Abstract
Aspergillus terreus isolates collected from patients at The M. D. Anderson Cancer Center in Houston, TX, and at The University Hospital of Innsbruck, Austria, were analyzed using random amplification of polymorphic DNA-PCR with three different primers. No strain similarity in either institution was detected, indicating great genetic diversity of A. terreus.
Invasive aspergillosis (IA) has emerged as a leading cause of morbidity and mortality in immunocompromised patients (5). Aspergillus fumigatus is frequently isolated from IA, followed by Aspergillus flavus, Aspergillus niger, and Aspergillus terreus (6, 9). A. terreus in particular is an amphotericin B-resistant fungus that has been recognized as a cause of lethal infections (11, 19). Although A. terreus accounts for a minority of cases of IA, ranging in incidence from 1% (14) to 6% to 12% (7, 8), it is a rather common cause of IA in some geographically disparate institutions, such as The University of Texas M. D. Anderson Cancer Center (MDACC) in Houston, TX (6), and The University Hospital of Innsbruck (UHI), Austria (9).
Whether this increased incidence of IA caused by A. terreus in these two institutions has resulted from spread of a clonal population or reflects unique ecological factors is unknown. To answer this question, we analyzed all consecutive A. terreus isolates collected from patients with IA at MDACC (n = 33; 1998 to 2005) and UHI (n = 26; 1999 to 2005) over the past years using molecular genotyping.
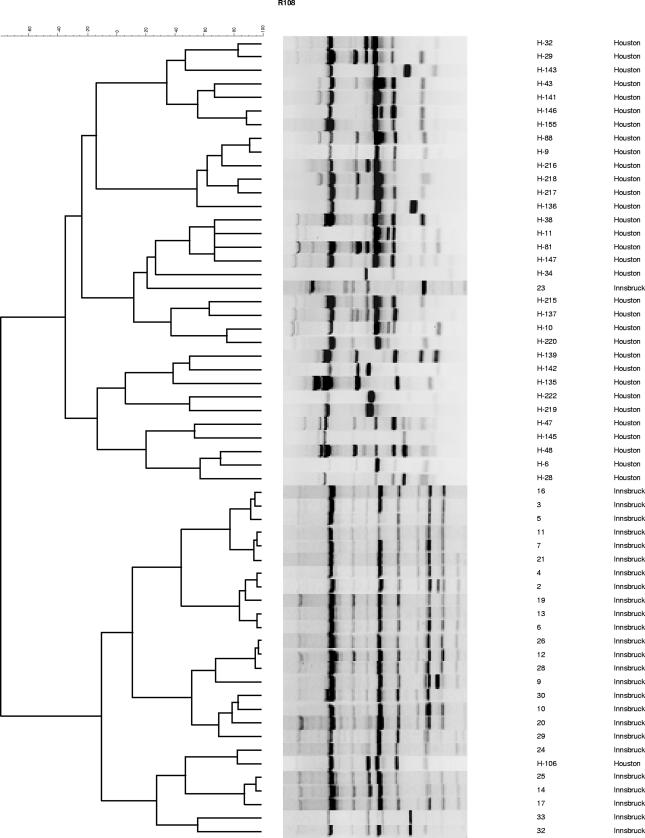
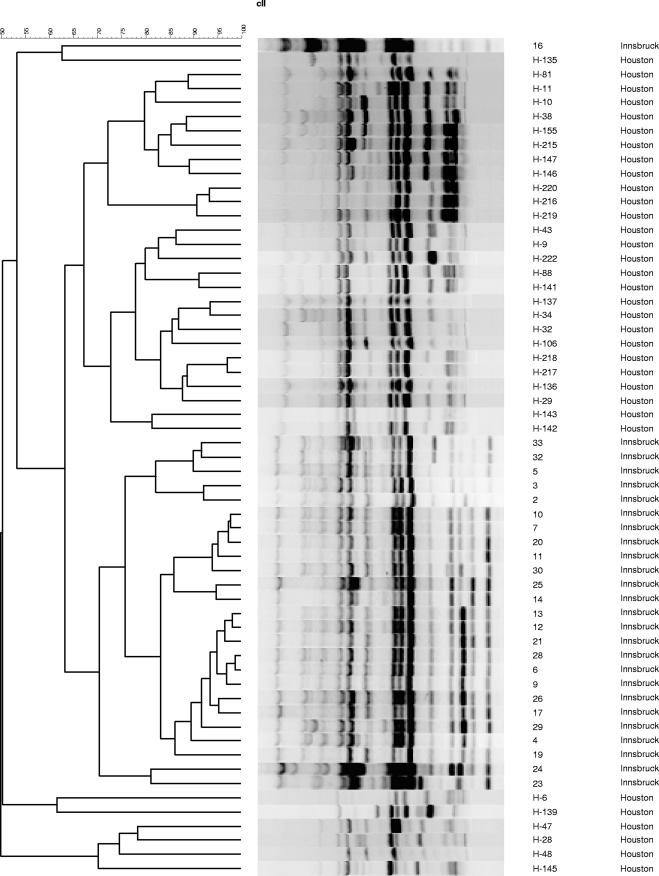
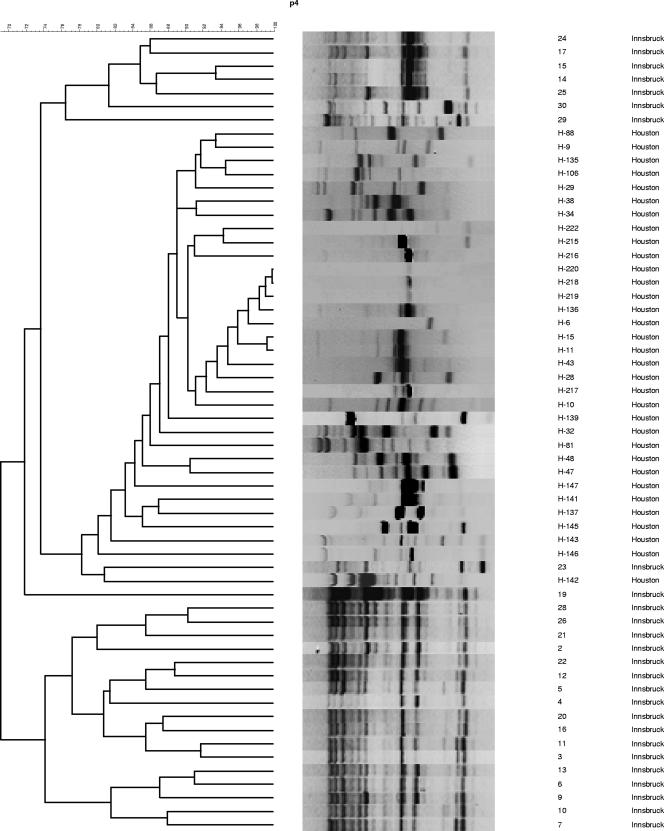
All 59 isolates were obtained from 57 patients with hematological malignancies and IA (European Organization for Research and Treatment of Cancer /Mycoses Study Group criteria) (1). Morphological identification of A. terreus was performed using standard methods (15). Isolates were stored either in water at room temperature or in 20% glycerol solution at −80°C and subcultured on Sabouraud agar at 30°C. Chromosomal DNA was extracted using the QIAamp tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer's recommendations. The clonality of the isolates was determined using random amplification of polymorphic DNA (RAPD)-PCR with primers R108 (5′-GTATTGCCCT-3′), CII (5′-GCGCACGG-3′), and P4 (5′-GATAGATAGATAGAT-3′) (10). A 5-μl template containing 50 ng of chromosomal DNA, 2.5 μl of each primer (20 pmol), and 17.5 μl of water was mixed with Ready-To-Go RAPD analysis beads (Amersham Pharmacia Biotech, Freiburg, Germany)-containing PCR buffer (30 mM KCl, 10 mM Tris-HCl, pH 8.3, 2.5 μg of bovine serum albumin, 3 mM MgCl2, 0.4 mM [each] deoxynucleoside triphosphates) and 1 U of AmpliTaq DNA polymerase (Roth, Graz, Austria) for amplification. Thermocycling was performed using a PCR cycler (UNO II; Biometra, Göttingen, Germany), and amplification products were analyzed using gel electrophoresis on a horizontal 1.8% agarose gel in 0.5 M Tris-borate-EDTA running buffer for 6 h at 80 V at room temperature. DNA band patterns were analyzed using the GelCompar II software program (Applied Maths, Sint-Martens-Latem, Belgium). Highly related strains were defined as those having at least 90% homology based on banding patterns (3). Each RAPD reaction, for each of the three primers used in this study, was performed at least twice to assess the stability and reproducibility of the profiles before an arbitrary type was assigned. In the MDACC A. terreus strain collection, we identified 18, 7, and 28 distinct strains using primers R108, CII, and P4, respectively; in the UHI strain collection, we identified 12, 16, and 19 strains using R108, CII, and P4, respectively (Fig. 1, Fig. 2, and Fig. 3). By combining the results of our analysis using these three primers, we identified 33 (types I to XXXIII) and 26 (types 1 to 26) distinct profiles in the MDACC and UHI collections. We found no strain similarity in the two collections, indicating great genomic diversity of A. terreus. Some (4) but not all (17) studies examining the genetic diversity of A. fumigatus strains have had similar findings. Thus far, we have not identified homology within the MDACC and UHI A. terreus isolates, indicating that patients were infected with unique strains, making nosocomial transmission of IA highly unlikely. In two patients, IA was caused by two A. terreus strains having different genotypes, a phenomenon also described in patients infected with A. fumigatus (13).
