Abstract
Sixty-one Salmonella enterica serovar Typhimurium isolates of animal and human origin, matched by phage type, antimicrobial resistance pattern, and place of isolation, were analyzed by microbiological and molecular techniques, including pulsed-field gel electrophoresis (PFGE) and plasmid profiling. PFGE identified 10 profiles that clustered by phage type and antibiotic resistance pattern with human and animal isolates distributed among different PFGE profiles. Genomic DNA was purified from 23 representative strains and hybridized to the composite Salmonella DNA microarray, and specific genomic regions that exhibited significant variation between isolates were identified. Bioinformatic analysis showed that variable regions of DNA were associated with prophage-like elements. Subsequently, simple multiplex PCR assays were designed on the basis of these variable regions that could be used to discriminate between S. enterica serovar Typhimurium isolates from the same geographical region. These multiplex PCR assays, based on prophage-like elements and Salmonella genomic island 1, provide a simple method for identifying new variants of S. enterica serovar Typhimurium in the field.
Salmonella enterica is a significant cause of morbidity and mortality for human and veterinary medicine, with multiantibiotic-resistant S. enterica serovar Typhimurium being an emerging problem. An important source of S. enterica serovar Typhimurium in human infections is contaminated food of animal origin, particularly meat products derived from cattle (9). S. enterica serovar Typhimurium can survive in the environment, and once established on a farm, contamination can be difficult to eradicate. Salmonella may spread from farm to farm through the exchange of livestock, by wildlife, or in the runoff from fields, and it can disseminate in food chains as a consequence of further cross-contamination at slaughterhouses. Food-borne transmission of common types of S. enterica serovar Typhimurium found in cattle, such as definitive phage type 104 (DT104), is well documented for human outbreaks, with sources ranging from roast beef to unpasteurized milk (29). Moreover, animals infected with antibiotic-resistant Salmonella are an important source of resistance determinants that can transfer to human-infective Salmonella serovars.
Many methods have been developed to phenotypically distinguish between S. enterica serovar Typhimurium isolates, including antibiotic susceptibility profiling, phage typing (1), pulsed-field gel electrophoresis (PFGE) (7), and plasmid profiling (26) as well as various PCR-based techniques (8, 15, 19). However, since the genome sequences of several S. enterica strains, including different S. enterica serovar Typhimurium strains, are now available, it should be possible to design rational DNA tools based on fully annotated DNA sequences for use in the field to monitor strain diversity. Here, we have used some of the existing classical typing techniques to analyze a matched collection of S. enterica serovar Typhimurium strains isolated from animal and human sources and have extended these approaches to include DNA microarray analysis. Using these techniques, we have been able to identify and map regions of variation on the chromosome of S. enterica serovar Typhimurium that discriminate between isolates circulating in the same geographical region. Using this information, we have designed multiplex PCR assays that are simple to use and that are able to rapidly distinguish between S. enterica serovar Typhimurium isolates in a cost-effective manner. We believe that similar PCR assays, constructed on the basis of regions of variation in other Salmonella serovars, have the potential to improve the local epidemiological analysis of Salmonella outbreaks.
MATERIALS AND METHODS
Bacterial isolates.
Thirty isolates of S. enterica serovar Typhimurium of animal origin (prefix A) (26 from cattle feces, 2 from pig feces, and 2 from crow feces) isolated between February 2000 and August 2002 from eight farms were selected from the Wellcome Trust International Partnership Research Award in Veterinary Epidemiology consortium collection. They were chosen to represent a number of phage types and phenotypic antibiotic sensitivity patterns (Table 1). Thirty-one well-characterized human isolates (prefix H) from the Scottish Salmonella Reference Laboratory (Glasgow) subsequently were selected to match the animal strains by phage type, antibiotic resistance pattern, and place and time of isolation, where possible. The human isolates had been received by the Scottish Salmonella Reference Laboratory from 12 regional laboratories between August 1996 and November 2002. Most human isolates (29/31) were from sporadic cases, but one was part of a family outbreak and one patient had a recent travel history. Identification by culture, serology (based on standard laboratory agglutination tests), and phage typing (1) was performed at the aforementioned laboratory. Additional laboratory reference strains included in the analyses were S. enterica serovar Typhimurium DT104 (NCTC 13348), S. enterica serovar Typhimurium LT2 (ATCC 700220), and S. enterica serovar Typhimurium SL1344 (NCTC 13347).
TABLE 1.
Origin of 30 animal and 31 human S. enterica serovar Typhimurium isolates
| Isolate | Source | Sample date (day/mo/yr) | Location (animal health district or sending laboratorya) | Farm code or location of patient | Note on infection type |
|---|---|---|---|---|---|
| Animal | |||||
| AWF002005 | Bovine | 18/02/2000 | Central | A03 | |
| AWF002008 | Bovine | 18/02/2000 | Central | A03 | |
| AWF007246 | Bovine | 15/11/2001 | Highlands | S009 | |
| AWF007581 | Bovine | 06/12/2001 | Highlands | S009 | |
| AWF009126 | Bovine | 16/08/2002 | Highlands | S011 | |
| AWF009144 | Bovine | 16/08/2002 | Highlands | S011 | |
| AWF009147 | Bovine | 16/08/2002 | Highlands | S011 | |
| AWX003485 | Bovine | 30/08/2001 | Highlands | S009 | |
| AWX003658 | Bovine | 30/08/2001 | Highlands | S009 | |
| AWX004814 | Bovine | 13/12/2001 | Highlands | S009 | |
| AWX004816 | Bovine | 13/12/2001 | Highlands | S009 | |
