Abstract
To enhance the sensitivity of the available real-time PCR systems for the detection of Mycoplasma pneumoniae, we established a method to amplify copies of the repetitive element repMp1. In a study of respiratory tract samples, we found that, compared to the use of the conserved part of the P1 adhesin gene as a monocopy target, the use of the repMp1-PCR showed an increase in the detected genome equivalents by a factor of 22.
Mycoplasma pneumoniae is one of the most common causes of upper and lower respiratory tract infections in children and adults. Between 10 and 20% (up to 50%) of all cases of community-acquired pneumonia can be attributed to this cell wall-less pathogen. In addition to the frequent occurrence of time-independent endemic outbreaks in susceptible populations, worldwide epidemics showing 3- to 7-year intervals have been recorded (6, 18). While the culture of M. pneumoniae is unreliable and time-consuming, serological tests require the availability of paired sera showing the problem of an age- and time-dependent occurrence of immunoglobulin A and M antibodies (1). PCR approaches have been found to be useful for the rapid, sensitive, and specific detection of M. pneumoniae in respiratory tract specimens. Because of speed and reduced risk of laboratory contaminations, real-time PCR systems increase the significance of the results in the diagnostic procedure to confirm M. pneumoniae infections. Different PCR protocols have been developed targeting in most cases the ATPase gene and the gene of the main P1 adhesin (11). In the latter gene, two copies of repetitive elements could be found occurring all over the genome of the completely sequenced strain M129 (7). The four different repetitive sequences—repMp1, repMp2/3, repMp4, and repMp5—differ in size (147 to 2,701 bp), number of copies (8 to 14), and sequence (15). Investigation of the rep sequences not only is the basis for the differentiation of clinical isolates of M. pneumoniae into subtypes and variants (3, 8, 16) but also may increase the sensitivity of a PCR approach by amplifying a multicopy target. We sought here to evaluate the sensitivity of a real-time PCR system based on amplification of a part of the repetitive element repMp1 of M. pneumoniae and compare the results with a P1 gene-derived approach targeting a conserved monocopy sequence.
M. pneumoniae reference strains M129 (subtype 1, ATCC 29342), FH (subtype 2, ATCC 15531), and the patient isolates 4817 (variant 1; kindly provided by S. A. J. Zaat, Academic Medical Center, Amsterdam, The Netherlands) and ST (characterized as variant 2) (4) were cultivated as described previously (4). To determine the detection limit of the real-time PCR, freshly grown cells of strain M129 were homogenized by using a 27G syringe and tenfold diluted in phosphate-buffered saline. Aliquots (200 μl) of the dilutions were used to determine the CFU on PPLO agar (Becton Dickinson, Sparks, MD) and in parallel to prepare the genomic DNAs. Respiratory tract specimens (bronchoalveolar lavage fluids and nasopharyngeal or pharyngeal swabs) were collected from patients with symptoms of community-acquired pneumonia during the years from 2004 to 2006. M. pneumoniae in the samples was typed culture independently as described recently (5). DNA was extracted from all patient materials and M. pneumoniae strains (200 μl each) with a QIAamp DNA minikit (QIAGEN, Hilden, Germany) using the protocol for blood and body fluids (elution volume, 200 μl). The DNA concentration was measured photometrically. With the primers MprepF (M. pneumoniae sequence, 5′-AAA CTT TAT ATT CGT TAA ATT