Abstract
Efficient and sensitive diagnostic methods are needed in the management of virus infections in the central nervous system. There is a demand for an evaluation of the sensitivity of PCR methods for early diagnosis of meningitis due to herpes simplex type 2 (HSV-2) and varicella-zoster virus (VZV). The objective of this study was to evaluate real-time PCR in the detection of HSV-2 and VZV DNA from cerebrospinal fluid (CSF) for etiological diagnoses in clinically well-characterized cases of primary and recurrent aseptic meningitis. Samples from 110 patients, 65 of whom were diagnosed with or were strongly suspected of having HSV-2 meningitis and 45 with acute aseptic meningitis of unknown causes, were analyzed. Results were compared with the outcome of nested PCR for HSV-2 infection. Clinical parameters were analyzed in relation to CSF viral load. With real-time PCR, HSV-2 DNA was found in CSF from 80% (52/65) of patients with clinical HSV-2 meningitis compared to 72% (47/65) found by nested PCR. The sensitivity of real-time HSV-2 PCR was found to be 87% (33/38) in primary and 70% (19/27) in recurrent meningitis. The HSV-2 viral load was significantly higher in primary than in recurrent meningitis and correlated with the degree of inflammation. VZV DNA was detected in 2 of 45 samples (4.4%). Real-time PCR for the diagnosis of HSV-2 meningitis was evaluated in a large, clinically well-characterized sample of material and found to identify more cases than nested PCR in the group of patients with recurrent meningitis. Quantification of DNA enables further research of disease prognosis and treatment.
Herpes simplex virus type 2 (HSV-2) infection may give symptoms from the skin and mucous membranes and/or the nervous system. Neurological complications appear with or without mucocutaneous lesions. After the primary infection, the virus remains latent in the nervous ganglia, where it can reactivate. HSV-2 meningitis carries a considerable risk of future neurological morbidity and is the major cause of recurrent aseptic meningitis (3, 30).
The overall incidence of aseptic meningitis is estimated to be 20 cases/100,000/year. The incidence of meningitis is probably underestimated due to unawareness and to previous limitations of diagnostic methods. HSV was previously detected by isolation from the cerebrospinal fluid (CSF) of between 0.5 and 3% of patients with aseptic meningitis. With detection of the viral genome in CSF by PCR, additional cases were identified (1, 3, 9, 21a, 27, 30). Using nested PCR, 5 to 17% of patients tested positive for HSV-2 DNA (13, 18, 20, 23). Since PCR assays were introduced, they have undergone further development. Real-time PCR has proved to be an efficient and reliable means for the detection and quantification of viral genomes (19, 26).
Aseptic meningitis and meningoencephalitis may be caused by varicella-zoster virus (VZV) infection, frequently without the zoster rash. In studies using nested PCR, it is suggested that VZV is an important etiological agent in acute aseptic meningitis (AAM) (11, 17, 18).
An evaluation of the sensitivity of PCR in early diagnosis by using CSF from patients with primary and recurrent HSV-2 aseptic meningitis is hitherto lacking. In this study, we evaluated the real-time PCR method for the detection of HSV-2 and VZV DNA in CSF from clinically well-characterized patients with aseptic meningitis.
MATERIALS AND METHODS
Patients and samples.
CSF samples from patients were collected consecutively and analyzed retrospectively from patients treated at the Departments of Infectious Diseases at Danderyd Hospital and Karolinska University Hospital, Stockholm, from 1988 to 2004.
Patients >15 years of age with clinical signs of meningitis and CSF pleocytosis (>5 × 106 mononuclear leukocytes/liter) and cultures negative for bacteria and fungi and belonging to one of two main groups, as described below, were included.
