Abstract
Using the BacT/Alert automated system, we conducted a 1-year retrospective study on blood cultures, focusing on the relevance of routine use of the anaerobic bottle. The rate of patients with positive blood cultures was 19.7%. Among these, 13.5% had a positive anaerobic bottle in the absence of any aerobic bottle, and 2/3 of these grew with nonobligate anaerobes. These patients were hospitalized in 20 out of 26 wards of the hospital group. For 65.4% of the monomicrobial-positive blood cultures growing Enterobacteriaceae, the anaerobic bottle detected growth earlier than the corresponding aerobic bottle. These data suggest that, in our institution, the use of an anaerobic bottle is still relevant.
Blood cultures remain the cornerstone for the diagnosis of bacteremia. Classically, two bottles are collected routinely: an aerobic bottle, allowing preferential growth of aerobic and facultative anaerobic microorganisms, and an anaerobic bottle, allowing preferential growth of strict anaerobic bacteria. BacT/Alert (bioMérieux, Lyon, France) is an automated system used for the incubation and detection of positive blood cultures (16). The main improvements introduced with this system were the replacement of glass with plastic bottles and the introduction of FAN medium containing charcoal and Fuller's earth. These components were supposed to adsorb antibiotics present in blood samples but showed additional properties improving recovery of microorganisms (9, 10, 15, 17).
Different parameters have been evaluated to improve the performance and the cost related to usage of blood cultures. For pediatric patients, since anaerobic bacteria are rarely implicated, the usefulness of the anaerobic bottle seemed limited and it was recommended that the entire blood volume should be collected only in aerobic bottles (20). For adults, it was also shown that the frequency of obligate anaerobic bacteremia declined significantly and that, with the exception of obligate anaerobic bacteria, many organisms grew preferentially in aerobic bottles (5, 12, 14). Taking into account these results and the comparison of bacteriological and clinical data (13), the routine use of two aerobic blood cultures with only selective use of anaerobic bottles was proposed previously (12).
On the other hand, although different studies have compared the times of detection between different automatic systems or different media (18, 21), few studies have compared, using the same system, the time differences for growth detection between the aerobic and anaerobic bottles according to the microorganism isolated.
Thus, using the BacT/Alert system and FAN bottles, we conducted a 1-year retrospective study to evaluate whether putative gains existed in terms of detection of the most common microorganisms by use of both aerobic and anaerobic bottles and also in terms of time of detection of positivity between the two bottles when the same blood culture grew with the same organism.
The study was conducted in a 750-bed, acute-care teaching hospital including 23 wards (12 medical wards, six surgical wards, and five intensive care units) accounting for approximately 35,000 admissions and three long-term-care hospitals corresponding to 850 beds. All of the blood cultures sampled in 2004 were incubated in a BacT/Alert system with 40 ml FAN aerobic and anaerobic media (17). Since the recommendation was always to collect aerobic and anaerobic bottles concomitantly, only pairs of aerobic and anaerobic bottles inoculated simultaneously were taken into account to compare recovery and rapidity of growth of the different organisms in each bottle. Using an aliquot of ca. 20 ml blood from patients with suspected bacteremia, 10 ml of blood was introduced in each bottle. After comparison of 200 pairs of blood cultures, no statistical difference was found between the quantities of blood introduced in the aerobic and the anaerobic bottles (data not shown). All bottles were placed at 37°C in the BacT/Alert system for a 7-day incubation period and monitored in accordance with the manufacturer's recommendations. All positive bottles were systematically plated on Columbia blood-sheep agar and incubated under aerobic and anaerobic conditions. Bacterial identification was performed using standard procedures (3). Coagulase-negative staphylococci, Corynebacterium spp., Bacillus spp., and Propionibacterium spp. were not considered clinically significant if isolated in only one bottle or if the same bacterium isolated in several bottles showed different antibiotic-resistant phenotypes. The number of hours needed for automatic detection of the microbial growth for each bottle was flagged by the instrument (Bactview software, interface between BacT/Alert incubators and laboratory information system).
