Abstract
Epstein-Barr virus (EBV) is a gammaherpesvirus infecting the majority of the human adult population in the world. TLR2, a member of the Toll-like receptor (TLR) family, has been implicated in the immune responses to different viruses including members of the herpesvirus family, such as human cytomegalovirus, herpes simplex virus type 1, and varicella-zoster virus. In this report, we demonstrate that infectious and UV-inactivated EBV virions lead to the activation of NF-κB through TLR2 using HEK293 cells cotransfected with TLR2-expressing vector along with NF-κB-Luc reporter plasmid. NF-κB activation in HEK293-TLR2 cells (HEK293 cells transfected with TLR2) by EBV was not enhanced by the presence of CD14. The effect of EBV was abrogated by pretreating HEK293-TLR2 cells with blocking anti-TLR2 antibodies or by preincubating viral particles with neutralizing anti-EBV antibodies 72A1. In addition, EBV infection of primary human monocytes induced the release of MCP-1 (monocyte chemotactic protein 1), and the use of small interfering RNA targeting TLR2 significantly reduced such a chemokine response to EBV. Taken together, these results indicate that TLR2 may be an important pattern recognition receptor in the immune response directed against EBV infection.
The innate immune response is mediated by cellular receptors programmed to recognize several conserved motifs on invading pathogens. A major molecular mechanism for innate immune recognition of pathogens is now known to involve the Toll-like receptor (TLR) gene family (1, 29). The TLR(s) recognizes pathogen-associated molecular patterns that are common to many organisms. Several studies have identified bacterial ligands and viral ligands recognized by a TLR(s), demonstrating that TLRs play a critical role in protective immunity against microorganisms (10, 43).
Regarding the Herpesviridae family, previous reports have demonstrated recognition of human members of this family by a TLR(s). Using HEK293 cells stably transfected with TLR2 expression vector, Compton et al. (13) showed that live or UV-inactivated human cytomegalovirus (CMV) virions trigger inflammatory cytokine production through TLR2-dependent activation of NF-κB. Authors also demonstrated that TLR2 activation was facilitated by the presence of CD14. Herpes simplex virus type 1 (HSV-1) was also reported to interact with TLR2 to mediate the release of inflammatory cytokines (30). Recognition of HSV-1 virions by TLR2 was demonstrated by using TLR2-transfected cells and by stimulating peritoneal cells isolated from TLR2-deficient mice with HSV-1. By using a mouse model of encephalitis, authors concluded that HSV-induced neuropathogenesis was associated with an inflammatory response mediated by a TLR2-dependent pathway. Moreover, it was reported that TLR2 is required for the production of inflammatory cytokines by microglial cells in response to HSV-1, thus supporting a role for TLR2 in the pathogenesis of HSV-1-induced encephalitis (2). More recently, varicella-zoster virus (VZV) was also found to induce the release of interleukin-6 (IL-6) in primary human monocytes in a TLR2-dependent manner (45). Thus, it seems likely that TLR2 can recognize pathogen-associated molecular patterns conserved on virions from different subfamilies of herpesvirus.
Epstein-Barr virus (EBV) is a human gammaherpesvirus recognized as the etiological agent of infectious mononucleosis, a lymphoproliferative disorder generally observed in young adults. Although EBV infection is benign in the majority of the population, EBV is associated with the development of undifferentiated nasopharyngeal carcinoma and Burkitt's lymphoma. Detection of viral DNA in a significant fraction of neoplastic tissues has also associated EBV with other types of cancer, such as Hodgkin's lymphoma, adult T-cell leukemia, and different epithelial cell malignancies (reviewed in reference 31). While B cells are the key EBV target leading to expansion of EBV-positive cells and establishment of latency, growing evidence shows that many other cell types are permissive to EBV infection. We have previously reported that EBV infects and modulates the profile of proinflammatory cytokines released by primary human monocytes (39; reviewed in reference 41). For many viruses, including EBV, the first wave of cytokine/chemokine production is an important step for the eventual outcome of the infection. EBV can trigger cells of the immune system to produce inflammatory mediators by two main mechanisms: the first is mediated by the interaction of a protein(s) of the EBV envelope with cellular surface proteins or receptors that may activate cytokine gene expression, while the second requires viral protein expression or viral replication following virus entry into the cell (for a review, see reference 41).