Patients with HSV-2 meningitis (HSM) or meningitis with strongly suspected HSV-2 infection etiology who met one or more of the study criteria (n = 65) comprised one group. This group was further subdivided into those with primary infection (i.e., infection appearing clinically for the first time) and those with recurrent infection. Inclusion criteria were as follows: (i) HSV-2 DNA in CSF, detected with nested PCR; (ii) present seroconversion against HSV-2; (iii) concurrent herpetic genital lesions and negative differential diagnosis; (iv) recurrent meningitis and earlier, verified HSV-2 meningitis; and (v) recurrent meningitis and a history of meningitis of unknown origin and HSV-2 antibodies in acute-phase serum and negative differential diagnosis.
Patients with AAM, i.e., those who were negative in diagnostic tests for enteroviruses, Borrelia burgdorferi, tick-borne encephalitis (TBE) and HSV-1 and who did not meet the above criteria for HSV-2 meningitis (n = 45) comprised the second group. Demographic and clinical data were collected from medical records.
Viral DNA preparation.
CSF specimens were analyzed for HSV-1 and HSV-2 by nested PCR upon arrival at the laboratory. Viral DNA was prepared by freeze-boiling for 10 min prior to amplification by nested PCR. Thereafter, the CSF samples were stored at −70°C. For real-time PCR, viral DNA was extracted from 200 μl of CSF with a Biorobot M48 using a MagAttract virus M48 mini-kit and eluted in 100 μl of buffer.
Nested PCR.
Routine diagnostics for HSV-1 and HSV-2 were performed with all material, using a previously described, qualitative, nested PCR assay (1a, 2).
Real-time PCR.
The design of the real-time PCR assays was based on a previously published paper (26), with some modifications. Primers were selected from glycoprotein D for HSV-1, glycoprotein G for HSV-2, and open reading frame (ORF) 29 for VZV (29) (Table 1). The HSV primers were optimized to work in a duplex format where HSV-1 and HSV-2 present melting temperatures of approximately 81°C ± 0.5°C and 87°C ± 0.5°C, respectively. The assays were performed in a LightCycler 1.2 (Roche). Amplifications were carried out in a 20-μl volume containing 5 μl of sample, 10 μl of 2× Quantitect SYBR Green PCR master mix (QIAGEN), 1 μM of primer HSV 1:2 and HSV 1:3, and 0.5 μM of HSV 2:1 and HSV 2:4. Solutions of 1.0 μM primers were used in the VZV real-time PCR. Both HSV and VZV real-time PCR assays used the same amplification profile. The cycling conditions were 15 min at 95°C and 45 cycles at 95°C for 5 s, 60°C for 15 s, and 72°C for 20 s, followed by a melting curve analysis. In this study, however, the HSV-1 primers were omitted to eliminate any possible interaction of the HSV-1 primers with the quantification of HSV-2.
TABLE 1.
Real-time PCR primers
| PCR primer | Target | Sequence | Nucleotide positionsa |
|---|---|---|---|
| HSV 1:3 | Glycoprotein D | 5′-CCATACCGACCACACCGACGA-3′ | 55-74 |
| HSV 1:2 | Glycoprotein D | 5′-CATACCGGAACGCACCACAC-3′ | 243-224 |
| HSV 2:1 | Glycoprotein G | 5′-TCAGCCCATCCTCCTTCGGCAGTA-3′ | 138240-138262 |
| HSV 2:4 | Glycoprotein G | 5′-CGCGCGGTCCCAGATCGGCA-3′ | 138398-138380 |
| VZV fw | ORF 29 | 5′-TGTCCTAGAGGAGGTTTTATCTG-3′ | 53941-53963 |
| VZV rev | ORF 29 | 5′-CATCGTCTGTAAGACTTAACCAG-3′ | 54142-54120 |
Derived from GenBank accession numbers J02217 for HSV-1, Z86099 for HSV-2, and X04370 for varicella-zoster virus.
Quantification.
Commercial DNA standards (Advanced Biotechnology) were used for quantification, in which serial dilutions ranging from 101 to 104 copies/reaction of the standards were run in parallel with the clinical samples (Table 2).
TABLE 2.