Positive rate of blood cultures.
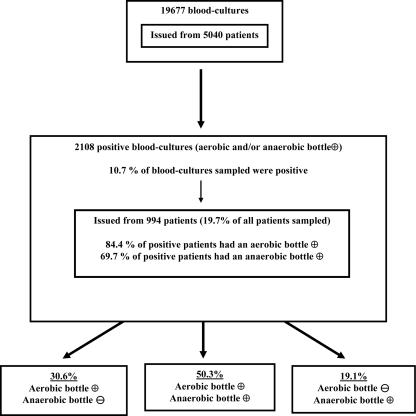
Only blood cultures for which simultaneous pairs of aerobic and anaerobic bottles were collected were counted in this study, corresponding to 95% of all blood cultures sampled. A total of 19,677 blood cultures (39,354 bottles) were collected from 5,040 patients during the study period (Fig. 1), corresponding to an average of four pairs of blood cultures per patient. A total of 2,108 positive blood cultures (10.7%), a number within the range (8.9% to 11.6%) of those determined by previous studies (6, 17, 20), were obtained from 994 patients, corresponding to 19.7% of patients. Eighty-four percent of patients had a positive aerobic bottle, while 69.7% had a positive anaerobic bottle. Among all positive blood cultures, aerobic or anaerobic bottles alone were detected positive in 30.6% (collected from 454 patients) and 19.1% (collected from 315 patients) of cases, respectively (Fig. 1).
FIG. 1.
Rates of positive blood culture bottles. +, positive; −, negative.
Positive anaerobic bottle(s) without any concomitant positive aerobic bottle(s).
For 137 patients, representing 13.7% of the patients with positive blood cultures (2.7% of all sampled patients), significant organisms were isolated only in an anaerobic bottle(s) without any concomitant positive aerobic bottle (Table 1). These 137 patients were localized in 20 out of 26 wards of the hospital group. Fifty-two obligate anaerobic bacteria were isolated from 48 patients, but most interestingly 91 nonobligate anaerobic bacteria, absent in all aerobic bottles collected for each patient, were isolated from the remaining 91 patients.
TABLE 1.
Numbers of significant bacterial isolates recovered from patients with positive anaerobic bottles without a positive aerobic bottle and vice versa
| Organism(s) | No. of isolates from anaerobic bottles without a positive aerobic bottle (no. of patients) | No. of isolates from aerobic bottles without a positive anaerobic bottle (no. of patients) |
|---|---|---|
| Anaerobic bacteria | 50 (46) | 1 (1) |
| Strict aerobic bacilli | 1 (1) | 48 (47) |
| Enterobacteriaceae | 38 (38) | 32 (32) |
| Streptococcus/Enterococcus | 26 (26) | 22 (21) |
| Staphylococcus spp. | 14 (14)a | 14 (14)b |
| Yeast | 0 | 22 (22) |
| Other | 10 (10) | 8 (8) |
| Anaerobic bacteria + Streptococcus spp. | 4 (2) | 0 |
| Strict aerobic bacilli + Streptococcus spp. | 0 | 2 (1) |
| Strict aerobic bacilli + Enterobacteriaceae | 0 | 4 (2) |
| Strict aerobic bacilli + yeast | 0 | 2 (1) |
| Staphylococcus spp. + Streptococcus spp. | 0 | 2 (1) |
| Streptococcus spp. + Enterobacteriaceae | 0 | 4 (1) |
| Total | 143 (137) | 161 (151) |
Including 13 S. aureus isolates.
Including 11 S. aureus isolates.