FIG. 1.
RAPD-PCR analysis of 56 A. terreus isolates using primer R108. Samples were collected from patients in Houston, TX, and Innsbruck, Austria.
FIG. 2.
RAPD-PCR analysis of 56 A. terreus isolates using primer CII. Samples were collected from patients in Houston, TX, and Innsbruck, Austria.
FIG. 3.
RAPD-PCR analysis of 56 A. terreus isolates using primer P4. Samples were collected from patients in Houston, TX, and Innsbruck, Austria.
RAPD-PCR typing of A. terreus has proven useful in many epidemiological investigations (2, 16). Varga et al. (18) applied RAPD-PCR and showed a high degree of variability among A. terreus isolates, even among those indistinguishable based on their internal transcribed spacer sequences. From 1993 to 1995, A. terreus infections accounted for 30% of cases of IA in UHI and were associated with hospital plants (10). RAPD-PCR identified the A. terreus isolates from four patients to be identical to those found in the environment. Yet, these epidemic strains were no longer observed, as the source of infection had been removed. The clonal isolates from that outbreak showed no homology with the latter native strains (data not shown) from the present survey. Although the incidence of IA decreased after a laminar airflow unit was built, A. terreus infections still occurred, albeit at a lower frequency.
The fact that infections caused by A. terreus have been prevalent at MDACC and UHI for more than 10 years and that we were not able to detect a clonal population in either center in the present study supports the notion that most cases of IA caused by A. terreus, at least in our respective geographical regions, are caused by native strains. Our findings cannot exclude the possibility of the existence of A. terreus clades within both geographic regions or even the possibility of some elements of geographic isolation. Only examination of a larger set of isolates could answer these questions.
The reason for the high incidence of A. terreus infections in specific geographic pockets remains enigmatic. Many factors, such as unique environmental exposure, specific host-related characteristics, the net state of immunosuppression of the affected patients, and extensive use of systemic antifungal agents for prophylaxis and empirical therapy (12) may partially account for this trend. Further studies are needed to determine which environmental niches are associated with exposure to A. terreus. Routine surface monitoring and air sampling for A. terreus may be useful in identifying potential exposure to and sources of this species. A limitation of the present study was that environmental isolates were not typed.
In conclusion, our molecular typing results with a sizable collection of A. terreus from geographically distant centers—a specialty oncology center in the southern United States and a tertiary care general hospital in Central Europe—show that A. terreus displayed tremendous genetic diversity and that nosocomial acquisition of an A. terreus clonal strain was highly unlikely.
(This work was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2006.)