Detection limits of HSV-2 by real-time PCR compared to those of nested PCR
| No. of copies per reaction | No. of patients positive for HSV-2/total no. of patients (%)
|
|
|---|---|---|
| Real-time PCR | Nested PCR | |
| HSV-2 DNA standard | ||
| 1,100-6,000 | 16/6 | 2/2 |
| 110-600 | 9/9 | 8/8 |
| 55-60 | 9/9 | 3/3 |
| 15-30 | 3/3 | NDa |
| 2.5-12 | 6/12 (50) | 1/8 (12) |
| HSV-2 in CSF | ||
| 110-600 | 6/6 | 6/6 |
| 55-60 | 4/4 | 3/4 |
| 15-30 | 3/4 | 1/4 |
| 2.5-12 | 1/4 | 0/4 |
ND, not done.
Diagnosis of enterovirus meningitis, TBE, and neuroborreliosis.
Enterovirus was detected by isolation of the virus in CSF or by enteroviral real-time PCR in CSF (12). A commercial indirect immunoassay was used to demonstrate TBE immunoglobulin M (IgM) and IgG antibodies (Immunize FSME, Progen Biotechnik GmbH). Intrathecal antibody production of neuroborreliosis was measured by a capture enzyme immunoassay (IDEIA Lyme neuroborreliosis; DAKO).
Statistics.
Comparisons were made using the t test and the χ2 test.
RESULTS
Demographic and clinical findings.
Of 65 patients with HSM (16 men and 49 women), 38 had experienced first-time meningitis, and 27 had recurrent meningitis. Of 45 patients with AAM (17 men and 28 women), 42 (93%) had first-time meningitis. The group with first-time HSM was significantly younger (P < 0.05; Table 3). Mucocutaneous lesions in association with the meningitis were noted in 23 out of 38 (61%) patients with primary and 16 out of 27 (59%) patients with recurrent HSM. The mean time with neurological symptoms before lumbar puncture was significantly shorter for the recurrent HSM group.
TABLE 3.
CSF findings in patients with primary and recurrent HSM and AAM of unknown origina
| Patient parameter | Reference value | Primary HSMa
|
Recurrent HSM
|
AAM
|
|||
|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | ||
| Age (yr) | 36.9** | 22-75 | 41** | 22-69 | 41.1** | 17-88 | |
| Days with symptoms | 2.5** | 0-9 | 1.7** | 0-11 | 3.1** | 0-9 | |
| Leukocytes (n × 106/liter) | 0-5 | 415** | 10-1,600 | 195** | 13-722 | 286 | 6-2,240 |
| Monocytes (n × 106/liter) | 0-5 | 368** | 10-1,504 | 190** | 13-710 | 156** | 6-940 |
| Protein (g/liter) | <0.50 | 1.03 | 0.4-2.60 | 0.99 | 0.41-2.96 | 1 | 0.2-2.7 |
| Albumin (mg/liter) | <320 | 648** | 250-1,580 | 434** | 200-950 | 495 | 104-1,336 |
| Glucose (mmol/liter) | 2.2-3.9 | 3.4 | 2.4-7 | 3 | 2-4 | 3.7 | 2.3-6.5 |
| Lactate (mmol/liter) | <3 | 2.8** | 1.3-7.1 | 2.1** | 0.8-3.4 | 2.2** | 0.5-3.5 |
Primary meningitis was defined as the first-time clinical experience of meningitis. Days with symptoms was defined as the number of days the patient had neurological symptoms before undergoing lumbar puncture. **, values showed statistically significant differences (P < 0.05).
Cerebrospinal fluid findings.
Significant differences were shown, with larger numbers of leukocytes and monocytes and higher levels of lactate and albumin in the primary HSM group then in the recurrent group (Table 3).
Comparison of nested PCR and real-time PCR.
The detection sensitivities of nested PCR and real-time PCR were compared by testing serial dilutions of an HSV-2 DNA standard as well as by serial dilutions of HSV-2 isolates in cerebrospinal fluid. Real-time PCR and nested PCR detected DNA standard and HSV-2 dilutions equally well in CSF down to 55 to 60 copies per reaction. Below this level, real-time PCR gave more positive reactions than nested PCR. When 2.5 to 12 copies/reaction were tested, 7/16 (44%) patients tested positive by real-time PCR compared to 1/12 (8%) patients by nested PCR (P < 0.05), combining the results of a standard and HSV-2 in CSF (Table 2).
