks (B) of treatment and HCV RNA levels on day 21 in relation to individual slope values (C).
Second-phase slope and response.
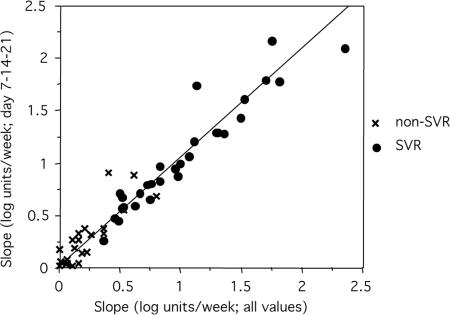
There was a strong correlation between the second-phase slope and the SVR: 22/23 patients (95%) with a slope steeper than 0.70 log units/week and 9/14 patients (64%) with a slope between 0.35 and 0.70 log units/week obtained SVR, while, conversely, all 16 of the patients with a slope below 0.35 log units/week were non-SVR (Fig. 2C, P < 0.0001). To evaluate how many samples were required for the calculation of the slope, we plotted slopes based on all available values (up to week 8) relative to slopes based on values from days 7, 14, and 21 (Fig. 3). The strong correlation (R2 = 0.93; P < 0.0001) indicated that slopes based on the three values were accurate for most patients: based on three time points, a slope below 0.35 log units/week had a negative predictive value (NPV) of 94%, and a slope above 0.70 log units/week had a positive predictive value (PPV) of 92% for the prediction of SVR (Table 2).
FIG. 3.
Correlation between slope based on all available values and only those from days 7, 14, and 21 (P < 0.001; correlation coefficient, R2 = 0.93).
TABLE 2.
Details of kinetics and treatment for patient with borderline responses to Peg-IFN and RBV therapy
| Patient no. | SVR or non-SVR | Age (yr) | Wt (kg) | Log HCV RNA level at wk 12a | Amplicor detectionb
|
No. of treatment wks | Mean weekly dose of Peg-IFN (μg) | Mean daily dose of RBV (g) | Slope (log LU/wk) | Calculated no. of treatment wks using:
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 12 | Wk 24 | This study's algorithmc | Drusano-Preston modeld | |||||||||
| Pat 5 | Non-SVR | 48 | 61 | 3.2 | Pos | Neg | 48 | 180 | 1.0 | 0.22 | 73 | 59 |
| Pat 42 | Non-SVR | 59 | 80 | 4.2 | Pos | Pos | 28e | 180 | 1.1 | 0.24 | 77 | 61 |
| Pat 31 | Non-SVR | 48 | 71 | 2.9 | Pos | Neg | 48 | 83 | 1.0 | 0.27 | 67 | 57 |
| Pat 27 | Non-SVR | 49 | 82 | 2.6 | Pos | Neg | 48 | 180 | 1.2 | 0.37 | 56 | 52 |
| Pat 32 | Non-SVR | 54 | 97 | 1.9 | GZg | Neg | 48 | 180 | 1.2 | 0.37 | 56 | 52 |
| Pat 1 | Non-SVR | 35 | 90 | 1.1 | Pos | Pos | 48 | 180 | 1.2 | 0.41 | 46 | 48 |
| Pat 21 | Non-SVR | 58 | 85 | 1.8 | Pos | Pos | 27e | 180 | 0.086f | 0.54 | 42 | 46 |
| Pat 13 | Non-SVR | 45 | 70 | 1.1 | Pos | Pos | 32h | 180 | 0.34h | 0.62 | 41 | 46 |
| Pat 33 | Non-SVR | 36 | 77 | Neg | Neg | Neg | 48 | 139 | 0.99 | 0.80 | 36 | 44 |
| Pat 28 | SVR | 47 | 94 | 3.8 | Pos | Neg | 48 | 180 | 1.2 | 0.36 | 63 | 56 |
| Pat 22 | SVR | 47 | 79 | Neg | Neg | Neg | 48 | 180 | 1.2 | 0.46 | 47 | 48 |
| Pat 44 | SVR | 60 | 75 | Neg | Neg | Neg | 48 | 180 | 1.2 | 0.48 | 44 | 47 |
| Pat 38 | SVR | 57 | 65 | 1.2 | Neg | Neg | 48 | 180 | 1.0 | 0.50 | 48 | 49 |
| Pat 2 | SVR | 48 | 68 | 1.7 | Neg | Neg | 40 | 180 | 1.0 | 0.52 | 48 | 49 |
| Pat 24 | SVR | 56 | 91 | Neg | Pos | Neg | 48 | 126 | 1.2 | 0.52 | 47 | 49 |
| Pat 6 | SVR | 52 | 88 | Neg | Neg | Neg | 48 | 180 | 1.0 | 0.53 | 42 | 46 |
| Pat 30 | SVR | 57 | 86 | Neg | Neg | Neg | 48 | 180 | 1.2 | 0.62 | 40 | 46 |
| Pat 7 | SVR | 30 | 78 | Neg | Neg | Neg | 48 | 180 | 1.2 | 0.66 | 36 | 43 |
| Pat 34 | SVR | 59 | 67 | Neg | Neg | Neg | 48 | 180 | 0.97 | 0.73 | 31 | 41 |
| Pat 9 | SVR | 29 | 78 | Neg | Neg | Neg | 48 | 180 | 1.2 | 0.75 | 32 | 43 |
| Pat 8 | SVR | 47 | 78 | Neg | Neg | Neg | 48 | 180 | 1.0 | 0.76 | 32 | 42 |
Cobas TaqMan detection limit, 15 IU/ml.