Since 13 additional patients had positive cultures with obligate anaerobic bacteria in the two bottles, a total of 61 patients, corresponding to 1.2% of all the sampled patients, grew blood cultures with obligate anaerobic bacteria, in accordance with previously published data (4, 13). Fifteen of those 61 patients belonged to medical and chirurgical digestive wards, while the others (46/61) were present in 15 out of the 26 wards of the hospital group. These results suggest that the selective use of anaerobic bottles according to the type of medical or surgical ward cannot generally be recommended and depends on the type of patient present in the different wards of the hospital. Moreover, unanticipated sepsis with anaerobic bacteria could place patients at high risk for serious, life-threatening infection. No change in anaerobic bacteremia rate was observed to occur between 2001 and 2004 (5.0% and 4.9%, respectively) (Table 2). These rates, which are slightly superior to those of recent and older studies, which varied from 2.5 to 3.3% (1, 11, 12), show that the proportion of positive anaerobic blood cultures, which has decreased since the 1970s, has now stabilized. Furthermore, a very recent study showed a rate that increased from 5.4% to 10.4% between 1993 and 2004, suggesting a potential reemergence of anaerobic bacteremia (8). Moreover, no significant difference in anaerobic species repartition was noted for this period (Table 2). As found in recent studies (1, 8, 19), Bacteroides spp. and Clostridium spp. remain the anaerobes isolated most frequently from blood cultures (Table 2).
TABLE 2.
Species of anaerobes isolated from blood cultures between 2001 and 2004
| Organism(s) | No. (%) of anaerobes isolated in yr:
|
|||
|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | |
| Gram-negative bacilli | ||||
| Bacteroides spp. | ||||
| B. caccae | 2 | |||
| B. distasonis | 1 | 1 | 1 | |
| B. fragilis | 12 | 18 | 14 | 32 |
| B. thetaiotaomicron | 3 | 6 | 1 | 2 |
| B. uniformis | 1 | 2 | ||
| B. vulgatus | 2 | |||
| Other Bacteroides spp. | 3 | 5 | 3 | |
| Leptotrichia spp. | 1 | |||
| Prevotella spp. | ||||
| P. loescheii | 1 | |||
| P. melaninogenica | 1 | |||
| P. oralis | 1 | |||
| Other Prevotella spp. | 1 | 1 | 1 | |
| Fusobacterium spp. | ||||
| F. mortiferum | 1 | |||
| F. naviforme | 1 | |||
| F. necrophorum | 1 | 2 | ||
| F. nucleatum | 2 | 1 | ||
| Other Fusobacterium spp. | 1 | 1 | 1 | 2 |
| Veillonella spp. | ||||
| V. parvula | 2 | |||
| V. ratti | 1 | |||
| Other Veillonella spp. | 2 | |||
| Other gram-negative bacilli | 4 | 1 | 5 | 1 |
| All gram-negative bacilli | 31 | 37 | 32 | 45 |
| Gram-positive bacilli | ||||
| Clostridium spp. | ||||
| C. bifermentans | 1 | |||
| C. clostridioforme | 1 | |||
| C. difficile | 1 | |||
| C. fimetarium | 1 | |||
| C. hastiforme | 1 | |||
| C. innocuum | 1 | |||
| C. paraputrificum | 1 | |||
| C. perfringens | 1 | 1 | 1 | 4 |
| C. ramosum | 1 | 2 | ||
| C. tertium | 1 | |||
| Other Clostridium spp. | 1 | 4 | 2 | 1 |
| Peptococcus spp. | 2 | 2 | 2 | |
| Peptostreptococcus spp. | ||||
| P. micros | 2 | |||
| Other Peptostreptococcus spp. | 4 | 4 | 4 | 4 |
| Eubacterium spp. | ||||
| E. aerofaciens | 1 | |||
| E. lentum | 2 | 1 | ||
| Other Eubacterium spp. | 2 | |||
| Other gram-positive bacilli | 2 | 4 | ||
| All gram-positive bacilli | 10 | 13 | 17 | 22 |
| Unidentified anaerobic bacteria | 11 | 2 | 4 | 1 |
| All anaerobic bacteria isolated from blood cultures | 52 (5.0) | 52 (3.8) | 53 (4.1) | 68 (4.9) |
| All bacteria isolated from blood cultures | 1,030 | 1,353 | 1,296 | 1,389 |
Mirroring the above-mentioned observation, for 151 patients, corresponding to 14.9% of patients with positive blood cultures, significant organisms were isolated only in aerobic bottles without any anaerobic bottles (Table 1). As expected, predominantly strictly aerobic bacteria or yeasts were isolated from these aerobic bottles. Interestingly, somewhat similar proportions of Enterobacteriaceae, staphylococci, and streptococci/enterococci were isolated either in an aerobic or in an anaerobic bottle alone (Table 1).