In the present study, using TLR2-transfected HEK293 cells and human monocytes, we provide evidence that EBV can specifically activate cells via TLR2-dependent signaling, a process that may contribute to the production of inflammatory cytokines during EBV infection.
MATERIALS AND METHODS
Cells and culture conditions.
Peripheral blood mononuclear cells (PBMCs) were derived from fresh heparinized blood samples from healthy adult donors after they gave informed consent. PBMCs were isolated over a lymphocyte separation medium density gradient. PBMCs were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and submitted to an adherence phase on autologous serum-treated petri dish in order to isolate monocytes from lymphocytes. Monocytes were further enriched using a cell-sorting procedure (Epics Elite-ESP; Beckman Coulter, Ontario, Canada). This resulted in 99% pure monocyte suspensions and cell viability of ∼99%, as evaluated by trypan blue dye exclusion procedure.
Human embryonic kidney HEK293 cell line used in this study was grown in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated FBS.
Viral preparations.
Preparation of EBV (strain B95.8) was produced as described previously (4). Briefly, B95.8-infected cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS in the presence of 20 ng/ml phorbol myristate acetate (PMA), a known inducer of viral replication. Viral titers were evaluated and expressed as transforming units per milliliter. Cell-free supernatants collected from B95.8-infected cells not exposed to PMA were processed as described above and used as mock controls. Similarly, Raji and BJAB cell lines were disrupted by sonication, and the sonicates were ultracentrifuged and used to treat transfected HEK293 cell cultures when indicated. Viral preparations from EBV-positive Akata cells stimulated with anti-immunoglobulin G (anti-IgG) antibodies, IgG-stimulated EBV-negative Akata cells (mock controls), EBV-positive AGS cells stimulated with PMA, and PMA-stimulated EBV-negative AGS cells (mock controls) were produced as described previously (9, 24). In selected experiments, EBV virions were inactivated by heating (1 h, 60°C) (8, 36) or by exposure to UV (1 h, 265 nm) (3) prior to cell treatment. The specificity of the EBV response was evaluated by pretreatment of viral preparations with the neutralizing monoclonal antibody 72A1 (ATCC, Manassas, VA) raised against the viral envelope gp350/220 of EBV as described previously (23). 72A1 F(ab′)2 fragments were generated using an immobilized pepsin kit (Pierce, Rockford, IL) following the manufacturer's instructions.
Plasmids.
pUNO-hTLR2-HA, pUNO-hTLR4-HA, and pDUO2-hMD2-CD14 expression vectors were purchased from Invivogen (San Diego, CA).
Luciferase assay.
HEK293 cells were transiently cotransfected with selected expression plasmids using Escort V transfection reagent (Sigma-Aldrich, Oakville, ON, Canada) along with NF-κB luciferase reporter plasmid. Briefly, cells were seeded at 5 × 104 cells per well in a 24-well plate 1 day prior to transfection. Transfection efficiencies of pUNO-hTLR2-HA, pUNO-hTLR4-HA, and pDUO2-hMD2-CD14 in HEK293 cells were evaluated by flow cytometry analysis using antihemagglutinin (anti-HA) and anti-CD14 monoclonal antibodies. Forty-eight hours posttransfection, cells were mock infected or infected with EBV for the indicated times and multiplicity of infection (MOI) or treated with cellular membrane preparations. In some cases, cells were pretreated with polymyxin B (10 μg/ml) (Sigma-Aldrich, Oakville, ON, Canada) for 30 min prior to mock stimulation or EBV stimulation. For positive controls, some samples were stimulated for 8 h with lipoteichoic acid (LTA from Staphylococcus aureus, a TLR2 ligand) at 20 μg/ml or with lipopolysaccharide (LPS) (Escherichia coli 0111:B4, a natural ligand of TLR4) (Sigma-Aldrich, Oakville, ON, Canada). In some experiments, cells were pretreated for 1 h prior to EBV stimulation with blocking anti-TLR2 antibodies (HyCult Biotechnology, Uden, The Netherlands) at 6 μg/ml or stimulated with EBV particles preincubated with 72A1 F(ab′)2 fragments at the indicated concentrations. Following stimulation, cells were lysed in luciferase buffer (1% Triton, 10% glycerol, 20 mM Tris phosphate, pH 7.8). Luciferase activity was measured by luminometry, and relative light units (RLU) were normalized by protein dosage using BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
siRNA transfection.