Detection limit, 50 IU/ml. Pos, positive; Neg, negative.
Our model algorithm: treatment weeks = 24 + 2 × [(log HCV RNA day 21 + 0.5)/log decline per week].
Drusano-Preston model algorithm: treatment weeks = 36 + (log HCV RNA day 21 − 0.5)/log decline per week.
These patients discontinued because of insufficient response and because of side effects.
RBV therapy was discontinued after 1 week because of side effects.
GZ, gray-zone reaction.
RBV therapy was discontinued after 10 weeks because of side effects.
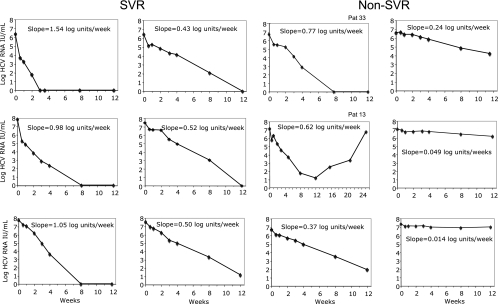
Figure 4 shows individual patterns of viral decay, with slope values ranging from 1.54 log units/week (rapid response) to 0.014 log units/week (flat response). For most patients, there was a clear log-linear decline of viremia, as also illustrated by the regression coefficient (R2) values above 0.96 with 38 patients for slopes based on three time points only. In particular, the R2 values for individual slopes were high for SVR (above 0.97 for all but two patients).
FIG. 4.
Individual graphs describing viral kinetics during the first 12 weeks of therapy for SVR and non-SVR patients representing different rates of second-phase decline. A longer time span is shown for patient Pat 13 who discontinued RBV therapy after 10 weeks.
In order to better understand the outcome in patients with borderline responses, we recorded dose reductions and other factors that might influence response for the 21 patients with slopes ranging between 0.20 and 0.80 log units/week (Table 2). There were no differences in age and weight between SVR and non-SVR patients in this subgroup of patients. At week 12 of treatment, 8 of 9 non-SVR patients were HCV RNA positive (including 1 with a gray-zone reaction) by Cobas Amplicor, compared to only 2 of 12 in the SVR group. The three non-SVR patients with slopes above 0.50 log units/week had not taken the treatment as scheduled: one patient with a slope of 0.77 log units/week (Fig. 4, Pat 33) had forgotten to bring the Peg-IFN medication during vacation and missed 2 weeks of treatment (weeks 18 to 19) and also had required a significant dose reduction of Peg-IFN from week 12 of treatment because of neutropenia (thereby receiving 77% of the planned total dose). Two other patients, with slopes of 0.62 and 0.54 log units/week, had stopped RBV therapy after 10 weeks and 1 week, respectively, because of intensive skin rash. The patient that stopped RBV after 10 weeks (Fig. 4, Pat 13) had a positive HCV RNA result at week 12 (but was negative by Cobas Amplicor Monitor) and then developed a virological breakthrough with viremia increasing to a high level at week 24 (13). All nine SVR patients with slopes below 0.70 log/week had taken full doses of Peg-IFN and RBV, but one had stopped treatment after 40 weeks because of side effects.
Models.