| AWX005861 | Bovine | 16/04/2002 | Highlands | S009 | |
| AWX006747 | Pig | 29/04/2002 | Highlands | S010 | |
| AWX006748 | Pig | 29/04/2002 | Highlands | S010 | |
| AWX008764 | Crow | 16/08/2002 | Highlands | S011 | |
| AWX008765 | Crow | 16/08/2002 | Highlands | S011 | |
| AWF004105 | Bovine | 12/12/2000 | North East | S001 | |
| AWF004135 | Bovine | 12/12/2000 | North East | S001 | |
| AWF004526 | Bovine | 16/01/2001 | North East | S001 | |
| AWF004602 | Bovine | 16/01/2001 | North East | S001 | |
| AWX000826 | Bovine | 10/10/2000 | North East | S001 | |
| AWX000836 | Bovine | 10/10/2000 | North East | S001 | |
| AWX000841 | Bovine | 10/10/2000 | North East | S001 | |
| AWX000847 | Bovine | 10/10/2000 | North East | S001 | |
| AWX001996 | Bovine | 23/01/2001 | North East | S006 | |
| AWX001997 | Bovine | 23/01/2001 | North East | S006 | |
| AWX002732 | Bovine | 19/06/2001 | North East | S300 | |
| AWX002743 | Bovine | 19/06/2001 | North East | S300 | |
| AWX002821 | Bovine | 19/06/2001 | North East | S301 | |
| AWX002823 | Bovine | 19/06/2001 | North East | S301 | |
| Human | |||||
| H20003447 | Human | 16/08/2000 | Aberdeen | Aberdeen | Sporadic |
| H20003919 | Human | 14/09/2000 | Aberdeen | Aberdeen | Sporadic |
| H20021958 | Human | 29/07/2002 | Aberdeen | Aberdeen | Sporadic |
| H20004019 | Human | 21/09/2000 | Aberdeen | Elgin | Sporadic |
| H20010345 | Human | 13/01/2001 | Aberdeen | Elgin | Sporadic |
| H20022475 | Human | 21/08/2002 | Aberdeen | Elgin | Sporadic |
| H19994475 | Human | 15/11/1999 | Aberdeen | Fraserburgh | Sporadic |
| H20020688 | Human | 23/03/2002 | Aberdeen | Not available | Sporadic |
| H19992292 | Human | 12/08/1999 | Arbroath | Arbroath | Sporadic |
| H19991017 | Human | 13/05/1999 | Ayr | Ayr | Family outbreak |
| H20014835 | Human | 13/12/2001 | Ayr | Cumnock | Sporadic |
| H20021651 | Human | 21/06/2002 | Ayr | Irvine | Sporadic |
| H19990100 | Human | 26/01/1999 | Ayr | Stewarton | Sporadic |
| H20002800 | Human | 08/07/2000 | Dumfries | Not available | Sporadic |
| H20004164 | Human | 05/10/2000 | Dumfries | Not available | Sporadic |
| H19990457 | Human | 15/03/1999 | Dumfries | Thornhill | Sporadic |
| H20021876 | Human | 25/07/2002 | Dundee | Angus | Sporadic |
| H20023824 | Human | 14/11/2002 | Dundee | Angus | Sporadic |
| H19990170 | Human | 08/02/1999 | Dundee | Dundee | Travel history |
| H20020895 | Human | 03/04/2002 | Dundee | Dundee | Sporadic |
| H20021856 | Human | 24/07/2002 | Dundee | Dundee | Sporadic |
| H19963477 | Human | 09/08/1996 | Edinburgh | Not available | Sporadic |
| H20003668 | Human | 28/08/2000 | Fife | Edinburgh | Sporadic |
| H20012056 | Human | 08/06/2001 | Fife | Edinburgh | Sporadic |
| H19990749 | Human | 20/04/1999 | Hawick | Hawick | Sporadic |
| H19991651 | Human | 09/07/1999 | Monklands | East Kilbride | Sporadic |
| H20004937 | Human | 24/11/2000 | Monklands | Strathaven | Sporadic |
| H20023530 | Human | 25/10/2002 | Monklands | Strathaven | Sporadic |
| H19992329 | Human | 13/08/1999 | Paisley | Paisley | Sporadic |
| H20004467 | Human | 20/10/2000 | Stirling | Alloa | Sporadic |
| H20013531 | Human | 04/09/2001 | Stirling | Dunblane | Sporadic |
Note that the sending laboratory is the local diagnostic microbiology laboratory that submitted the isolate to the reference laboratory. In Scotland, the local diagnostic microbiology laboratory may cover very large geographical areas, particularly if the outlying islands are considered. Thus, the location of the patient was taken from the first three letters/numbers of the regional postal code of the address given. The complete postal code was not available due to patient confidentiality.
Antibiotic resistance.
The phenotypic sensitivity pattern of each isolate was determined by breakpoint antibiotic agar incorporation (10). The antimicrobials and breakpoints (in milligrams per liter) were as follows: ampicillin (A), 128 (high-level breakpoint only); chloramphenicol (C), 8; gentamicin (G), 4; kanamycin (K), 16; streptomycin (S), 16; sulfonamides (sulfathiazole) (Su), 64; spectinomycin (Sp), 64; tetracyclines (T), 128 (high-level breakpoint only); trimethoprim (Tm), 2; furazolidone (Fu), 8; nalidixic acid (Nx), 16; ciprofloxacin, 0.125 and 1.0; cephalexin, 16; cephradine, 16; cefuroxime, 16; ceftriaxone, 1; and cefotaxime, 1. Breakpoint antibiotic agar incorporation was performed by diluting 10 μl test culture (obtained by overnight culture on Luria-Bertani [LB] agar at 37°C) with 0.5 ml normal saline in the well of a multipoint inoculator plate. Antibiotic-containing Isosensitest agar plates (Oxoid) were inoculated together with a control plate (no antibiotic). An antibiotic-sensitive S. enterica serovar Typhimurium strain (42R500) was cultured on all plates as a control. After overnight incubation, growth was recorded on the basis of its variation from the growth on the control plate.
Plasmid DNA extraction.
Plasmid DNA was extracted from each isolate using a modified Kado and Liu protocol (14). Each isolate was cultured in nutrient broth at 37°C overnight, and then 1 ml of culture was centrifuged at 13,000 rpm for 5 min and the supernatant was removed. The pellet was resuspended in 20 μl Tris-EDTA buffer and then mixed with 100 μl 0.05 M Tris-3% sodium dodecyl sulfate (SDS). After the mixture was heated at 55°C for 30 min, 100 μl phenol-chloroform was added and emulsified by brief shaking. After being centrifuged at 13,000 rpm for 5 min, 25 μl of the upper aqueous layer was loaded onto a 0.6% agarose gel containing the Escherichia coli transconjugant 39R861 (27), which harbors plasmids of 7.5, 39, 68, and 160 kb, as a size marker. Plasmid molecular masses were calculated by logarithmic comparison to the mass of this marker. The plasmid preparation was repeated for a number of isolates to assess reproducibility.
Genomic DNA extraction.
Bacteria were cultured in LB broth or on LB agar overnight at 37°C. Genomic DNA was extracted using either the cetyltrimethylammonium bromide method (Sigma-Aldrich, Ltd., Dorset, United Kingdom) (3) or a QIAGEN tissue DNA extraction kit according to manufacturer's instructions.
Macrorestriction of genomic DNA visualized by PFGE.