Repartition of positive aerobic and anaerobic bottles for patients sampled for only one blood culture.
To evaluate whether an advantage to collecting pairs of aerobic and anaerobic bottles exists, patients for whom only a single pair of bottles was sampled were analyzed. Out of 1,902 patients, 118 (6.2%) had a positive blood culture. For 80 of them, a significant isolate grew in the blood culture (Table 3). Among those, for 12 and 21 patients, respectively, bacteria grew only in the aerobic or the anaerobic bottle. These results emphasize the respective role of each type of bottle. If anaerobic bottles had not been sampled systematically, 25% of the single pair of positive blood cultures, among which 50% grew nonobligate anaerobic bacteria, would not have been diagnosed.
TABLE 3.
Repartition of significant isolates recovered from patients for whom only one blood culture was sampled
| Group | No. of isolates (no. of patients)a
|
||
|---|---|---|---|
| Aer+/Ana− | Aer+/Ana+ | Aer−/Ana+ | |
| Strict aerobic bacilli | 2 | 1 | 0 |
| Anaerobic bacteria | 0 | 1 | 8 |
| Enterobacteriaceae | 5 | 25 | 7 |
| Staphylococcus spp. | 2 | 11 | 2 |
| Streptococcus/ Enterococcus spp. | 4 | 14 | 2 |
| Other | 0 | 0 | 2 |
| Total | 13 (12) | 52 (47) | 21 (21) |
Aer+, positive aerobic bottle; Aer−, negative aerobic bottle; Ana+, positive anaerobic bottle; Ana−, negative anaerobic bottle.
Time of detection for aerobic and anaerobic positive bottles.
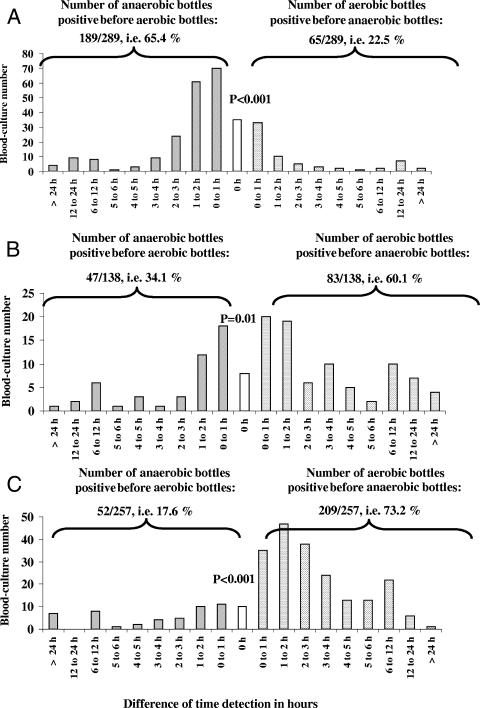
Another question raised during this study was, as far as the time of detection before positivity was concerned, to find the possible advantage of growth in anaerobic or aerobic bottles. Out of 2,108 positive blood cultures, 1,060 corresponded to a positive pair of aerobic and anaerobic bottles, and for 894 of them, the same unique organism grew in the two bottles: Enterobacteriaceae in 289 pairs, Staphylococcus aureus in 257 pairs, either Streptococcus spp. or Enterococcus spp. in 138 pairs, and other microorganisms, mainly coagulase-negative staphylococci, in 210 pairs. The difference in the times of detection of growth between the two bottles is represented in Fig. 2. For bottles with Enterobacteriaceae, the Streptococcus/Enterococcus group, and S. aureus, a gain of >6 h of growth for one bottle over the other was found for 15.1% of the monomicrobial blood cultures (6.4% grew in the anaerobic bottle first and 8.7% grew in the aerobic bottle first). A gain of >2 h was found for 40.8% of the monomicrobial blood cultures (14.6% grew in the anaerobic bottle first and 26.2% grew in the aerobic bottle first). Looking at the overall range of detection for Enterobacteriaceae, 65.4% of the anaerobic bottles grew cultures before the aerobic bottles while only 22.5% of aerobic bottles grew cultures before the anaerobic bottles (P < 0.001) (Fig. 2A). In contrast, for the Enterococcus/Streptococcus group and S. aureus, respectively, 60.1% and 73.2% of the aerobic bottles grew cultures before the anaerobic bottles (P = 0.01 and P < 0.001, respectively) (Fig. 2B and C).