C-3′) and MprepR (5′-GAA TTC CAT GAC ATG GTA-3′) (Biomers, Ulm, Germany), a 184-bp product including repMp1-1, and with the primers MpP1F (5′-GGA TGG CAG TTG CTG GC-3′) and MpP1R (5′-AGG GTG TGA AGA GTT GCA AGT-3′), a 177-bp product in the conserved inter-repetitive part of the P1 gene of M. pneumoniae strain M129 were amplified by PCR. Both amplificates were cloned into the pET-30 vector, and Escherichia coli Nova Blue cells (Novagen, Madison, WI) were transformed as recommended by the manufacturer. Plasmids pET-30-P1 and pET-30-repMp1 were extracted from the bacterial cultures by using a QIAprep miniprep kit (QIAGEN), and the concentration of DNA was quantified by measuring the optical density at 260 nm. For the generation of the standard curve, tenfold dilutions (102 to 106 genome equivalents/5 μl) of both of the plasmid DNAs containing the target sequences were prepared. For amplification of the inter-repetitive region of the P1 gene (bases 1804 to 1894 from the start codon in strain M129), the primers MpLCP1F (5′-CGC TTA CTG TAC GAT GAA CTT GAA A-3′; calculated Tm with the nearest-neighbor method of 67.5°C) and MpLCP1R (5′-AGG GTG TGA AGA GTT GCA AGT CT-3′; Tm = 66.4°C) were derived to amplify a 91-bp amplicon within the inserted P1 sequence of the plasmid pET-30-P1. By using the data of Himmelreich et al. (7; GenBank accession no. U00089) the 14 repMp1 sequences were aligned, and the primers MpLCrepF (Tm = 58.0°C) and MpLCrepR (Tm = 59.8°C) were designed (Fig. 1) for amplification of an 89-bp product within the plasmid pET-30-repMp1. The probes MpLCP1S (5′-CAA CCT GAA CTT AGT AGC GCA AGG CCA A-3′; P1 gene; Tm = 77.9°C) and MpLCrepS (repMp1; Tm = 67.8°C) were labeled with FAM (5′) and TAMRA (3′). With a LightCycler 1.5 instrument (Roche, Mannheim, Germany) real-time PCR was performed in a final volume of 20 μl containing 4.6 μl of water (PCR grade; Roche), 2.4 μl of MgCl2 (25 mM; Roche), 2 μl of LightCycler FastStart DNA Master HybProbe mix (Roche), 2 μl of each primer (5 pmol), 2 μl of probe (2 pmol), and 5 μl of DNA. The filled glass capillaries were incubated under the following cycling conditions: preincubation at 95°C (10 min), followed by 55 cycles of denaturation at 95°C (15 s), hybridization at 63°C (P1 gene, 5 s) and 53°C (repMp1; 5 s), and elongation at 72°C (5 s). The reactions were cooled down to 40°C for 1 min. The data were analyzed with the LightCycler software version 3.5 (Roche). By using our collection of subtyped M. pneumoniae strains (4), we selected three patient isolates of subtype 1, subtype 2 (16), and variant 2 (8) and the variant 1 isolate 4817 (3) to confirm the occurrence and sequence identity of the repetitive elements in non-M129 strains. All 14 repMp1 copies in these 10 isolates were amplified by PCR and sequenced. Sequencing was done by using the BigDye terminator cycle sequencing kit and the DNA sequencer ABI Prism 377 or 3100 (Perkin-Elmer/Applied Biosystems, Foster City, CA).
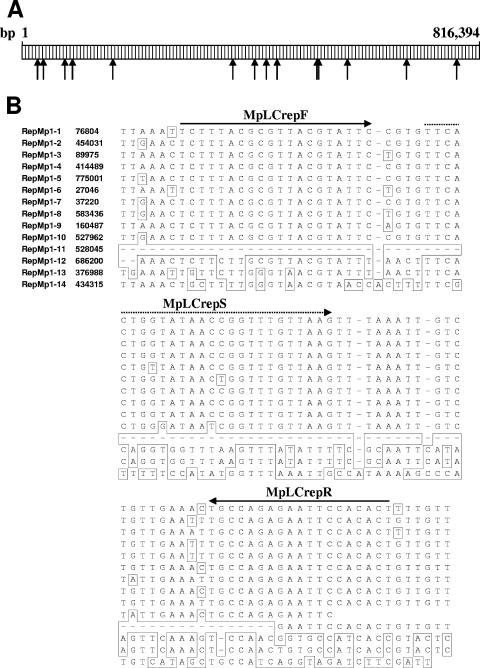
FIG. 1.
(A) Schematic representation showing the location of the different repMp1 copies (indicated by arrows) in the genome of M. pneumoniae strain M129. (B) Partial alignment of the 14 repMp1 copies of M. pneumoniae strain M129 and the location of the primer (drawn arrow) and of the probe (dashed arrow) used for real-time PCR. Differing bases and gaps are indicated by boxes. Genome positions are derived from the published sequence (7; GenBank accession no. U00089).
The repetitive element repMp1 occurred with the highest number of 14 copies compared to all other repMp sequences in the genome of M. pneumoniae strain M129 (7, 15).