One milliliter of the overnight culture was resuspended in 1 ml 0.85% NaCl and centrifuged at 13,000 rpm for 5 min. The pellet was resuspended in 0.85% NaCl at a cell density equivalent to a McFarland standard of 3.0. To prepare the plugs, 500 μl 2% chromosomal agarose (Bio-Rad, Hemel Hempstead, United Kingdom) at 55°C was mixed with an equal volume of cells; 500 μl of the mixture was dispensed into plug molds and cooled at 4°C for 20 min. The plugs were placed in 2 ml lysis solution (500 mM EDTA, 1% [wt/vol] sarkosyl [aqueous]) overnight at 55°C, with 40 μl 1-mg/ml proteinase K. The following day, plugs were washed at 55°C three times with water and four times with ET10 (10 mM Tris-Cl, 10 mM EDTA, pH 8); each wash was 15 min long. Plugs were digested with 1 U of XbaI (Roche) overnight at 37°C. The digested plugs were loaded onto a 1% agarose gel and run on a contour-clamped homogeneous electric field DRII system (Bio-Rad) at 14°C with 2 liters of 0.5× TBE (50 mM Tris, 50 mM boric acid, 0.5 mM EDTA). The switch times ranged from 6 to 72 s during a 44-h run at 5.4 V/cm. XbaI-digested DNA from S. enterica serovar Braenderup H9812 was used as a molecular reference marker every five lanes (22).
Statistical analyses of restriction patterns were performed with BioNumerics software (Applied Maths, St. Martens-Latern, Belgium) using the dice similarity coefficient. A dendrogram was calculated by the unweighted pair group method of averages, with a position tolerance of 1%. Fragments smaller than 30 kb were not included in the final analysis, as recommended by the European guidelines for standardization (20). Different XbaI-PFGE profiles were assigned to different PFGE groups according to a single band difference in restriction pattern (11, 20) rather than according to the criteria of Tenover et al. (24).
Salmonella microarray.
Salmonella Microarray Generation III is an extension of the previously described Salmonella Microarray Generation I array constructed at the Wellcome Trust Sanger Institute (2, 4, 25). Generation III includes additional coding sequences from the Salmonella genomes being sequenced at The Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/Projects/Salmonella/). Thus, Microarray Generation III is an essentially nonredundant array that contains features representing the following eight genomes: S. enterica serovar Typhi CT18, S. enterica serovar Typhi Ty2, S. enterica serovar Typhimurium LT2 (ATCC 700220) (17), S. enterica serovar Typhimurium DT104 (NCTC 13348), S. enterica serovar Typhimurium SL1344 (NCTC 13347), S. enterica serovar Enteritidis PT4 (NCTC 13349), S. enterica serovar Gallinarum 287/91 (NCTC 13346), and S. bongori 12419 (ATCC 43975).
Hybridization procedures.
DNA was extracted from 23 isolates that had been selected to represent PFGE profiles 1 to 10, and the different phage types, resistance patterns, and plasmid profiles from animal and human origins, where available. Genomic DNA was competitively hybridized to Salmonella Microarray Generation III against S. enterica serovar Typhimurium DT104 (NCTC 13348) genomic DNA as the control. Genomic DNA was sonicated (10 s, level 2; Virsonic sonicator) and then fluorescently labeled with Cy5 (test) or Cy3 (control) using the Bioprime kit (Gibco-BRL). Dye-swap labeling experiments also were performed for each test sample. Labeled DNA was purified using an Autoseq G-50 Amersham column, denatured, and precipitated, and the resulting probes were hybridized to the microarray slide for 16 h at 49°C in a hybridization chamber (Genetix X2530). Three slides per isolate were hybridized. Washing procedures were stringent (two washes at 65°C in 2× SSC-0.1% SDS for 30 min and two washes at 65°C in 0.1× SSC for 30 min [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]).
Microarray analysis.
Hybridization to microarray slides was detected by using a Genepix 4000B scanner (Axon Instruments, Inc.) and quantified by using Genepix Pro software (Axon Instruments, Inc.). Signal intensities were corrected by subtracting the local background values. Normalization was performed across all features on the array before any filtering took place. Data were ordered and labeled according to the gene names of the genomes from which each array feature was derived, and BLASTN analysis, using the array feature for DNA sequence as the query, was used to identify the gene in the S. enterica serovar Typhimurium DT104 (NCTC 13348) genome (loci SDT0001 to SDT4571) that was most similar to the tested gene. Signal ratios were analyzed by three methods to establish the cutoff values for designating genes present, absent, and uncertain: (i) twofold variation, which is the default on GeneSpring microarray analysis software V7.2 (Silicon Genetics); (ii) median value (per strain) ± 3 standard deviations (28); and (iii) the gene-calling program GACK, which is based on log2 ratios, with trinary analysis (16). The sensitivity, specificity, and positive and negative predictive values of each method using the E score and bit score were determined for the hybridization results of S. enterica serovar Typhimurium DT104 (NCTC 13348) compared to those of S. enterica serovar Typhimurium LT2. GACK analysis, using mirroring, produced the highest sensitivity, specificity, and positive and negative predictive values, and it was used to assign the present, absent, or uncertain status to each of the 4,167 loci representing the chromosomal features of S. enterica serovar Typhimurium DT104. Information concerning Salmonella Microarray Generation III has been deposited in ArrayExpress as A-SGRP-4, and experimental data are available under the accession number E-SGRP-6 (http://www.ebi.ac.uk/arrayexpress/).
Multiplex PCR.
Oligonucleotides for PCR analyses were designed to explore the main regions of variation highlighted by the microarray data (see Table 3). These regions were Salmonella genomic island 1 (SGI1), which is well described for S. enterica serovar Typhimurium DT104 (5, 6, 18), and five prophage-like elements. Multiplex PCR was performed on the 23 isolates to confirm the microarray hybridization results. PCRs contained template DNA (1 to 4 μl), primer pairs (0.5 to 1 μl), and Invitrogen Platinum PCR Supermix (23 to 46 μl). The following PCR protocol was used for all oligonucleotide pairs, except for pairs P8, P9, and P10: 94°C for 1 min; 30 cycles of 94°C for 30 s (denaturation step), 65°C for 1 min (annealing step), and 72°C for 5 min; and 72°C for 2 min. For oligonucleotide pairs P8, P9, and P10, the annealing step was 61°C for 1 min.
TABLE 3.