Negative-control scrambled small interfering RNA (siRNA) and TLR2-targeting siRNA (sense, GCCUUGACCUGUCCAACAtt; the lowercase letters represent two deoxy bases that serve as overhangs for cleavage by dicer) were predesigned by Ambion Inc. (Austin, TX) and used following the manufacturer's instructions. Transfection of monocytes was performed using Escort V transfection reagent (Sigma-Aldrich). Monocytes were seeded at 2 × 106 cells per well in a 12-well plate. The next day, cells were transfected with 100 nM siRNAs for 4 h. Medium was then replaced, and cells were kept in culture for an additional 48 h prior to stimulation.
RPA.
Monocytes (10 × 106 cells) were stimulated or not with infectious EBV or UV-treated EBV (MOI of 0.5) for 4 h, before total RNA was extracted using TRIzol reagent (Invitrogen Canada Inc., Burlington, ON, Canada). Chemokine mRNAs were measured by a RNase protection assay (RPA) as previously described (27). The probe set hCK5 from BD Pharmingen (San Diego, CA) was used. It hybridizes to lymphotaxin, RANTES, IP-10 (interferon-inducible protein of 10 kDa), MIP-1α/β (macrophage inflammatory protein 1α/β), MCP-1 (monocyte chemotactic protein 1), and IL-8, as well as to the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Enzyme-linked immunosorbent assay (ELISA).
Forty-eight hours posttransfection of siRNA, monocyte cultures were stimulated or not with EBV or TLR2 ligand for 8 h. Supernatants from mock-stimulated, LTA-stimulated, and EBV-stimulated monocyte cultures were harvested, and the presence of MCP-1 was determined using a commercially available enzyme immunometric assay (eBIOSCIENCES, San Diego, CA). The detection limit for MCP-1 was 7 pg/ml.
Statistical analysis.
Data were analyzed by one-tailed analysis of variance followed by Newman-Keuls post-hoc test using PRISM3 software. Differences were considered significant at a P of ≤0.05.
RESULTS
EBV activates NF-κB through TLR2.
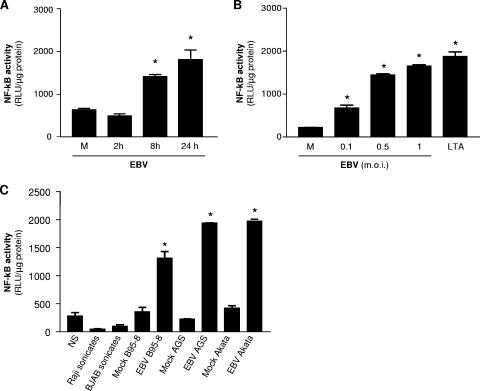
Different members of the herpesvirus family have been reported to lead to NF-κB activation via TLR2 signaling. In the first set of experiments, HEK293 cells were transiently cotransfected with TLR2 and NF-κB luciferase reporter plasmids and stimulated or not with EBV or LTA, a natural ligand for TLR2. As shown in Fig. 1A, EBV treatment activates NF-κB in a time-dependent manner via TLR2. Furthermore, TLR2-dependent NF-κB activation by EBV was also found to be dose dependent as reflected by the luciferase activities measured in cotransfected HEK293 cells stimulated with increasing quantities of EBV virions (Fig. 1B). We also tested the possibility that EBV preparations from different cellular origins have the capacity to activate NF-κB via TLR2 signaling. As shown in Fig. 1C, all three different EBV preparations, B95-8, Akata, and AGS, were able to activate NF-κB via TLR2 compared to their respective mock treatments. Treatment of TLR2-transfected HEK293 cells with sonicates from Raji and BJAB cells were not found to induce NF-κB activation. This control experiment using cell sonicates demonstrates that EBV-mediated activation of NF-κB via TLR2 is virus specific and is not the result of contaminating membranes or cell organelles present in virus preparations. In addition, treatment of TLR2 expressing HEK293 cells with cell-free supernatants from PMA-stimulated Raji, BJAB, and Akata EBV-negative cells was found to have no effect on NF-κB activation (data not shown).