Using real-time PCR, HSV-2 DNA was detected in 53 out of 110 (48%) patients compared with 47 out of 110 (42%) by nested PCR (Table 4). Only one patient in the AAM group was positive for HSV-2 DNA by real-time PCR but negative by nested PCR. HSV-2 DNA was found in CSF from 52 out of 65 (80%) patients with clinical HSV-2 meningitis compared to 47 out of 65 (72%) with nested PCR. The detection rate of HSV-2 by real-time PCR was increased in the group of patients with recurrent HSM, 70% compared to 52%, although the difference was not statistically significant. In the primary meningitis group, 87% of patients were positive by both methods.
TABLE 4.
Results of quantitative real-time PCR and qualitative nested PCR for detection of HSV-2 in CSF in two groups of patients with acute aseptic meningitis
| Group | No. of patients | Real-time PCR
|
Nested PCR
|
||
|---|---|---|---|---|---|
| No. positive (%) | No. negative | No. positive (%) | No. negative | ||
| HSM total | 65 | 52 (80) | 13 | 47 (72) | 18 |
| HSM primary | 38 | 33 (87) | 5 | 33 (87) | 5 |
| HSM recurrent | 27 | 19 (70) | 8 | 14 (52) | 13 |
| AAM | 45 | 1 | 44 | 0 | 45 |
| Total | 110 | 53 (48) | 57 | 47 (42) | 63 |
Ten samples were positive with real-time PCR only (three primary patients, six recurrent patients, and one AAM patient). Four samples (three primary patients, one recurrent patient) were positive by nested PCR but negative by real-time PCR. These four samples were subsequently reanalyzed in duplicate. As a result, two samples, both from primary meningitis patients, were found to be positive, having viral loads of <1,000 copies/ml. Inhibitory factors may influence the efficiency of PCR. In the four discrepant cases, no inhibition was found when samples were assessed by adding HSV-2 to the CSF.
Among the 10 samples that were positive by real-time PCR only, five had <1,000 copies/ml, and five had between 1,000 and 10,000 copies/ml.
Viral load in primary versus recurrent HSV-2 meningitis and correlation with inflammatory changes.
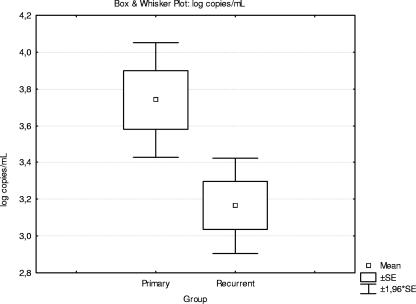
The number of viral copies found in patients with primary versus recurrent HSV-2 meningitis is shown in Fig. 1. The viral load reached significantly higher levels in primary meningitis than in recurrent meningitis (P < 0.05). Viral loads of >10,000 copies were found exclusively in patients with primary HSV-2 meningitis, while viral loads of <1,000 or between 1,000 and 10,000 copies were found in all patients with recurrent HSV-2 meningitis. Correlation was found between the viral load and the amount of leukocytes, monocytes, protein, and albumin in CSF.
FIG. 1.
Box and whisker plot showing viral loads (log copies/ml) in patients with primary (32 patients) and recurrent (19 patients) HSV-2 meningitis.
Detection of VZV DNA in CSF by real-time PCR.
VZV DNA was detected in samples from two AAM patients by real-time PCR, with an average of 4,850 copies/ml. Analysis of acute-phase serum showed high levels of VZV IgG antibodies, indicating reactivated infections.
DISCUSSION
PCR for the diagnosis of primary and recurrent HSV-2 meningitis was evaluated in a large and clinically well-characterized sample of material. Real-time PCR detected more cases with HSV-2 meningitis than nested PCR in the group of patients with recurrent meningitis.