Multiplex PCR for detection of the five prophages harbored by S. enterica serovar Typhimurium DT104 (NCTC 13348) and SGI1
| Primer | Sequence (5′-3′) | Genome coordinates of product (DT104 [NCTC13348]) | Product size (bp) | Target |
|---|---|---|---|---|
| P1For | CGGAAGAGCTGGATAACGAGTTTCTG | 365087-367366 | 2,179 | Prophage 1/ST104 |
| P1Rev | GCGAATTATGTTCCGGGAGTATGAC | |||
| P2For | TCACAAGCAATGCGTGGTGTGCAAC | 381790-382891 | 1,101 | Prophage 1/ST104 |
| P2Rev | GCAGCACGAACACATGACATTACTAG | |||
| P3For | GGTGAAACGCGCATTGGCATAGAAG | 406501-407203 | 702 | Prophage 1/ST104 |
| P3Rev | ACGCGGGTAGGATCAGAGTACATAG | |||
| P4For | GCAGTACGAACCGTACCCGATACAG | 1111820-1112670 | 850 | Prophage 2 |
| P4Rev | CAGTCCGTCACGCCATGCTCAAACT | |||
| P5For | GCTTTCTCGTAACGCCTGCGATTTT | 1953476-1954662 | 1,186 | Prophage 3 |
| P5Rev | CGAATTTATTGGGGCAGGTGATGCG | |||
| P6For | TGAAGGCTCTCAGCATATCACCCGTA | 1965128-1965939 | 811 | Prophage 3 |
| P6Rev | ATCCACTGCCGAACGTTATCGTGGT | |||
| P7For | AATAGCCCGACCGGGAATATTCATC | 1995172-1995776 | 604 | Prophage 3 |
| P7Rev | GCTTTGTGATCCATCCAATAGCTGAC | |||
| P8For | ATGCTGACGGAAGCGTTCGGGATT | 2129791-2128102 | 1,689 | Prophage 4 |
| P8Rev | GATGCGTGGAGATCAGCTTATGCA | |||
| P9For | TAATACCGCGCTCACGCTCAAGATC | 2796632-2797808 | 1,176 | Prophage 5 |
| P9Rev | CTATAACAGTACCGGGAACTGTTCG | |||
| P10For | CTGGCGCTTAGTCATGTCGGTAATT | 2827013-2827557 | 544 | Prophage 5 |
| P10Rev | ACGTTTGGTAAACGCCTTACGCCTT | |||
| P11For | AGTGAAGTAAACGGTCACTCACTGG | 4083151-4083812 | 661 | SGI1 |
| P11Rev | ATTGTGCTGACGCTCTGCTTGTGTC | |||
| P12For | CAACTTCCGTAAGTTCAGCTACAGC | 4096995-4097976 | 981 | SGI1 |
| P12Rev | TAGCTCTATCCAGCAATGCGGATTG | |||
| P13For | AATTAGTCGGGCTACTTGCATTTGCT | 4129495-4129852 | 357 | SGI1 |
| P13Rev | ACTTATCTACAGCGTTCTGTCTGCC |
RESULTS
Origin of Salmonella strains and microbiological analysis.
Sixty-one S. enterica serovar Typhimurium strains collected in Scotland were subjected to typing using established phenotypic techniques. These approaches allowed us to assess the amount of variation present in an S. enterica serovar Typhimurium population gathered in a similar time period from the same geographical region. Thirty S. enterica serovar Typhimurium strains, representing five different phage types, were obtained from animals (26 from cattle, 2 from pigs, and 2 from crows) between 2000 and 2002 (Tables 1 and 2). Four of these isolates were nontypeable. The 31 isolates from humans were obtained from 12 Scottish laboratories between 1996 and 2002 and represented four phage types (Tables 1 and 2), with four isolates being nontypeable. Antimicrobial susceptibility testing revealed eight resistance patterns and included multiresistant isolates, with some exhibiting reduced susceptibility to ciprofloxacin (Table 2).
TABLE 2.
Results of microbiological analysis of S. enterica serovar Typhimurium isolates
| Isolate | PFGE profile | Phage type | Antibiotic resistance profilea | Plasmid profile (kb) | Hybridized to microarray |
|---|---|---|---|---|---|
| Animal | |||||
| AWF002005 | 1 | 104 | ACSSuSpT | 96, 3.0 | No |
| AWF002008 | 1 | 104 | ACSSuSpT | 96, 3.0 | Yes |
| AWF004526 | 1 | 104 | ACSSuSpT | 96 | No |
| AWF004602 | 1 | 104 | ACSSuSpT | 99 | No |
| AWX000836 | 1 | 104 | ACSSuSpT | 99 | No |
| AWX000847 | 1 | 104 | ACSSuSpT | 99 | No |
| AWX001996 | 1 | 104 | ACSSuSpT | 97 | No |
| AWX001997 | 1 | 104 | ACSSuSpT | 97 | No |
| AWX002732 | 1 | 104 | ACSSuSpTNxpCp | 97 | No |
| AWX002743 | 1 | 104 | ACSSuSpTNxpCp | 97 | No |
| AWX002821 | 1 | 104 | ACSSuSpTNxpCp | 97 | Yes |
| AWX002823 | 1 | 104 | ACSSuSpTNxpCp | 97 | No |
| AWX000826 | 1 | 104b | ACSSuSpT | 99 | Yes |
| AWF004105 | 1 | 104b | ACSSuSpT | 96 | No |
| AWX000841 | 1 | Nontypeable | ACSSuSpT | 99 | Yes |
| AWF004135 | 1 | Nontypeable | ACSSuSpT | 96 | No |
| AWF007246 | 2 | 170 | Sensitiveb | Plasmid free | No |
| AWF009144 | 2 | 170 | Sensitive | Plasmid free | No |
| AWF009147 | 2 | 170 | Sensitive | Plasmid free | Yes |
| AWX003485 | 2 | 170 | Sensitive | Plasmid free | No |
| AWX005861 | 2 | 170 | Sensitive | Plasmid free | No |
| AWX008764 | 2 | 170 | Sensitive | Plasmid free | No |
| AWX008765 | 2 | 170 | Sensitive | Plasmid free | No |
| AWX003658 | 2 | 168a | Sensitive | Plasmid free | Yes |
| AWF007581 | 2 | Nontypeable | Sensitive | Plasmid free | Yes |
| AWX004816 | 2 | Nontypeable | Sensitive | 70 | Yes |
| AWX004814 | 4 | 193 | Sensitive | Plasmid free | Yes |
| AWX006747 | 7 | RDNC | ASSuT | 100 | Yes |
| AWX006748 | 7 | RDNC | ASSuT | 100 | Yes |
| AWF009126 | 10 | 170 | SuSpTm | 99 | Yes |
| Human | |||||
| H19990457 | 1 | 104 | ACSSuSpT | 96 | No |
| H19991017 | 1 | 104 | ACSSuSpT | 93 | No |
| H19992329 | 1 | 104 | ACSSuSpT | 93, 2.5 | No |
| H20002800 | 1 | 104 | ACSSuSpT | 93 | Yes |
| H20003447 | 1 | 104 | ACSSuSpT | 93 | No |
| H20003668 | 1 | 104 | ACSSuSpT | 93 | No |
| H20004164 | 1 | 104 | ACSSuSpT | 104 | No |
| H20010345 | 1 | 104 | ACSSuSpT | 104 | No |
| H20014835 | 1 | 104 | ACSSuSpT | 104 | No |
| H20013531 | 1 | 104 | ACSSuSpTNx | 104, 5.6, 4.0, 3.6, 3.2 | No |
| H19991651 | 1 | 104 | ACSSuSpTNxpCp | 93, 72 | No |
| H20020688 | 1 | 104 | ACSSuSpTNxpCp | 104 | No |
| H19990100 | 1 | 104b | ACSSuSpT | 96, 72, 5.7, 4.1, 3.1 | No |
| H20021651 | 1 | 104b | ACSSuSpT | 100, 6.8 | Yes |
| H19990749 | 1 | Nontypeable | ACSSuSpT | 93, 7.1 | No |