FIG. 2.
For monomicrobial blood cultures, difference between time of detection of growth for the anaerobic bottles and their corresponding aerobic bottles for Enterobacteriaceae (A), the Enterococcus/Streptococcus group (B), and S. aureus (C). P values correspond to the chi-square test and were used to determine if the number of aerobic bottles positive before the anaerobic bottle was statistically different from the number of anaerobic bottles positive before the aerobic bottle. The level of statistical significance was set at 0.05.
In conclusion, several issues concerning the usefulness of the anaerobic bottles were raised in this study. (i) Only 13.7% of the sampled patients had a significant positive blood culture detected by the anaerobic bottle alone in the absence of any growth in all aerobic bottles. Thus, a nonsystematic sampling of anaerobic bottles could lead to an underestimation of the number of cases of diagnosed bacteremia. (ii) For 2/3 of these patients, the isolated bacteria were not obligate anaerobes, suggesting that usefulness of the anaerobic bottle is not restricted to the isolation of strictly anaerobic bacteria. (iii) These patients were hospitalized in 20 out of 26 wards of our hospital group, making unpredictable selective sampling of anaerobic bottle in our institution. (iv) When found in the aero- and anaerobic bottles of the same blood culture, and in contrast to S. aureus, Enterobacteriaceae grew faster in the anaerobic bottle than in the aerobic one. Such an advantage remains to be evaluated in clinical practice, in particular, as far as the gain for the treatment decision is concerned. It is also well known that the quantity of blood introduced in the blood culture bottle plays a role (2, 7). It remains to be determined whether the distribution of the whole blood in one or two bottles would significantly change the range of positive blood cultures. Finally, this study underlines the necessity for each hospital to determine the combination of bottles that would be most efficient for its patient population.
Acknowledgments
We thank Vincent Jarlier for his relevant comments.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Blairon, L., Y. De Gheldre, B. Delaere, A. Sonet, A. Bosly, and Y. Glupczynski. 2006. A 62-month retrospective epidemiological survey of anaerobic bacteraemia in a university hospital. Clin. Microbiol. Infect. 12:527-532. [DOI] [PubMed] [Google Scholar]
- 2.Cockerill, F. R., III, J. W. Wilson, E. A. Vetter, K. M. Goodman, C. A. Torgerson, W. S. Harmsen, C. D. Schleck, D. M. Ilstrup, J. A. Washington II, and W. R. Wilson. 2004. Optimal testing parameters for blood cultures. Clin. Infect. Dis. 38:1724-1730. [DOI] [PubMed] [Google Scholar]
- 3.Comité de l'antibiogramme de la Société Française de Microbiologie. 2003. Comité de l'antibiogramme de la Société Française de Microbiologie report 2003. Int. J. Antimicrob. Agents 21:364-391. [DOI] [PubMed] [Google Scholar]
- 4.Cornish, N., B. A. Kirkley, K. A. Easley, and J. A. Washington. 1999. Reassessment of the routine anaerobic culture and incubation time in the BacT/Alert FAN blood culture bottles. Diagn. Microbiol. Infect. Dis. 35:93-99. [DOI] [PubMed] [Google Scholar]
- 5.Gransden, W. R., S. J. Eykyn, and I. Phillips. 1991. Anaerobic bacteremia: declining rate over a 15-year period. Rev. Infect. Dis. 13:1255-1256. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy, G. T., J. G. Barr, and C. Goldsmith. 1995. Detection of bacteraemia by the continuously monitoring BacT/Alert system. J. Clin. Pathol. 48:912-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamy, B., P. Roy, G. Carret, J. P. Flandrois, and M. L. Delignette-Muller. 2002. What is the relevance of obtaining multiple blood samples of culture? A comprehensive model to optimize the strategy for diagnosing bacteremia. Clin. Infect. Dis. 35:842-850. [DOI] [PubMed] [Google Scholar]