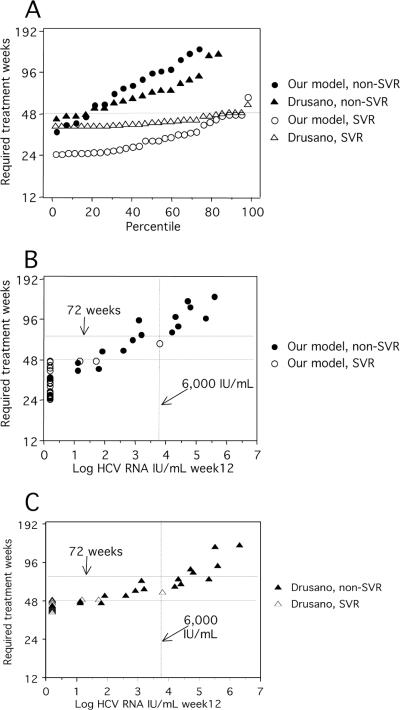
Figure 5 shows the required number of treatment weeks calculated for patients in the present study in relation to the observed outcome. According to our model, 14 of the 31 SVR patients (including the 11 who were HCV RNA negative at week 4) would have required less than 28 weeks, and another 8 SVR patients would have required 28 to 36 weeks of treatment. One patient who in fact responded to 48 weeks of treatment, according to the algorithm, would have required 63 weeks. The calculated treatment time was less than 48 weeks for four non-SVR (36, 41, 42, and 46 weeks), but three of these were the patients mentioned above who stopped RBV or reduced the dose of therapy. Required treatment times between 50 and 72 weeks were obtained for nine non-SVR patients by the Drusano and Preston model. In contrast, our model indicated that only three non-SVR patients would be cured by extended treatment, as shown in Fig. 5 and Table 2.
FIG. 5.
Required duration of treatment for the patients in the present study as calculated by algorithms described below (A). Treatment times in relation to HCV RNA levels at week 12 as calculated by our model (B) or by model adapted from Drusano and Preston (C). A dotted line representing 6,000 IU/ml at week 12 is included because Berg et al. (3) reported that only patients with HCV RNA below this level benefited from extended treatment. Our model, treatment weeks = 24 + 2 × [(log HCV RNA day 21 + 0.5)/log decline per week]. Drusano and Preston model, treatment weeks = 39 + (log HCV RNA day 21 − 0.5/log decline per week). Values for six patients with non-SVR are not shown because they were above 200 weeks.
DISCUSSION
Combination therapy with Peg-IFN and RBV has clearly improved the results of treatment of chronic HCV infection. However, the relatively low response rates for patients carrying genotype 1 virus remains a significant problem, especially in light of the costs and treatment-related toxicity. An improved prediction of SVR as well as non-SVR would therefore be important, and it has been proposed that the viral kinetics during treatment might be clinically useful for this purpose (1, 2, 4, 5, 16, 18, 20, 22, 25, 26). For example, the magnitude of HCV RNA reduction after only one dose of interferon is clearly associated with an SVR (10, 15), as is the rate of decline in viremia between day 7 and week 12 (second-phase slope) (1, 8). However, it has been difficult to include kinetic parameters in clinical practice, partly because the reliability of predictions for individual patients has been uncertain and partly due to concerns regarding the robustness of HCV RNA quantification. Thus, 48 weeks of therapy has remained the standard treatment for all HCV genotype 1-infected patients, and qualitative HCV RNA tests after 12 and 24 weeks of treatment have been used to decide whether to continue or discontinue therapy (21). Recently, it was proposed that 24 weeks of treatment may be sufficient for patients with low baseline viremia levels and/or undetectable levels of HCV RNA after 4 weeks of therapy (14, 24), but a truly tailored treatment has hitherto not been established.
The aim of the present study, which included genotype 1-infected patients treated with Peg-IFN alfa-2a and RBV, was to analyze how the early kinetics were related to the outcome of treatment and, possibly, to propose a model for tailored therapy. Out of 53 patients studied, 31 patients obtained an SVR (58.5%). The likelihood of response was associated with virological parameters such as baseline viremia, reduction of HCV RNA between day 1 and 4, second-slope decline, and HCV RNA levels at days 21 and 28 and week 12 (Table 1). All patients with baseline viremia levels below 400,000 IU/ml achieved SVR. This is in line with previous reports in which the response rates for this subset of patients, however, have not been as high as 100%.