| H19994475 | 1 | Nontypeable | ACSSuSpT | 93, 2.5 | No |
| H20021958 | 1 | Nontypeable | ACSSuSpT | 100, 15, 3.2, 2.0 | Yes |
| H19990170 | 2 | 170 | Sensitive | 3.5 | No |
| H20004019 | 2 | 170 | Sensitive | 58 | Yes |
| H20004937 | 2 | 170 | SSuSp | Plasmid free | No |
| H20012056 | 2 | 170 | Sensitive | Plasmid free | No |
| H20020895 | 2 | 170 | Sensitive | Plasmid free | No |
| H20021856 | 2 | 170 | Sensitive | Plasmid free | Yes |
| H20021876 | 2 | 170 | Sensitive | Plasmid free | No |
| H20022475 | 2 | 170 | Sensitive | Plasmid free | No |
| H19992292 | 3 | 170 | Sensitive | Plasmid free | Yes |
| H19963477 | 3 | 170 | SuTm | 7.9, 4, 1.9 | Yes |
| H20004467 | 5 | Nontypeable | ACSSuSpTFu | 141, 7.6, 3.5, 3.2 | Yes |
| H20023824 | 6 | 170 | Sensitive | Plasmid free | Yes |
| H20003919 | 8 | RDNC | ASSuT | 123, 49 | Yes |
| H20023530 | 9 | 193 | Sensitive | 100, 6.3 | Yes |
Antibiotic abbreviations: ampicillin, A; chloramphenicol, C; streptomycin, S; sulfonamides, Su; spectinomycin, Sp; tetracyclines, T; trimethoprim, Tm; furazolidone, Fu; nalidixic acid, Nx; low-level (partial) ciprofloxacin resistance, pCp.
Sensitive to all antibiotics tested (see Materials and Methods for the agents used).
The isolates were subjected to PFGE analysis using the restriction enzyme XbaI, and 10 PFGE profiles were identified, with DNA fragments ranging from 10 kb to approximately 882 kb. The unweighted-pair-group-method-of-averages dendrogram constructed using BioNumerics software demonstrated clustering of PFGE restriction profiles by antibiotic resistance pattern and phage type. Significantly, there were indistinguishable restriction profiles produced from animal and human isolates of the same phage type and resistance pattern. The most common PFGE profile, PFGE 1, was observed in 33 isolates (16 animal isolates and 17 human isolates), including DT104 (24 isolates), DT104b (4 isolates), and 5 nontypeable isolates. All 33 isolates in PFGE 1 were multiresistant: 26 exhibited the resistance pattern ACSSuSpT (12 animal isolates and 14 human isolates), 6 exhibited the resistance pattern ACSSuSpTNpxCp (4 animal isolates and 2 human isolates), and 1 exhibited the resistance pattern ACSSuSpTNx (a human isolate). The second most prevalent restriction pattern, PFGE 2, was observed for 18 isolates (10 animal isolates and 8 human isolates) that were all sensitive to antibiotics, with the exception of H20004937, which had the resistance pattern SSpSu. Fifteen PFGE 2 isolates were DT170 (7 animal isolates and 8 human isolates), one was DT168a (an animal isolate), and two were nontypeable (both animal isolates). The eight other PFGE profiles, PFGE 3 to 10, contained three isolates or fewer.
Nine isolates of animal origin and seven isolates of human origin were plasmid free (Table 2), sensitive to the antibiotics, and phage typed as DT170 (14), DT193 (1), or nontypeable (1). The remaining 45 isolates harbored between one and five plasmids, ranging in size from 2 kb to approximately 141 kb. PFGE 1 isolates harbored an approximately 100-kb plasmid of the same size as the serovar-specific plasmid. An approximately 100-kb plasmid was also detected in two RNDC (reacts but does not conform to designated types on phage typing) isolates, one DT170 type and one DT193 type. The plasmid content could be classified into 15 different profiles.
DNA microarray analysis.
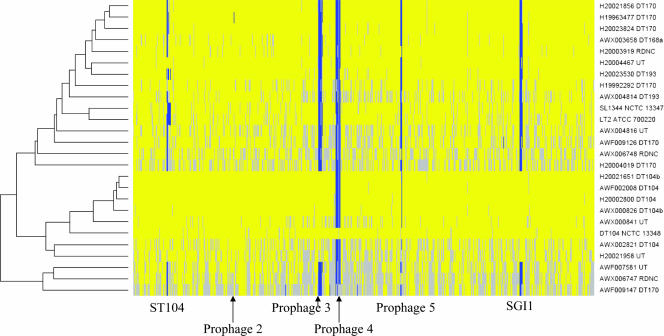
Although we could detect variations in the S. enterica serovar Typhimurium isolates, we were unable to genetically define these regions using the above approaches. Consequently, 23 S. enterica serovar Typhimurium isolates, representative of the different PFGE profiles, antibiotic resistance patterns, and phage sensitivity patterns, were subjected to DNA analysis using a composite Salmonella DNA microarray (Table 2). Not surprisingly, the microarray analysis revealed high levels of similarity between the genomes of the different S. enterica serovar Typhimurium isolates (Fig. 1). However, specific regions were variable, with some differences being present in the majority of isolates while others were restricted to particular strains or groups of strains. This limited variation allowed us to perform a simple cluster analysis of the isolates by using a restricted group of 4,167 genes present in S. enterica serovar Typhimurium DT104 (NTCC 13348), for which the complete genome sequence was available (Fig. 1).
FIG. 1.
Analysis of the microarray data by GACK. Test/reference ratios of the 24 S. enterica serovar Typhimurium isolates analyzed by microarray were assessed for the presence/absence of genes by using GACK software. The input data set was restricted to the 4,167 chromosomal features (SDT loci) expected to be present in one or more of the isolates. The heat map is plotted in the physical order of the SDT loci according to the order of the loci in the S. enterica serovar Typhimurium DT104 (NCTC 13348) genome. The presence of a gene in the test strain that is also present in the control strain (S. enterica serovar Typhimurium DT104 [NCTC 13348]) is shown in yellow, the absence or divergence of the gene from its presence in the control strain is shown in blue, and genes for which their presence was uncertain are in gray. The positions of prophage 1/ST104 and prophages 2 to 5 as well as SGI1 are indicated.