Viral meningitis remains an important cause of morbidity and financial burden and merits efforts to improve diagnostic, treatment, and prevention options (15, 22). The etiological diagnosis of HSV-2 meningitis is of particular importance as this condition carries a considerable risk of future neurological morbidity. HSV-2 proved to be the major cause of benign recurrent lymphocytic meningitis (30). A retrospective study (3) showed that during the year following the acute phase of HSV-2 meningitis, more than 30% of 40 patients suffered from recurrent neurological symptoms (one or several episodes of recurrent meningitis or myelitis or distinct attacks of severe headache). With HSV-2 diagnosis, accurate information about diagnosis, prognosis, and treatment options, i.e., early episodic treatment or suppressive prophylaxis in cases with frequent recurrences, is possible.
Isolation of HSV from CSF has been reported in cases of primary meningitis caused by both types of virus, i.e., HSV-1 and HSV-2 (5, 21, 23, 27, 28). In recurrent meningitis, attempts to isolate HSV in the CSF have been unsuccessful (5). The implementation of PCR for the detection of viral DNA in spinal fluid has resulted in considerable improvement. HSV-2 DNA has been detected by PCR in several patients with primary as well as recurrent aseptic meningitis, both with and without mucocutaneous lesions (3, 9, 25 and 30). However, an evaluation of the diagnostic sensitivity has not been documented in larger sized series. In a number of cases where an HSV-2 etiology is suspected on clinical grounds, e.g., in patients with previous genital blisters or recurrent episodes with a confirmed HSV-2 etiology, nested PCR assays fail to detect HSV-2 DNA.
In our study, real-time PCR verified a larger number of cases of HSV-2 meningitis than the previously used nested PCR. In the absence of a gold standard, using the above-stated criteria for HSV-2 meningitis, the sensitivities of real-time PCR for the detection of HSV-2 DNA in CSF were 87% (33/38) and 70% (19/27) in primary and recurrent meningitis.
However, 4 out of 47 patients were found to be negative by real-time PCR but positive by nested PCR. The missing cases as well as the discrepancy between the nested PCR and the real-time PCR results may be explained by the fact that the DNA in the investigated samples may be around the level of detection and may be unevenly distributed, as shown by analyses of portions of CSF drawn on the same occasion (2). Consequently, discordant results were noticed mainly for samples with low copy numbers.
Real-time PCR makes it possible to evaluate quantitative data in relation to the clinical course and antiviral treatment. The clinical symptoms of recurrent HSM are generally milder and of shorter duration than those of primary meningitis (4, 5). Accordingly, the viral loads in primary meningitis were significantly larger, and the inflammatory changes in the CSF were more pronounced in primary meningitis than in recurrent episodes.
Patients with first-time meningitis came to the clinic, on average, 1 day later than the patients with recurrent meningitis. This could be due to unawareness and misinterpretation of the clinical symptoms. In previous investigations of series of CSF samples, the viral load has been shown to decrease over time (unpublished data). In the present study, viral loads in the group of patients with recurrent HSM were lower than those in the primary group, although their samples were taken earlier, suggesting even greater differences in viral loads in primary versus recurrent meningitis.
Hypoglycorrhachia has previously been reported in HSM (4, 7, 8, 14). In our study, slightly increased lactate levels and lowered CSF/serum glucose ratios were found with a small proportion of HSM patients, in agreement with previous reports. This should be kept in mind in the clinical interpretation of acute meningitis.
With regard to VZV, our findings underline the fact that VZV should be considered in the etiological diagnosis of aseptic meningitis among adults with or without vesicular rash (6, 10).