The reduction in viremia after 4 days of treatment, which is considered to reflect mainly interferon efficiency, was strongly associated with the response, in agreement with the observation by Jessner et al., who reported that less than 0.5-log reduction was highly predictive for non-SVR (15). However, at this early stage, the differences in log reduction between SVR and non-SVR are relatively small and might be influenced by the test variability of HCV RNA quantification. We therefore believe that predicting response by this parameter alone may not be sufficiently reliable for large-scale clinical use.
The second-phase decline of viremia appears clinically more useful for predicting outcome. First, our data indicate that a decline rate slower than 0.35 log units/week is a good predictor of non-SVR. Thus, a slope generated by HCV RNA quantifications after 1, 2, and 3 weeks of treatment could be used to identify non-SVR at an early stage, instead of, as today, after 12 weeks. Moreover, all but one patient with a slope greater than 0.70 log units/week obtained SVR. The exception was a patient with a slope of 0.77 log units/week, but it is likely that the inability to achieve SVR in this case was partly due to dose reductions. Similarly, non-SVR in two patients with 0.62 and 0.54 log units/week was probably due to discontinuation of RBV after 1 and 10 weeks, respectively.
It has recently been proposed that a negative qualitative HCV RNA response after 4 weeks may be used to identify patients for whom 24 weeks of treatment would be sufficient (14, 24). In previous studies, around 25% of patients have had a rapid response (i.e., HCV RNA negative at week 4), and about 90% of these patients have achieved SVR (2, 6, 8, 24). In our study, only 21% were HCV RNA negative at week 4 (probably as an effect of a higher assay sensitivity, 15 IU/ml), and all of them achieved SVR. A level above 450,000 IU/ml at week 4 was recently described as useful for predicting non-SVR (2), and accordingly, all but one of our nine patients with such levels were non-SVR. However, although the HCV RNA response at week 4 may be an accurate predictor for both SVR and non-SVR, it was, with the limits given above, applicable for only 36% of patients, and we would prefer tailored treatment for all patients.
With this background, we evaluated two models for calculating the required treatment time, one adapted from Drusano and Preston (7) and one developed by us. The algorithms both include a calculation of the time to undetectable HCV RNA levels, using the HCV RNA level at day 21 and the second-phase decline. The Drusano and Preston model fitted well to the observed data, as patients that obtained SVR had calculated treatment times of 48 or fewer weeks, while the non-SVR would have required longer treatment. However, treatment times are never shorter than 39 weeks by the Drusano and Preston model, and it is known that some patients can be cured at only 24 weeks (14, 24) or even at 18 weeks (26) of treatment. Our alternative model predicted that around 25% of the SVR patients would have required 24 weeks of therapy, which is well in line with recent observations by others (14, 24). Also in accordance with recent studies (3, 23), our model predicted that prolonging the treatment time to 72 weeks would have been beneficial for only three patients who showed low but detectable viremia levels (<1,000 IU/ml) at week 12 and undetectable HCV RNA levels at week 24. This agrees with the observation by Berg et al. (3) that extended treatment seems to be beneficial only for patients with HCV RNA levels below 6,000 IU/ml at week 12. In contrast, the Drusano and Preston model predicted that treatment extended to 72 weeks would have cured nine additional non-SVR patients, four of whom, however, had HCV RNA levels above 15,000 IU/ml at week 12. Clearly, further study of the value of longer treatment regimens is required.
Our finding that the slope of viral decline is highly predictive for both SVR and non-SVR and that models for tailoring treatment appear to be accurate suggests that treatment may be individualized. An important issue is determining which time points should be used for slope calculation. We believe that measurements taken as early as days 7, 14, and 21 are appropriate. First, the majority of patients are HCV RNA positive at these time points. Second, day 7 is an adequate start point, because the first-phase decline is completed. It may be objected that some patients reach a plateau phase and show a delayed start of the second slope. We saw this in only a few patients (readily identified by a lower R2 for the slope) and conclude that the advantage of using day 14 as the starting point is marginal.
It is also important to consider to what degree test variability may influence the slope. Our observation that the R2 values of individual slopes were above 0.97 for almost all SVR indicates that changes in HCV RNA levels early during treatment were true variations rather than methodological artifacts. Thus, quantification results by a Cobas TaqMan assay appear to be sufficiently reliable and are possible to obtain within 1 week in a clinical routine.