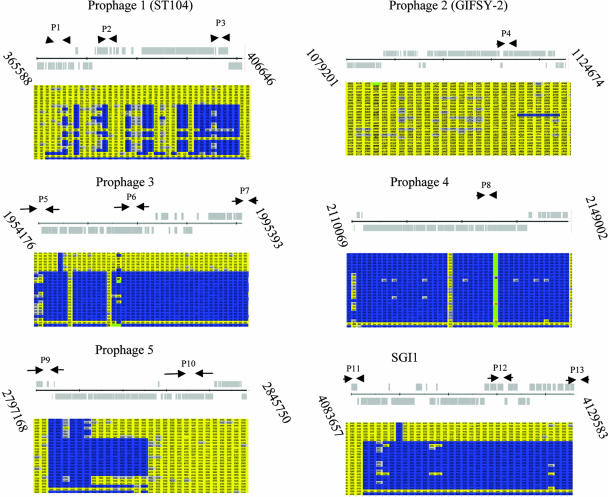
Seven PFGE 1 S. enterica serovar Typhimurium isolates clustered with the reference strain DT104 (NCTC 13348), including three of DT104, two of DT104b, and two that were nontypeable. Interestingly, one region of the reference strain DT104 (NCTC 13348), represented by prophage-like element 4, was absent from all other isolates as revealed by GACK analysis. Also, the SGI1 element of the reference DT104 strain was present only in all PFGE 1 isolates. Visual examination of the microarray profiles highlighted several other regions of the genome that represent significant variation between isolates. Detailed bioinformatic analysis of the microarray data revealed that the majority of these regions of variation corresponded to prophage-like elements within the genome of S. enterica serovar Typhimurium DT104 (NCTC 13348). The prophage in DT104 (NCTC 13348) included prophage 1 (also called ST104 [23]; hereafter it is referred to as prophage 1/ST104) (genome coordinates 365588.406646); prophage 2 (genome coordinates 1079201.1124674), which is related to the GIFSY-2 phage (17); prophage 3 (genome coordinates 1954176. 1995367); prophage 4 (genome coordinates 2109720.2149064); and prophage 5 (genome coordinates 2797168.2845750) (Fig. 1; Table 3). Analysis of the microarray data corresponding to the prophage elements indicated that prophages 1, 3, and 5 harbored the most variation, while prophages 2 and 4 were mostly conserved (Fig. 2). Thus, although we detected other types of variation, usually limited to single genes, variation was predominantly associated with some of the prophage-like elements, suggesting that these regions were driving recent evolution in these strains.
FIG. 2.
Schematic diagram of prophages 1 to 5 and SGI1, showing oligonucleotide target sites. The gray schematic diagrams represent the predicted open reading frames in the annotated genome of S. enterica serovar Typhimurium DT104 (NCTC 13348). The arrows show the location of the primer pairs P1 to P13, as listed in Table 3. The corresponding microarray results, processed by the gene-calling program GACK, are shown underneath each prophage or under SGI1. The columns represent the features harbored in each prophage or the genomic island. Yellow represents features that are present in the test strain as well as the control strain (S. enterica serovar Typhimurium DT104 [NCTC 13348]), blue represents genes that are in the control strain but are absent in the test strain, and gray indicates that the presence of the gene in the test strain is uncertain. The rows represent each S. enterica serovar Typhimurium isolate, in the following order: row 1, AWF002008; 2, AWX002821; 3, H20002800; 4, H20021651; 5, AWX000826; 6, AWX000841; 7, H20021958; 8, H20004019; 9, AWF009126; 10, AWF009147; 11, H20021856; 12, AWX003658; 13, AWX004816; 14, AWF007581; 15, H19963477; 16, H19992292; 17, AWX004814; 18, H20004467; 19, H20023824; 20 AWX006747; 21, AWX006748; 22, H20003919; 23, H20023530; 24, DT104 (NCTC 13348); 25, LT2 (ATCC 700220).
Multiplex PCR for phages and SGI1.
The microarray analysis identified several significant regions of variation within the genomes of the different S. enterica serovar Typhimurium isolates. This information was used to design PCR primers that could form the basis of simple PCR assays to discriminate between these isolates (Table 4). Primers were designed on the basis of different regions of prophages 1 to 5 and, additionally, on the basis of the variant SGI1 antibiotic resistance island known to be present in the antibiotic-resistant DT104 isolates. DNA sequences related to SGI1 were probed by using oligonucleotide pairs P11, P12, and P13. A product was obtained with the oligonucleotide pair P13 from all S. enterica serovar Typhimurium isolates, as the amplified region (genome coordinates 4129495.4129852) is located in the core chromosomal sequence downstream of SGI1. Hence, the P13 probes acted as a positive control for the PCR analysis. Products for P11 and P12 were generated from DNA for all seven PFGE 1 isolates and for no others.
TABLE 4.
Multiplex PCR results from 23 S. enterica serovar Typhimurium isolates selected for microarray analysis
| PFGE profile | Isolate | Phage type | Ability of oligonucleotide pair to detect:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prophage 1/ST104
|
Prophage 2
|
Prophage 3
|
Prophage 4
|
Prophage 5
|
SGI1
|
||||||||||
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | |||
| 1 | AWF002008 | DT104 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | AWX002821 | DT104 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | H20002800 | DT104 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | H20021651 | DT104b | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | AWX000826 | DT104b | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | AWX000841 | Nontypeable | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1 | H20021958 | Nontypeable | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | H20004019 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 2 | AWF009147 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 2 | H20021856 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 2 | AWX003658 | DT168a | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 2 | AWX004816 | Nontypeable | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 3 | AWF007581 | Nontypeable | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 3 | H19963477 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 3 | H19992292 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 4 | AWX004814 | DT193 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 5 | H20004467 | Nontypeable | + | + | + | + | − | − | + | − | + | − | − | − | + |
| 6 | H20023824 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
| 7 | AWX006747 | RDNC | − | − | + | + | − | − | + | − | + | + | − | − | + |
| 7 | AWX006748 | RDNC | − | − | + | + | − | − | + | faint | + | + | − | − | + |
| 8 | H20003919 | RDNC | − | − | + | + | − | − | + | − | + | + | − | − | + |
| 9 | H20023530 | DT193 | − | − | + | + | − | − | + | faint | + | − | − | − | + |
| 10 | AWF009126 | DT170 | − | − | + | + | − | − | + | + | + | − | − | − | + |
The presence of DNA sequences relating to prophage 1/ST104 was monitored using the oligonucleotide pairs P1, P2, and P3. P3 corresponds to DNA sequences encoding structural proteins of prophage 1/ST104, and a product was generated by using DNA from all 23 S. enterica serovar Typhimurium isolates. PCR signals for P1 and P2 were detected in the seven PFGE 1 isolates and one other isolate, H20004467, which is a nontypeable strain. Microarray results for the DT170 isolate H19992292 had indicated the presence of sequences relating to prophage 1/ST104 in this genome (Fig. 1), but no PCR product was generated with either the P1 or the P2 oligonucleotide pair, suggesting the presence of additional sequence variation. Thus, results from pairs P1 and P2, which are within the regulatory region, indicated divergence between different isolates.