In conclusion, the sensitivity of real-time PCR for the diagnosis of HSV-2 meningitis was evaluated and found to be high, though somewhat lower in recurrent infection than primary infection. Real-time PCR for the detection of HSV-2 and VZV DNA in CSF is a quick and efficient tool for the etiological diagnosis of aseptic meningitis and should be used in the first-line routine diagnosis. With doctors' increased awareness of the diagnosis, a thorough history taking, and the consistent use of novel diagnostic methods, HSV-2 and VZV etiologies will be revealed in an even higher percentage of cases.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Afonso, N., S. Gunasena, K. Galla, R. Podzorski, P. Chandrasekar, and G. Alangaden. 2007. Appropriate use of polymerase chain reaction for detection of herpes simplex virus 2 in cerebrospinal fluid of patients at an inner-city hospital. Diagn. Microbiol. Infect. Dis. 57:309-313. [DOI] [PubMed] [Google Scholar]
- 1a.Aurelius, E., B. Johansson, B. Sköldenberg, A. Staland, and M. Forsgren. 1991. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189-192. [DOI] [PubMed] [Google Scholar]
- 2.Aurelius, E. 1993. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during long-term follow-up. Scand. J. Infect. Dis. Suppl. 89:3-62. [PubMed] [Google Scholar]
- 3.Aurelius, E., M. Forsgren, E. Gille, and B. Sköldenberg. 2002. Neurologic morbidity after herpes simplex virus type 2 meningitis: a retrospective study of 40 patients. Scand. J. Infect. Dis. 34:278-283. [DOI] [PubMed] [Google Scholar]
- 4.Bachmeyer, C., A. de la Blanchardiere, J. Lepercq, R. Dhote, G. Grateau, M. Detilleux, M. Tournaire, and B. Christoforov. 1996. Recurring episodes of meningitis (Mollaret's meningitis) with one showing an association with herpes simplex virus type 2. J. Infect. 32:247-248. [DOI] [PubMed] [Google Scholar]
- 5.Bergström, T., A. Vahlne, K. Alestig, S. Jeansson, M. Forsgren, and E. Lycke. 1990. Primary and recurrent herpes simplex virus type 2-induced meningitis. J. Infect. Dis. 162:322-330. [DOI] [PubMed] [Google Scholar]
- 6.Bergström, T. 1996. Polymerase chain reaction for diagnosis of varicella zoster virus central nervous system infections without skin manifestations. Scan. J. Infect. Dis. Suppl. 100:41-45. [PubMed] [Google Scholar]
- 7.Brenton, D. W. 1980. Hypoglycorrhachia in herpes simplex type 2 meningitis. Arch. Neurol. 37:317. [DOI] [PubMed] [Google Scholar]
- 8.Chin, R. H., B. C. Ross, K. I. Taylor, A. P. Yung, and P. D. R. Johnson. 1996. Chronic meningitis due to herpes simplex virus in an immunocompetent host. Eur. J. Clin. Microbiol. Infect. Dis. 15:650-653. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, B. A., A. H. Rowley, and C. M. Long. 1994. Herpes simplex type 2 in a patient with Mollaret's meningitis: demonstration by polymerase chain reaction. Ann. Neurol. 35:112-116. [DOI] [PubMed] [Google Scholar]
- 10.Echevarria, J. M., I. Casas, A. Tenorio, F. de Ory, and P. Marinez-Martin. 1994. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J. Med. Virol. 43:331-335. [DOI] [PubMed] [Google Scholar]
- 11.Echevarria, J. M., I. Casas, and P. Martinez-Martin. 1997. Infections of the nervous system caused by varicella-zoster virus: a review. Intervirology 40:72-84. [DOI] [PubMed] [Google Scholar]
- 12.Glimåker, M., B. Johansson, P. Olcén, A. Ehrnst, and M. Forsgren. 1993. Detection of enteroviral RNA by polymerase chain reaction in cerebrospinal fluid from patients with aseptic meningitis. Scand. J. Infect. Dis. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 13.Günther, G., M. Haglund, L. Lindquist, M. Forsgren, and B. Sköldenberg. 1997. Tick-borne encephalitis in Sweden in relation to aseptic meningoencephalitis of other aetiology: a prospective study of clinical course and outcome. J. Neurol. 244:230-238. [DOI] [PubMed] [Google Scholar]