We are aware that the number of patients in the present study is relatively low, making firm conclusions regarding thresholds and predictive values difficult. If tailored treatment is used, one must carefully consider what levels of PPV and NPV are acceptable. A stopping rule based on >2 log reduction of HCV RNA at week 12 had a PPV of 72% and an NPV of 100% in the report by Davis et al. (6), and the corresponding values in our study were very similar (74% and 100%, respectively). If our proposed model for tailored treatment was applied, we would consider a PPV of 85% and an NPV of 95% as acceptable and realistic. However, one must keep in mind that this model was evaluated with the same patients that were investigated for construction of the algorithm and therefore has to be validated further.
In summary, we report that an SVR is highly associated with a baseline HCV RNA level below 400,000 IU/ml, a >1.5 log reduction of viremia after 4 days, and undetectable HCV RNA after 4 weeks of treatment. However, the second-phase decline appears to be the single most useful parameter, as it predicts SVR and non-SVR for the majority of patients. In addition, second-slope calculation in combination with the HCV RNA levels after 3 weeks of treatment may be used to determine an individualized duration of treatment.
Acknowledgments
The study was supported by ALF funds (grant ALFGBG-2983), by Western Region R&D funds (grant VGFOUREG-1869), and by Roche Pharma.
We thank Giuseppe Colucci at Roche Diagnostics and Ulrika Nillroth at Roche Pharma for their kind assistance and Catarina Skoglund, Kerstin Johansson, Lena Finlöf, Katarina Holmblad, Clementina Garcia, Lena Tollén, Lilly Namvar, Asieh Raisi, Lejla Dubicanac, Irma Szentivanyi and Christina Andersson for dedicated work in this project.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Bekkering, F. C., C. Stalgis, J. G. McHutchison, J. T. Brouwer, and A. S. Perelson. 2001. Estimation of early hepatitis C viral clearance in patients receiving daily interferon and ribavirin therapy using a mathematical model. Hepatology 33:419-423. [DOI] [PubMed] [Google Scholar]
- 2.Berg, T., C. Sarrazin, E. Herrmann, H. Hinrichsen, T. Gerlach, R. Zachoval, B. Wiedenmann, U. Hopf, and S. Zeuzem. 2003. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology 37:600-609. [DOI] [PubMed] [Google Scholar]
- 3.Berg, T., M. von Wagner, S. Nasser, C. Sarrazin, T. Heintges, T. Gerlach, P. Buggisch, T. Goeser, J. Rasenack, G. R. Pape, W. E. Schmidt, B. Kallinowski, H. Klinker, U. Spengler, P. Martus, U. Alshuth, and S. Zeuzem. 2006. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology 130:1086-1097. [DOI] [PubMed] [Google Scholar]
- 4.Buti, M., F. Sanchez-Avila, Y. Lurie, C. Stalgis, A. Valdes, M. Martell, and R. Esteban. 2002. Viral kinetics in genotype 1 chronic hepatitis C patients during therapy with 2 different doses of peginterferon alfa-2b plus ribavirin. Hepatology 35:930-936. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson, T., O. Reichard, G. Norkrans, J. Blackberg, P. Sangfelt, E. Wallmark, and O. Weiland. 2005. Hepatitis C virus RNA kinetics during the initial 12 weeks treatment with pegylated interferon-alpha 2a and ribavirin according to virological response. J. Viral Hepat. 12:473-480. [DOI] [PubMed] [Google Scholar]
- 6.Davis, G. L., J. B. Wong, J. G. McHutchison, M. P. Manns, J. Harvey, and J. Albrecht. 2003. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 38:645-652. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L., and S. L. Preston. 2004. A 48-week duration of therapy with pegylated interferon alpha 2b plus ribavirin may be too short to maximize long-term response among patients infected with genotype-1 hepatitis C virus. J. Infect. Dis. 189:964-970. [DOI] [PubMed] [Google Scholar]