FIG. 1.
EBV activates TLR2 in transfected HEK293 cells. (A) HEK293 cells were transiently transfected with TLR2 and NF-κB luciferase reporter plasmid. Forty-eight hours posttransfection, cells were stimulated with infectious EBV (MOI of 0.5) for 0 (mock stimulated [M]), 2, 8, and 24 h followed by luciferase assay. (B) HEK293 cells were transiently transfected with TLR2 and an NF-κB luciferase reporter plasmid. Forty-eight hours later, cells were mock stimulated (M) or stimulated with infectious EBV (MOI of 0.1 to 1) for 8 h or stimulated with LTA (20 μg/ml), and luciferase assay was performed. (C) HEK293 cells were transiently transfected with TLR2 and NF-κB luciferase reporter plasmid. Forty-eight hours posttransfection, cells were stimulated with infectious EBV (MOI of 0.5) B95-8, Akata, or AGS preparations or with their respective mock controls for 8 h followed by luciferase assay. Cells were also treated with sonicates from Raji and BJAB cells as described in Materials and Methods. Assays were performed in triplicate, and results are expressed in relative light units (RLU) per microgram of protein (mean plus standard error of the mean [error bar]). Values that were significantly different (P < 0.05) from the value for the mock control (M) are indicated (*). NS, not stimulated.
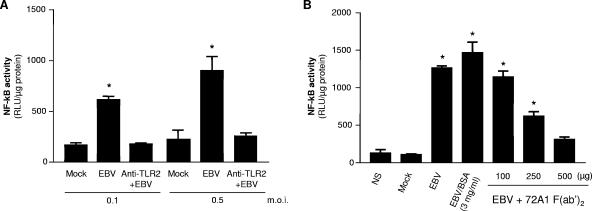
To further confirm that EBV is recognized by TLR2, we carried out two additional sets of experiments. First, blocking experiments were performed using anti-TLR2 antibodies. Because HEK293 cells do not constitutively express TLR2 and they were generated to express this receptor, blocking experiments using anti-TLR2 antibodies were thus chosen to confirm the action of EBV through TLR2. HEK293 cells were cotransfected with TLR2 expression vector and with NF-κB luciferase reporter plasmid and preincubated in the presence of blocking anti-TLR2 antibodies prior to treatment with EBV. While stimulation with EBV using two different MOIs leads to the activation of NF-κB (Fig. 2A), pretreatment of transfected HEK293 cells with blocking anti-TLR2 antibodies significantly reduced the effects of EBV, supporting the hypothesis that activation of NF-κB by EBV is mediated through TLR2. Second, the specificity of EBV-induced NF-κB activation in HEK293 cells transfected with TLR2 was also evaluated by pretreating EBV virions with different concentrations of F(ab′)2 fragments generated from digestion of 72A1 neutralizing antibodies prior to stimulation. 72A1 antibodies are known to recognize an epitope of EBV gp350/220, a heavily glycosylated protein of the viral envelope. F(ab′)2 fragments were used in order to rule out the possibility that inhibition of TLR2 signaling would be the result of a bivalent effect of complete 72A1 antibodies. Results obtained show that preincubation of virions with 72A1 F(ab′)2 fragments significantly abrogated the effect of EBV in a dose-dependent manner, reaching almost complete inhibition when 500 μg of F(ab′)2 fragments was used (Fig. 2B). The use of elevated concentrations of bovine serum albumin in EBV preparations did not affect EBV-mediated TLR2 activation, confirming that 72A1 inhibition is not the result of a nonspecific protein “crowding” effect. This fact further supports the hypothesis that the virus itself is responsible for TLR2-mediated activation of NF-κB. These results also suggest that an epitope of the viral envelope of EBV could be recognized by TLR2.