However, the shortness of the repM1 sequences ranging between 147 bp (repMp1-11) and 302 bp (repMp1-5) and the broad spectrum of identity rates between the 14 repMp1 copies in M. pneumoniae strain M129 (26.1 to 96.6%) cause limited possibilities for the design of an appropriate primer system targeting a sufficient number of repMp1 copies. With the established real-time PCR approach the amplification of at least 10 copies of repMp1 sequences can be expected. In a search of GenBank using the BLAST algorithm, no homology of the primer to other species was found. The reproducibility of the results of the real-time systems was determined by testing tenfold dilutions of the plasmid preparations containing the target sequences. The variability of both of the real-time PCR systems with three different dilutions of the plasmids ranged between 0.2 and 1.0 (Table 1), indicating low intra-assay variations. However, an interassay comparison showed significant higher crossing points after using the P1-based real-time PCR (P < 0.05 [Student t test]). No amplification was observed with DNA from Mycoplasma genitalium (ATCC 33530), Mycoplasma hominis (ATCC 23114), Ureaplasma urealyticum (ATCC 27818), Mycoplasma penetrans (ATCC 55252), Mycoplasma salivarium (ATCC 23064), Chlamydophila pneumoniae (strain TW-183), Legionella pneumophila (ATCC 33152), Haemophilus influenzae (ATCC 49247), Streptococcus pneumoniae (ATCC 6305), Staphylococcus aureus (ATCC 25923), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 43895), thus confirming the specificity of both of the real-time approaches to detect M. pneumoniae. None of the 52 respiratory tract specimens of pneumonia patients that have been tested negative by the P1-based real-time PCR approach gave positive results with the repMp1-derived assay.
TABLE 1.
Results of testing of different specimens with real-time PCR assays based on the amplification of parts of the P1 gene and the repetitive element repMp1 of M. pneumoniae
| Sample | Descriptiona | M. pneumoniae strain | Real-time PCR | CP ± SDb (n = 8) |
|---|---|---|---|---|
| Purified plasmid pET-30 (containing P1 sequence) | 102 GE/5 μl | M129 (subtype 1) | P1 | 36.45 ± 0.37 (1.0) |
| 104 GE/5 μl | 29.20 ± 0.08 (0.3) | |||
| 106 GE/5 μl | 22.21 ± 0.07 (0.3) | |||
| Purified plasmid pET-30 (containing repMp1 sequence) | 102 GE/5 μl | M129 (subtype 1) | RepMp1 | 35.19 ± 0.30 (0.8) |
| 104 GE/5 μl | 28.43 ± 0.14 (0.5) | |||
| 106 GE/5 μl | 21.55 ± 0.04 (0.2) | |||
| Extracted DNA from bouillon cultures | 0.2 CFU/5 μl | M129 (subtype 1) | P1 | NEc (CP > 42) |
| (0.4 fg of DNA/5 μl) | RepMp1 | 34.16 ± 0.22 (0.6) | ||
| 1.9 CFU/5 μl | P1 | 40.56 ± 1.25 (3.1) | ||
| (4.2 fg of DNA/5 μl) | RepMp1 | 33.64 ± 0.31 (0.9) | ||
| 19.0 CFU/5 μl | P1 | 36.44 ± 0.41 (1.1) | ||
| (42.2 fg of DNA/5 μl) | RepMp1 | 31.15 ± 0.48 (0.6) | ||
| Extracted DNA from bouillon cultures | FH (subtype 2) | P1 | 29.98 ± 0.08 (0.3) | |
| RepMp1 | 26.02 ± 0.06 (0.2) | |||
| 4817 (variant 1) | P1 | 30.95 ± 0.10 (0.3) | ||
| RepMp1 | 26.05 ± 0.09 (0.3) | |||
| ST (variant 2) | P1 | 29.58 ± 0.10 (0.3) | ||
| RepMp1 | 24.70 ± 0.04 (0.2) |
GE, genome equivalent.
The arithmetic mean of crossing points ± the standard deviation is indicated; the coefficient of variation (%) is given in parentheses. CP, crossing point.
NE, not evaluable.