Prophage 2 was found to be present in all 23 isolates probed with oligonucleotide pair P4, and hence GIFSY-2 phage-related DNA is conserved in this population. In contrast, prophage 3, which was probed for by using oligonucleotide pairs P5, P6, and P7, was present only in the seven PFGE 1 isolates.
Prophage 4 is present in the sequenced DT104 isolate, but interestingly it was not detected in any of the 23 other S. enterica serovar Typhimurium isolates, including other DT104 isolates, by microarray analysis. The presence of prophage 4-related sequences was probed for by using oligonucleotide pair P8, and interestingly a PCR product of the predicted size was produced by using DNA prepared from all but three isolates (H20004467, AWX006747, and H20003919), suggesting that these isolates encode sequences relating to prophage 4.
The PCR results obtained with prophage 5-based probe pairs corresponded to the information gleaned from the microarrays. DNA sequences related to prophage 5, probed by P9 and P10, were present in all the PFGE 1 isolates and the three RDNC isolates belonging to other PFGE types. All other S. enterica serovar Typhimurium isolates generated a product with P9 but not P10, suggesting that they harbored a variant form of prophage 5.
Thus, these sets of primers confirm aspects of the microarray analysis of variation in specific prophage regions of S. enterica serovar Typhimurium and may form the basis of a simple PCR assay for discriminating between different S. enterica serovar Typhimurium strains circulating in a geographical area.
DISCUSSION
We have used a variety of techniques to examine in detail the relationship between a matched group of S. enterica serovar Typhimurium strains isolated from animals and humans in Scotland between 1996 and 2002. One aim of this work was to further define the molecular basis of genome variation within Salmonella serovars and to exploit this information to discriminate between isolates in the field by using simple assays. Conventional techniques such as phage and plasmid typing, PFGE, and antibiotic resistance analysis were able to distinguish between different isolates but could not identify the genetic basis of such changes. In contrast, genomic approaches utilizing DNA microarrays were able to highlight hot spots of variation and to link these to annotated and genetically defined regions of DNA. This approach opens up the potential to rationally design specific probes for strain variation and to identify quickly novel genetic variants in the field. Obviously, PCR product-based DNA microarrays are not able to identify variations such as point mutations or DNA insertions not represented on the array. Nevertheless, with this technology we were able to rapidly identify several key areas of genomic variation and to demonstrate that these map to regions encoding prophage-like elements. This observation supports the hypothesis that bacteriophage are a key factor driving the microevolution of Salmonella (25), a process that readily can be detected in the sample of strains analyzed in this study.
Using the recently determined genome sequence of the DT104 isolate NCTC 13348, we were able to identify up to five different regions of the genome encoding prophage-like elements, complementing and extending studies carried out by others using non-genome-wide approaches (12, 13). Prophage 1/ST104, also known as PDT17 (21), previously has been described as a resident prophage in S. enterica serovar Typhimurium DT104 (23). Interestingly, a prophage 1-like element also was detected in the nontypeable S. enterica serovar Typhimurium isolates H20004467 and H19992292 (DT170), although only the former was confirmed by multiplex PCR. These observations are of interest, as prophage 1/ST104 previously has not been described outside of phage type DT104. Prophage 2 (GIFSY-2), which is a well-characterized S. enterica serovar Typhimurium prophage, was present in all 23 isolates and was thus of little value for discriminating between strains. Prophage 3 was present in the seven PFGE 1 isolates and, together with SGI1, could form the basis of a diagnostic test for DT104.
Significantly, although these techniques could distinguish between isolates of S. enterica serovar Typhimurium, organisms derived from humans or animals did not fall into two mutually exclusive groups. This observation serves to illustrate the close genetic relationship between animal and human Salmonella strains circulating in the same geographical region and emphasizes the zoonotic nature of these infections. It seems that very similar strains of S. enterica serovar Typhimurium were causing disease in animals and humans in the same geographical location and in the same time frame. Antibiotic-resistant strains of S. enterica serovar Typhimurium were present in both the human and animal populations. Interestingly, antibiotic-sensitive isolates from animals tended to cluster with sensitive isolates from humans, and antibiotic-resistant isolates also grouped together, irrespective of source.
In conclusion, the examination of the DNA sequences of the different prophages present in S. enterica serovar Typhimurium DT104 (NCTC 13348) allowed us to design PCR primer sets that could be used to interrogate the genomes of different field isolates in multiplex PCR assays. These simple tests were used to rapidly distinguish between different S. enterica serovar Typhimurium isolates by using basic equipment and reagents. Thus, we have developed a rational DNA sequence-based assay for discriminating between S. enterica serovar Typhimurium isolates that can be applied even in a simple laboratory environment. Importantly, we believe that such assays could be refined to form the basis of a simple rapid test to distinguish between S. enterica serovar Typhimurium isolates in a diagnostic or surveillance setting. Further sequence analyses are under way to define the prophage content of multiple S. enterica serovar Typhimurium isolates, with a view to improving the discriminatory power of these assays. This approach easily could be expanded to the design of similar PCR-based assays for additional Salmonella serovars. The advantage of this approach is that it is based on defined DNA sequences physically mapped in the Salmonella genomes, and it targets known hot spots for evolutionary change. Hence, these signatures reasonably might be expected to detect the early emergence of pathogenic variants of Salmonella in the field.
Acknowledgments
This work was supported by The Wellcome Trust and the IPRAVE consortium. F.J.C. is an MRC Clinical Research Training Fellow.
We thank Matloob Qureshi for his help with submission of the microarray data to ArrayExpress.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Anderson, E. S., L. R. Ward, M. J. Saxe, and J. D. de Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. (London) 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum, M. F., C. Marooney, M. Fookes, S. Baker, G. Dougan, A. Ivens, and M. J. Woodward. 2005. Identification of core and variable components of the Salmonella enterica subspecies I genome by microarray. Infect. Immun. 73:7894-7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. Kingston, D. Moore, J. Seidman, and J. Smith. 1995. Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., Brooklyn, NY.