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Ascioglu, S., J. Rex, B. De Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. Walsh, on behalf of the invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Baddley, J. W., P. Pappas, A. C. Smith, and S. Moser. 2003. Epidemiology of Aspergillus terreus at a university hospital. J. Clin. Microbiol. 41:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch, M., and D. W. Denning. 1999. Molecular typing of Aspergillus terreus by random amplification of polymorphic DNA. Eur. J. Clin. Microbiol. Infect. Dis. 18:838-841. [DOI] [PubMed] [Google Scholar]
- 4.Chazalet, V., J.-P. Debeaupuis, J. Sarfati, J. Lortholary, P. Ribaud, P. Shah, M. Cornet, H. Vu Thien, E. Gluckman, G. Brücker, and J.-P. Latge. 1999. Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J. Clin. Microbiol. 36:1494-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 6.Hachem, R. Y., D. P. Kontoyiannis, M. R. Boktour, C. Afif, C. Cooksley, G. P. Bodey, I. Chatzinikolaou, C. Perego, H. M. Kantarjian, and I. I. Raad. 2004. Aspergillus terreus: an emerging amphotericin B-resistant opportunistic mold in patients with hematologic malignancies. Cancer 101:1594-1600. [DOI] [PubMed] [Google Scholar]
- 7.Herbrecht, R., D. Denning, T. F. Patterson, D. Bennett, E. Greene, J.-W. Oestmann, W. Kern, A. K. Marr, P. Ribaud, O. Lortholary, R. Sylvester, J. Wigard, R. Rubin, P. Stark, C. Durand, D. Caillot, T. Eckhard, P. H. Chandrasekar, M. Hodges, H. Schlamm, P. Troke, and P. Ben de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 8.Iwen, P. C., M. E. Rupp, A. N. Langnas, E. C. Reed, and S. H. Hinrichs. 1998. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-years experience and review of the literature. Clin. Infect. Dis. 26:1092-1097. [DOI] [PubMed] [Google Scholar]
- 9.Lass-Flörl, C., K. Griff, A. Mayr, A. Petzer, H. Bonatti, M. Freund, G. Kropshofer, M. P. Dierich, and D. Nachbaur. 2005. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single center experience. Br. J. Haematol. 131:201-207. [DOI] [PubMed] [Google Scholar]
- 10.Lass-Flörl, C., P. M. Rath, D. Niederwieser, G. Kofler, R. Würzner, A. Kreczy, and M. P. Dierich. 2000. Aspergillus terreus infections in haematological malignancies: molecular epidemiology suggests association with in-hospital plants. J. Hosp. Infect. 46:31-35. [DOI] [PubMed] [Google Scholar]
- 11.Lass-Flörl, C., G. Kofler, G. Kropshofer, J. U. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 12.Lionakis, M., R. Russell, H. Torres, N. Albert, I. Raad, and D. Kontoyiannis. 2005. Increased frequency of non-fumigatus Aspergillus species in amphotericin B- or triazole-pre-exposed cancer patients with positive cultures for aspergilli. Diagn. Microbiol. Infect. Dis. 52:15-20. [DOI] [PubMed] [Google Scholar]
- 13.Menotti, J., J. Waller, O. Meunier, V. Letscher-Bru, R. Herbrecht, and E. Candolfi. 2005. Epidemiological study of invasive pulmonary aspergillosis in a haematology unit by molecular typing of environmental and patient isolates of Aspergillus fumigatus. J. Hosp. Infect. 60:61-68. [DOI] [PubMed] [Google Scholar]
- 14.Perfect, J., G. Cox, J. Y. Lee, C. A. Kauffman, L. Repentigny, S. W. Chapman, V. A. Morrison, P. Pappas, J. W. Hiemenz, D. Stevens, and Mycoses Study Group. 2001. The impact of culture isolation of Aspergillus species: a hospital based survey of aspergillosis. Clin. Infect. Dis. 33:1824-1833. [DOI] [PubMed] [Google Scholar]
- 15.Raper, K. B., and D. I. Fennel. 1965. Aspergillus terreus group, p. 293-344. In K. B. Raper and D. I. Fennel (ed.), The genus Aspergillus. The Williams & Wilkins Co., Baltimore, MD.
- 16.Rath, P. M., S. Kamphoff, and R. Ansorg. 1999. Value of different methods for the characterisation of Aspergillus terreus strains. J. Med. Microbiol. 48:161-166. [DOI] [PubMed] [Google Scholar]
- 17.Rydholm, C., G. Szakacs, and F. Lutzoni. 2006. Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot. Cell 5:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga, J., B. Toth, S. Kocsube, B. Farkas, G. Szakacs, J. Teren, and Z. Kozakiewicz. 2005. Evolutionary relationships among Aspergillus terreus isolates and their relatives. Antonie van Leeuwenhoek 88:141-150. [DOI] [PubMed] [Google Scholar]
- 19.Walsh, T., V. Petraitis, R. Petraitiene, A. Field-Ridely, D. A. Sutton, M. Ghannoum, T. Sein, R. Schaufele, J. Peter, J. Bacher, H. Casler, D. Armstrong, A. Espinel-Ingroff, M. Rinaldi, and C. A. Lyman. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305-319. [DOI] [PubMed] [Google Scholar]