- 8.Ferenci, P., M. W. Fried, M. L. Shiffman, C. I. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, M. Chaneac, and K. R. Reddy. 2005. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J. Hepatol. 43:425-433. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 10.Fukutomi, T., M. Nakamuta, M. Fukutomi, M. Iwao, H. Watanabe, K. Hiroshige, Y. Tanabe, and H. Nawata. 2001. Decline of hepatitis C virus load in serum during the first 24 h after administration of interferon-beta as a predictor of the efficacy of therapy. J. Hepatol. 34:100-107. [DOI] [PubMed] [Google Scholar]
- 11.Gavier, B., M. A. Martinez-Gonzalez, J. I. Riezu-Boj, J. J. Lasarte, N. Garcia, M. P. Civeira, and J. Prieto. 1997. Viremia after one month of interferon therapy predicts treatment outcome in patients with chronic hepatitis C. Gastroenterology 113:1647-1653. [DOI] [PubMed] [Google Scholar]
- 12.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann, E., J. H. Lee, G. Marinos, M. Modi, and S. Zeuzem. 2003. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology 37:1351-1358. [DOI] [PubMed] [Google Scholar]
- 14.Jensen, D. M., T. R. Morgan, P. Marcellin, P. J. Pockros, K. R. Reddy, S. J. Hadziyannis, P. Ferenci, A. M. Ackrill, and B. Willems. 2006. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kD)/ribavirin therapy. Hepatology 43:954-960. [DOI] [PubMed] [Google Scholar]
- 15.Jessner, W., M. Gschwantler, P. Steindl-Munda, H. Hofer, T. Watkins-Riedel, F. Wrba, C. Mueller, A. Gangl, and P. Ferenci. 2001. Primary interferon resistance and treatment response in chronic hepatitis C infection: a pilot study. Lancet 358:1241-1242. [DOI] [PubMed] [Google Scholar]
- 16.Layden, J. E., and T. J. Layden. 2001. How can mathematics help us understand HCV? Gastroenterology 120:1546-1549. [DOI] [PubMed] [Google Scholar]
- 17.Lindh, M., and C. Hannoun. 2005. Genotyping of hepatitis C virus by Taqman real-time PCR. J. Clin. Virol. 34:108-114. [DOI] [PubMed] [Google Scholar]
- 18.Makiyama, A., Y. Itoh, K. Yasui, K. Mori, M. Okita, M. Nakayama, J. Yamaoka, M. Minami, T. Nakajima, and T. Okanoue. 2006. First phase viral kinetic parameters and prediction of response to interferon alpha-2b/ribavirin combination therapy in patients with chronic hepatitis C. Hepatol. Res. 36:94-99. [DOI] [PubMed] [Google Scholar]
- 19.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Bauer, E., J. Crespo, M. Romero-Gomez, R. Moreno-Otero, R. Sola, N. Tesei, F. Pons, X. Forns, and J. M. Sanchez-Tapias. 2006. Development and validation of two models for early prediction of response to therapy in genotype 1 chronic hepatitis C. Hepatology 43:72-80. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Health. 2002. NIH consensus statement on management of hepatitis C. NIH Consens. State Sci. Statements 19:1-46. [PubMed] [Google Scholar]
- 22.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Tapias, J. M., M. Diago, P. Escartin, J. Enriquez, M. Romero-Gomez, R. Barcena, J. Crespo, R. Andrade, E. Martinez-Bauer, R. Perez, M. Testillano, R. Planas, R. Sola, M. Garcia-Bengoechea, J. Garcia-Samaniego, M. Munoz-Sanchez, and R. Moreno-Otero. 2006. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 131:451-460. [DOI] [PubMed] [Google Scholar]
- 24.Zeuzem, S., M. Buti, P. Ferenci, J. Sperl, Y. Horsmans, J. Cianciara, E. Ibranyi, O. Weiland, S. Noviello, C. Brass, and J. Albrecht. 2006. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J. Hepatol. 44:97-103. [DOI] [PubMed] [Google Scholar]
- 25.Zeuzem, S., J. H. Lee, A. Franke, B. Ruster, O. Prummer, G. Herrmann, and W. K. Roth. 1998. Quantification of the initial decline of serum hepatitis C virus RNA and response to interferon alfa. Hepatology 27:1149-1156. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem, S., J. M. Pawlotsky, E. Lukasiewicz, M. von Wagner, I. Goulis, Y. Lurie, E. Gianfranco, J. M. Vrolijk, J. I. Esteban, C. Hezode, M. Lagging, F. Negro, A. Soulier, E. Verheij-Hart, B. Hansen, R. Tal, C. Ferrari, S. W. Schalm, and A. U. Neumann. 2005. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J. Hepatol. 43:250-257. [DOI] [PubMed] [Google Scholar]
- 27.Zeuzem, S., J. M. Schmidt, J. H. Lee, B. Ruster, and W. K. Roth. 1996. Effect of interferon alfa on the dynamics of hepatitis C virus turnover in vivo. Hepatology 23:366-371. [DOI] [PubMed] [Google Scholar]