- 4.Bishop, A. L., S. Baker, S. Jenks, M. Fookes, P. O. Gaora, D. Pickard, M. Anjum, J. Farrar, T. T. Hien, A. Ivens, and G. Dougan. 2005. Analysis of the hypervariable region of the Salmonella enterica genome associated with tRNAleuX. J. Bacteriol. 187:2469-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd, D., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd, D. A., G. A. Peters, L. Ng, and M. R. Mulvey. 2000. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica Typhymurium DT104. FEMS Microbiol. Lett. 189:285-291. [DOI] [PubMed] [Google Scholar]
- 7.Corbett-Feeney, G., and U. N. Riain. 1998. The use of pulsed-field gel electrophoresis for subdivision of Salmonella typhimurium in an outbreak situation. J. Infect. 36:175-177. [DOI] [PubMed] [Google Scholar]
- 8.Ebner, P. D., and A. G. Mathew. 2001. Three molecular methods to identify Salmonella enterica serotype Typhimurium DT104: PCR fingerprinting, multiplex PCR and rapid PFGE. FEMS Microbiol. Lett. 205:25-29. [DOI] [PubMed] [Google Scholar]
- 9.Evans, S., and R. Davies. 1996. Case control study of multiple-resistant Salmonella typhimurium DT104 infection of cattle in Great Britain. Vet. Rec. 139:557-558. [PubMed] [Google Scholar]
- 10.Frost, J. 1994. Testing for resistance to antibacterial drugs, p. 73-82. In H. Chart (ed.), Methods in practical laboratory bacteriology. CRC Press, Boca Raton, FL.
- 11.Gatto, A. J., T. M. Peters, J. Green, I. S. Fisher, O. N. Gill, J. O'Brien, S. C. Maguire, C. Berghold, I. Lederer, P. Gerner-Smidt, M. Torpdahl, A. Siitonen, S. Lukinmaa, H. Tschape, R. Prager, I. Luzzi, A. M. Dionisi, W. K. van der Zwaluw, M. Heck, J. Coia, D. Brown, M. Usera, A. Echeita, and E. J. Threlfall. 2006. Distribution of molecular subtypes within Salmonella enterica serotype Enteritidis phage type 4 and S. Typhimurium definitive phage type 104 in nine European countries, 2000-2004: results of an international multi-centre study. Epidemiol. Infect. 134:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermans, A. P., T. Abee, M. H. Zwietering, and H. J. Aarts. 2005. Identification of novel Salmonella enterica serovar Typhimurium DT104-specific prophage and nonprophage chromosomal sequences among serovar Typhimurium isolates by genomic subtractive hybridization. Appl. Environ. Microbiol. 71:4979-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans, A. P., A. M. Beuling, A. H. van Hoek, H. J. Aarts, T. Abee, and M. H. Zwietering. 2006. Distribution of prophages and SGI-1 antibiotic-resistance genes among different Salmonella enterica serovar Typhimurium isolates. Microbiology 152:2137-2147. [DOI] [PubMed] [Google Scholar]
- 14.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidgell, C., U. Reichard, J. Wain, B. Linz, M. Torpdahl, G. Dougan, and M. Achtman. 2002. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2:39-45. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C. C., E. A. Joyce, K. Chan, and S. Falkow. 2002. Improved analytical methods for microarray-based genome-composition analysis. Genome Biol. 3:RESEARCH0065. [DOI] [PMC free article] [PubMed]
- 17.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 18.Mulvey, M. R., D. A. Boyd, A. B. Olson, B. Doublet, and A. Cloeckaert. 2006. The genetics of Salmonella genomic island 1. Microbes Infect. 8:1915-1922. [DOI] [PubMed] [Google Scholar]
- 19.Olsen, J. E., M. N. Skov, O. Angen, E. J. Threlfall, and M. Bisgaard. 1997. Genomic relationships between selected phage types of Salmonella enterica subsp. enterica serotype Typhimurium defined by ribotyping, IS200 typing and PFGE. Microbiology 143:1471-1479. [DOI] [PubMed] [Google Scholar]
- 20.Peters, T. M., C. Maguire, E. J. Threlfall, I. S. Fisher, N. Gill, and A. J. Gatto. 2003. The Salm-gene project-a European collaboration for DNA fingerprinting for. Euro Surveill. 8:46-50. [DOI] [PubMed] [Google Scholar]
- 21.Schicklmaier, P., T. Wieland, and H. Schmieger. 1999. Molecular characterization and module composition of P22-related Salmonella phage genomes. J. Biotechnol. 73:185-194. [DOI] [PubMed] [Google Scholar]
- 22.Swaminathan, B., T. J. Barrett, S. B. Hunter, and R. V. Tauxe. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka, K., K. Nishimori, S. Makino, T. Nishimori, T. Kanno, R. Ishihara, T. Sameshima, M. Akiba, M. Nakazawa, Y. Yokomizo, and I. Uchida. 2004. Molecular characterization of a prophage of Salmonella enterica serotype Typhimurium DT104. J. Clin. Microbiol. 42:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson, N., S. Baker, D. Pickard, M. Fookes, M. Anjum, N. Hamlin, J. Wain, D. House, Z. Bhutta, K. Chan, S. Falkow, J. Parkhill, M. Woodward, A. Ivens, and G. Dougan. 2004. The role of prophage-like elements in the diversity of Salmonella enterica serovars. J. Mol. Biol. 339:279-300. [DOI] [PubMed] [Google Scholar]
- 26.Threlfall, E. J., M. D. Hampton, S. L. Schofield, L. R. Ward, J. A. Frost, and B. Rowe. 1996. Epidemiological application of differentiating multiresistant Salmonella typhimurium DT104 by plasmid profile. Commun. Dis. Rep. CDR Rev. 6:R155-R159. [PubMed] [Google Scholar]
- 27.Threlfall, E. J., B. Rowe, J. L. Ferguson, and L. R. Ward. 1986. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J. Hyg. (London) 97:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witney, A. A., G. L. Marsden, M. T. Holden, R. A. Stabler, S. E. Husain, J. K. Vass, P. D. Butcher, J. Hinds, and J. A. Lindsay. 2005. Design, validation, and application of a seven-strain Staphylococcus aureus PCR product microarray for comparative genomics. Appl. Environ. Microbiol. 71:7504-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright, J. G., L. A. Tengelsen, K. E. Smith, J. B. Bender, R. K. Frank, J. H. Grendon, D. H. Rice, A. M. Thiessen, C. J. Gilbertson, S. Sivapalasingam, T. J. Barrett, T. E. Besser, D. D. Hancock, and F. J. Angulo. 2005. Multidrug-resistant Salmonella Typhimurium in four animal facilities. Emerg. Infect. Dis. 11:1235-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]