- 8.Lassmann, B., D. R. Gustafson, C. M. Wood, and J. E. Rosenblatt. 2007. Reemergence of anaerobic bacteremia. Clin. Infect. Dis. 7:895-900. [DOI] [PubMed] [Google Scholar]
- 9.McDonald, L. C., J. Fune, L. B. Gaido, M. P. Weinstein, L. G. Reimer, T. M. Flynn, M. L. Wilson, S. Mirrett, and L. B. Reller. 1996. Clinical importance of increased sensitivity of BacT/Alert FAN aerobic and anaerobic blood culture bottles. J. Clin. Microbiol. 34:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirrett, S., M. J. Joyce, and L. B. Reller. 2005. Validation of performance of plastic versus glass bottles for culturing anaerobes from blood in BacT/ALERT SN medium. J. Clin. Microbiol. 43:6150-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris, A. J., M. L. Wilson, S. Mirrett, and L. B. Reller. 1993. Rationale for selective use of anaerobic blood cultures. J. Clin. Microbiol. 31:2110-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray, P. R., P. Traynor, and D. Hopson. 1992. Critical assessment of blood culture techniques: analysis of recovery of obligate and facultative anaerobes, strict aerobic bacteria, and fungi in aerobic and anaerobic blood culture bottles. J. Clin. Microbiol. 30:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz, E., and M. A. Sande. 2000. Routine use of anaerobic blood cultures: are they still indicated? Am. J. Med. 108:505-506. [DOI] [PubMed] [Google Scholar]
- 14.Pottumarthy, S., and A. J. Morris. 1997. Assessment of the yield of anaerobic blood cultures. Pathology 29:415-417. [DOI] [PubMed] [Google Scholar]
- 15.Snyder, J. W., G. K. Munier, G. D. Bostic, P. S. Bozigar, and R. Hanna. 2002. Evaluation of a plastic nonvented aerobic blood culture bottle for use with the BacT/ALERT microbial detection system. J. Clin. Microbiol. 40:4757-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorpe, T. C., M. L. Wilson, J. E. Turner, J. L. DiGuiseppi, M. Willert, S. Mirrett, and L. B. Reller. 1990. BacT/Alert: an automated colorimetric microbial detection system. J. Clin. Microbiol. 28:1608-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson, M. L., M. P. Weinstein, S. Mirrett, L. G. Reimer, R. J. Feldman, C. R. Chuard, and L. B. Reller. 1995. Controlled evaluation of BacT/alert standard anaerobic and FAN anaerobic blood culture bottles for the detection of bacteremia and fungemia. J. Clin. Microbiol. 33:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson, M. L., M. P. Weinstein, L. G. Reimer, S. Mirrett, and L. B. Reller. 1992. Controlled comparison of the BacT/Alert and BACTEC 660/730 nonradiometric blood culture systems. J. Clin. Microbiol. 30:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahar, J. R., H. Farhat, E. Chachaty, P. Meshaka, S. Antoun, and G. Nitenberg. 2005. Incidence and clinical significance of anaerobic bacteraemia in cancer patients: a 6-year retrospective study. Clin. Microbiol. Infect. 11:724-729. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi, A. K., A. L. Knaut, S. Mirrett, and L. B. Reller. 1995. Value of routine anaerobic blood cultures for pediatric patients. J. Pediatr. 127:263-268. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler, R., I. Johnscher, P. Martus, D. Lenhardt, and H. M. Just. 1998. Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections. J. Clin. Microbiol. 36:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]