- 14.Jensenius, M., B. Myrvang, G. Storvold, A. Bucher, K. B. Hellum, and A.-L. Bruu. 1998. Herpes simplex virus type 2 DNA detected in cerebrospinal fluid of 9 patients with Mollaret's meningitis. Acta Neurol. Scand. 98:209-212. [DOI] [PubMed] [Google Scholar]
- 15.Khetsuriani, N., E. S. Quiroz, R. C. Holman, and L. J. Anderson. 2003. Viral meningitis-associated hospitalizations in the United States, 1988-1999. Neuroepidemiology 22:345-352. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Koskiniemi, M., T. Rantalaiho, H. Piiparinen, C. H. von Bonsdorff, M. Farkkila, A. Jarvinen, E. Kinnunen, S. Koskiniemi, L. Mannonen, M. Muttilainen, K. Linnavuori, J. Porras, M. Puolakkainen, K. Raiha, E. M. Salonen, P. Ukkonen, A. Vaheri, V. Valtonen, and Study Group. 2001. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J. Neurovirol. 7:400-408. [DOI] [PubMed] [Google Scholar]
- 18.Kupila, L., T. Vuorinen, R. Vainionpaa, V. Hukkanen, R. J. Marttila, and P. Kotilainen. 2006. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 66:75-80. [DOI] [PubMed] [Google Scholar]
- 19.Niesters Hubert, G. M. 2001. Quantitation of viral load using real-time amplification techniques. Methods 25:419-429. [DOI] [PubMed] [Google Scholar]
- 20.Nowak, D. A., R. Boehmer, and H. H. Fuchs. 2003. A retrospective clinical, laboratory and outcome analysis in 43 cases of acute aseptic meningitis. Eur. J. Neurol. 10:271-280. [DOI] [PubMed] [Google Scholar]
- 21.Olson, L. C., E. L. Beuscher, M. S. Artenstein, and P. D. Parkman. 1967. Herpes virus infections of the human central nervous system. N. Engl. J. Med. 277:1271-1277. [DOI] [PubMed] [Google Scholar]
- 21a.O'Sullivan, C. E., A. J. Aksamit, J. R. Harrington, W. S. Harmsen, P. S. Mitchell, and R. Patel. 2003. Clinical spectrum and laboratory characteristics associated with detection of herpes simplex virus DNA in cerebrospinal fluid. Mayo Clin. Proc. 78:1347-1352. [DOI] [PubMed] [Google Scholar]
- 22.Read, S. J., K. J. Jeffery, and C. Bangham. 1997. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J. Clin. Microbiol. 35:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read, S. J., and J. B. Kurtz. 1999. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J. Clin. Microbiol. 37:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Schlesinger, Y., P. Tebas, M. Gaudreault-Keener, R. S. Buller, and G. A. Storch. 1995. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin. Infect. Dis. 20:842-848. [DOI] [PubMed] [Google Scholar]
- 26.Schmutzhard, J., H. Merete Riedel, B. Zweygberg Wirgart, and L. Grillner. 2004. Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. J. Clin. Virol. 29:120-126. [DOI] [PubMed] [Google Scholar]
- 27.Sköldenberg, B., S. Jaensson, and S. Wolontis. 1973. Herpes simplex virus in acute aseptic meningitis. Br. Med. J. 2:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sköldenberg, B., S. Jaensson, and S. Wolontis. 1975. Herpes simplex virus type 2 and acute aseptic meningitis. Scand. J. Infect. Dis. 7:227-232. [DOI] [PubMed] [Google Scholar]
- 29.Stöcher, M. V. Leb, M. Bozic, H. Kessler, G. Halwachs-Bauermann, O. Landt, H. Stekel, and J. Berg. 2003. Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J. Clin. Virol. 26:85-93. [DOI] [PubMed] [Google Scholar]
- 30.Tedder, D. G., R. Ashley, K. L Tyler, and M. J. Levin. 1994. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann. Intern. Med. 121:334-338. [DOI] [PubMed] [Google Scholar]