FIG. 2.
Blocking experiments inhibit EBV activation of NF-κB in HEK293 cells transfected with TLR2. (A) TLR2/NF-κB-transfected HEK293 cells were preincubated for 1 h at 37°C with blocking anti-TLR2 antibodies (6 μg/ml) prior to mock stimulation or stimulation with infectious EBV for 8 h, and the luciferase assay was performed. (B) Transiently transfected HEK293 cells were mock stimulated or stimulated with infectious EBV or with EBV particles preincubated with F(ab′)2 fragments of 72A1 monoclonal antibodies (37°C, 60 min) prior to cell treatment. In both panels A and B, assays were performed in triplicate, and results are expressed in relative light units (RLU) per microgram of protein (mean plus standard error of the mean [error bar]). Values that were significantly different (P < 0.05) from the value for the mock control (M) are indicated (*). NS, not stimulated; BSA, bovine serum albumin.
TLR4 does not recognize EBV virions.
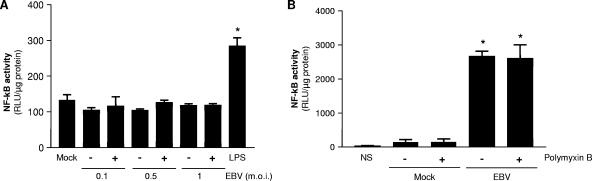
To further demonstrate that EBV-induced activation of NF-κB is specifically mediated through TLR2, we tested the effects of EBV treatment on HEK293 cells transfected with TLR4/CD14/MD-2 expression vectors. As shown in Fig. 3A, NF-κB activation was detected only in cells stimulated with LPS (a TLR4 ligand), as opposed to all EBV doses tested.
FIG. 3.
EBV is not recognized by TLR4. (A) HEK293 cells were transiently transfected with human TLR4, CD14/MD-2, and NF-κB luciferase reporter plasmid and were mock stimulated (−) or stimulated with EBV (+) or LPS (1 μg/ml), a natural ligand of TLR4, for 8 h. (B) HEK293 cells were transiently transfected with TLR2 and pretreated with polymyxin B (+) (10 μg/ml) (30 min, 37°C) before stimulation. Assays were performed in triplicate, and results are expressed in relative light units (RLU) per microgram of protein (mean plus standard error of the mean [error bar]). In panel A, values that were significantly different (P < 0.05) from the values for the mock-treated and EBV-treated cells are indicated (*). In panel B, values that were significantly different (P < 0.05) from the values for the mock-treated cells and cells that were not stimulated (NS) are indicated (*).
We next tested whether the effects of EBV observed in the viral preparations could be attributed to the presence of contaminating LPS. Viral preparations from B95-8 cells were treated with polymyxin B prior to stimulation of HEK293 cells transfected with TLR2 (Fig. 3B). The use of polymyxin B was found to have no effect on EBV-induced activation of NF-κB in HEK293 cells expressing TLR2.
Intact EBV virions are recognized by TLR2.