Both real-time PCR systems were effective for detecting all of the known subtypes (1 and 2) and variants (1 and 2) of M. pneumoniae. The repMp1-based real-time PCR system was able to detect 0.2 CFU/5 μl, whereas with the P1-based approach the detection of 2 CFU/5 μl resulted in mean crossing-point values of more than 40. The sensitivity of the repMp1-based real-time PCR was further tested by amplifying serial dilutions of DNA of M. pneumoniae strain M129. The proportion of positive samples (n = 10) ranged from 100% (0.3 CFU or 0.83 fg of DNA/reaction), 50% (0.03 CFU or 0.08 fg of DNA), 30% (0.015 CFU or 0.04 fg of DNA), and 0% (0.003 CFU or 0.008 fg of DNA [data not shown]). Accurate comparison of the detection limits of described real-time PCR systems with the results in the literature is difficult because of the differences in the units on which the data are based (CFU, DNA concentration, genome copies, and color changing units) (11). To increase the information about the demonstrated sensitivity of the real-time assay, the CFU of the used M. pneumoniae suspensions and the concentration of the extracted M. pneumoniae DNA were measured in parallel. Furthermore, comparison of the sensitivities must consider the occurrence of repetitive elements in the target sequences. Whereas in the ATPase gene (2, 10) and in the 16S rRNA gene (9) no repetitive elements exist, the amplification results of parts of the P1 gene can be influenced by the repetitive sequences repMp4 and repMp2/3. Based on the data of Himmelreich et al. (7) and the real-time PCR systems to detect M. pneumoniae described by Welti et al. (19) and Pitcher et al. (14), the P1-based system described in the present study amplifies sequences outside of the repMp4 and the repMp2/3 in the P1 gene representing a monocopy target. In contrast, the method published by Ursi et al. (17) based on primer pairs inside the repMp2/3 and the SYBR green LightCycler procedure of Maltezou et al. (12) use primers derived from the repMp4 of the P1 gene. With the latter two approaches the amplification of more than a single target per bacterium cannot be excluded. In these real-time PCR systems, detection limits of between 10 and 20 copies (corresponding to at least two color changing units) were determined (14, 17, 19).
The investigation of 25 respiratory tract specimens of pneumonia patients confirmed the higher sensitivity of the repMp1-based real-time PCR compared to the P1-derived approach (Table 2). Compared to the mean concentration of genome equivalents in the respiratory tract samples, the results of both of the systems differed by a factor of 21.6 (arithmetic mean).
TABLE 2.
Comparison of the results (arithmetic mean of three replicates) of the P1- and repMp1-based real-time PCR in DNA extracts of respiratory tract samples (bronchoalveolar lavage fluids and nasopharyngeal and pharyngeal swabs) of pneumonia patients
| M. pneumoniae straina | Sample | Genome equivalents/reaction ± SD
|
|
|---|---|---|---|
| Real-time PCR (P1 based) | Real-time PCR (RepMp1 based) | ||
| Subtype 1 | CAP-1 | 7.3 × 101 ± 4.0 × 101 | 3.4 × 103 ± 4.5 × 101 |
| CAP-2 | 1.3 × 102 ± 3.2 × 101 | 7.1 × 103 ± 2.6 × 102 | |
| CAP-3 | 9.8 × 103 ± 1.2 × 103 | 1.3 × 105 ± 4.7 × 102 | |
| CAP-4 | 5.0 × 104 ± 1.8 × 103 | 7.4 × 105 ± 1.6 × 104 | |
| CAP-5 | 7.3 × 103 ± 5.2 × 102 | 1.4 × 105 ± 2.1 × 103 | |
| CAP-6 | 1.4 × 104 ± 2.1 × 103 | 2.5 × 105 ± 5.4 × 104 | |
| CAP-7 | 1.8 × 103 ± 4.3 × 101 | 2.0 × 104 ± 1.0 × 104 | |
| CAP-8 | 1.0 × 104 ± 5.8 × 102 | 2.0 × 105 ± 1.3 × 104 | |
| CAP-9 | 2.3 × 105 ± 2.0 × 104 | 1.1 × 107 ± 3.0 × 106 | |
| CAP-10 | 2.7 × 103 ± 1.8 × 102 | 2.0 × 104 ± 4.0 × 103 | |
| CAP-11 | 2.0 × 106 ± 5.2 × 105 | 7.7 × 107 ± 2.0 × 106 | |
| CAP-12 | 4.2 × 103 ± 1.7 × 103 | 1.2 × 104 ± 2.1 × 103 | |
| Subtype 2 | CAP-13 | 4.4 × 102 ± 1.0 × 101 | 1.9 × 103 ± 1.1 × 102 |
| CAP-14 | 8.0 × 104 ± 2.8 × 103 | 9.8 × 105 ± 1.0 × 104 | |
| CAP-15 | 7.0 × 102 ± 2.1 × 101 | 1.2 × 104 ± 8.5 × 102 | |
| CAP-16 | 4.0 × 103 ± 6.8 × 101 | 6.9 × 104 ± 5.2 × 103 | |
| CAP-17 | 2.7 × 103 ± 6.5 × 101 | 6.2 × 104 ± 1.4 × 103 | |
| CAP-18 | 3.9 × 103 ± 3.5 × 102 | 4.9 × 104 ± 1.2 × 104 | |
| Variant 2 | CAP-19 | 4.0 × 103 ± 1.2 × 102 | 1.2 × 105 ± 7.6 × 102 |
| CAP-20 | 9.5 × 104 ± 5.4 × 103 | 2.4 × 106 ± 9.8 × 104 | |
| CAP-21 | 5.0 × 104 ± 8.0 × 103 | 1.3 × 106 ± 8.3 × 104 | |
| CAP-22 | 2.7 × 103 ± 3.8 × 102 | 5.6 × 104 ± 1.8 × 103 | |
| CAP-23 | 1.6 × 105 ± 2.6 × 103 | 2.2 × 106 ± 1.2 × 105 | |
| CAP-24 | 1.8 × 105 ± 3.0 × 103 | 4.5 × 106 ± 1.0 × 106 | |
| CAP-25 | 2.2 × 105 ± 1.6 × 104 | 4.1 × 106 ± 3.4 × 105 | |
Tested M. pneumoniae positive and subtyped according to the method of Dumke et al. (5).
Sequencing of the 14 repMp1 sequences in the 10 patient isolates of M. pneumoniae resulted in only small sequences differences. Five of fourteen repetitive elements (repMp1-3, -10, -12, -13, and -14) showed identical sequences in all strains, whereas in six (repMp1-1, -2, -4, -6, -9, and -11) and two (repMp1-5 and -8) sequences a single and two nucleotide exchanges were found. In the repMp1-7 (241 bp) a higher degree of heterogeneity with 17 nucleotide exchanges was detected. Twenty-three of the twenty-seven differences were subtype or variant specific. Only one and two of these nucleotide exchanges are located inside the sequence of the primer and the probe, respectively, and are considered to have limited influence on the results of the repMp1-based PCR. In addition, the sequencing data confirmed the stability of the M. pneumoniae genome not only in genes coding for housekeeping and structural proteins (4) but also in the repetitive elements.
In conclusion, the sensitive repMp1-based real-time PCR approach seems to be an alternative method particularly for the investigation of samples in which low concentrations of M. pneumoniae DNA can be expected, e.g., serum samples of pneumonia patients (2), cerebrospinal fluid samples of patients with extrapulmonary complications of M. pneumoniae infections (13), or respiratory tract samples of pneumonia patients after pretreatment with antibiotics. The test system combines the common advantages of real-time PCR in the diagnosis of infectious diseases with an optimization of the sensitivity for detecting M. pneumoniae.
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (JA 399/10-1) and the BMBF network of competence (CAPNETZ).
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Daxboeck, F., R. Krause, and C. Wenisch. 2003. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin. Microbiol. Infect. 9:263-273. [DOI] [PubMed] [Google Scholar]
- 2.Daxboeck, F., G. Khanakah, C. Bauer, M. Stadler, H. Hofmann, and G. Stanek. 2005. Detection of Mycoplasma pneumoniae in serum specimens from patients with mycoplasma pneumonia by PCR. Int. J. Med. Microbiol. 295:279-285. [DOI] [PubMed] [Google Scholar]
- 3.Dorigo-Zetsma, J. W., B. Wilbrink, J. Dankert, and S. A. J. Zaat. 2001. Mycoplasma pneumoniae P1 type 1- and type 2-specific sequences within the P1 cytadhesin gene of individual strains. Infect. Immun. 69:5612-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumke, R., I. Catrein, E. Pirkl, R. Herrmann, and E. Jacobs. 2003. Subtyping of Mycoplasma pneumoniae isolates based on extended genome sequencing and on expression profiles. Int. J. Med. Microbiol. 292:513-525. [DOI] [PubMed] [Google Scholar]
- 5.Dumke, R., P. C. Lück, C. Schaefer, C. Noppen, H. von Baum, R. Marre, and E. Jacobs. 2006. Culture-independent molecular subtyping of Mycoplasma pneumoniae in clinical samples. J. Clin. Microbiol. 44:2567-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerschlag, M. R. 2001. Mycoplasma pneumoniae infections. Curr. Opin. Infect. Dis. 14:181-186. [DOI] [PubMed] [Google Scholar]
- 7.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenri, T., R. Taniguchi, Y. Sasaki, N. Okazaki, M. Narita, K. Izumikawa, M. Umetsu, and T. Sasaki. 1999. Identification of a new variable sequence in the P1 cytadhesin gene of Mycoplasma pneumoniae: evidence for the generation of antigenic variation by DNA recombination between repetitive sequences. Infect. Immun. 67:4557-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna, M., J. Fan, K. Pehler-Harrington, C. Waters, P. Douglass, J. Stallock, S. Kehl, and K. J. Henrickson. 2005. The pneumoplex assays, a multiplex PCR-enzyme hybridization assay that allows simultaneous detection of five organisms, Mycoplasma pneumoniae, Chlamydia (Chlamydophila) pneumoniae, Legionella pneumophila, Legionella micdadei, and Bordetella pertussis, and its real-time counterpart. J. Clin. Microbiol. 43:565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, G., D. Talkington, B. S. Fields, O. S. Levine, Y. Yang, and M. L. C. Tondella. 2005. Chlamydia pneumoniae and Mycoplasma pneumoniae in young children from China with community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 52:7-14. [DOI] [PubMed] [Google Scholar]
- 11.Loens, K., D. Ursi, H. Goossens, and M. Ieven. 2003. Molecular diagnosis of Mycoplasma pneumoniae respiratory tract infections. J. Clin. Microbiol. 41:4915-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maltezou, H. C., B. La-Scola, H. Astra, I. Constantopoulou, V. Vlahou, D. A. Kafetzis, A. G. Constantopoulos, and D. Raoult. 2004. Mycoplasma pneumoniae and Legionella pneumophila in community-acquired lower respiratory tract infections among hospitalized children: diagnosis by real-time PCR. Scand. J. Infect. Dis. 36:639-642. [DOI] [PubMed] [Google Scholar]
- 13.Padovan, C. S., H. W. Pfister, S. Bense, V. Fingerle, and M. Abele-Horn. 2001. Detection of Mycoplasma pneumoniae DNA in cerebrospinal fluid of a patient with M. pneumoniae infection-“associated” stroke. Clin. Infect. Dis. 33:119-121. [DOI] [PubMed] [Google Scholar]
- 14.Pitcher, D., V. J. Chalker, C. Sheppard, R. C. George, and T. G. Harrison. 2006. Real-time detection of Mycoplasma pneumoniae in respiratory samples with an internal processing control. J. Med. Microbiol. 55:149-155. [DOI] [PubMed] [Google Scholar]
- 15.Ruland, K., R. Wenzel, and R. Herrmann. 1990. Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome. Nucleic Acids Res. 18:6311-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su, C. J., A. Chavoya, S. F. Dallo, and J. B. Baseman. 1990. Sequence divergency of the cytadhesin gene of Mycoplasma pneumoniae. Infect. Immun. 58:2669-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ursi, D., C. Dirven, K. Loens, M. Ieven, and H. Goossens. 2003. Detection of Mycoplasma pneumoniae in respiratory tract samples by real-time PCR using an inhibition control. J. Microbiol. Methods 55:149-153. [DOI] [PubMed] [Google Scholar]
- 18.Waites, K. B., and D. F. Talkington. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17:697-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welti, M., K. Jaton, M. Altwegg, R. Sahli, A. Wenger, and J. Bille. 2003. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae in respiratory tract secretions. Diagn. Microbiol. Infect. Dis. 45: 85-95. [DOI] [PubMed] [Google Scholar]