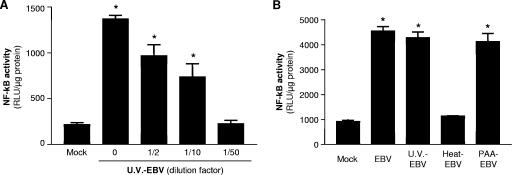
We have previously reported that PBMCs produce IL-6 in response to in vitro EBV infection (20, 21) and that monocytes were the predominant source of IL-6 among the circulating blood cells. Furthermore, we also demonstrated that viral adsorption was sufficient to trigger IL-6 expression by monocytes. Therefore, we evaluated the capacity of UV-irradiated EBV particles to trigger TLR2 and activate NF-κB. HEK293 cells were cotransfected with TLR2- and NF-κB-Luc-expressing vectors and stimulated with infectious EBV or with different doses of UV-irradiated virions. In addition, as in many other viruses, the viral envelope of EBV is sensitive to heat (36), and cellular activation is completely inhibited by heat denaturation. Heat-inactivated EBV particles were then used as negative controls. While UV-irradiated EBV lead to NF-κB activation in a dose-dependent manner (Fig. 4A), disruption of viral particles by heat was found to prevent activation of NF-κB in cotransfected HEK293 cells, supporting the need of intact virions in triggering TLR2 intracellular signaling (Fig. 4B). Since HEK293 cells are permissive to EBV infection (19), cells were also pretreated with phosphonoacetic acid (PAA), an inhibitor of viral DNA polymerase, prior to EBV treatment. As expected, EBV-mediated NF-κB activation was still detectable in TLR2-expressing cells treated with PAA, demonstrating that viral replication or expression of late viral genes are not required to activate TLR2-dependent intracellular events (Fig. 4B).
FIG. 4.
TLR2 activation by EBV is dependent on cell binding. (A) HEK293 cells were transiently transfected with TLR2 and NF-κB luciferase reporter plasmid and mock stimulated or stimulated with different concentrations of UV-irradiated EBV (U.V.-EBV) preparations. (B) TLR2-transfected cells were treated with infectious EBV or UV-irradiated or inactivated EBV or pretreated with phosphonoacetic acid (PAA) for 30 min (200 μg/ml) before stimulation with infectious EBV (PAA-EBV). Luciferase assays were performed in triplicate, and results are expressed in relative light units (RLU) per microgram of protein (mean plus standard error of the mean [error bar]). Values that were significantly different (P < 0.05) from the value for the mock control are indicated (*).
CD14 is not required for EBV activation of NF-κB through TLR2.
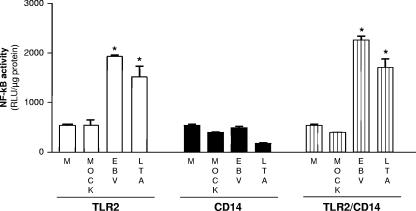
CD14 is a component of TLR2 and TLR4 complexes which was reported to facilitate ligand binding and signaling efficacy (reviewed in references 5 and 45). In order to evaluate whether triggering of TLR2 by EBV requires the presence of CD14, HEK293 cells were transiently transfected with either TLR2 or CD14/MD-2 expression vector or cotransfected with both expression vectors (TLR2/CD14/MD-2) along with NF-κB luciferase reporter plasmid and stimulated with EBV or LTA as a positive control. CD14 was transfected in HEK293 cells because this cell type is known to lack endogenous CD14 expression (17, 33). As shown in Fig. 5, NF-κB activation by EBV was clearly measured in TLR2-expressing cells. However, this activation was not significantly enhanced by the presence of CD14/MD2, suggesting that CD14 is not essential for EBV-mediated TLR2 activation. As expected, no signal was observed in HEK293 cells solely transfected with CD14/MD2 plasmid.
FIG. 5.
CD14 is not essential for TLR2 activation by EBV. HEK293 cells were transiently cotransfected with TLR2 and/or CD14/MD-2 and NF-κB luciferase reporter plasmid. Cells were mock stimulated or stimulated for 8 h with infectious EBV or LTA (20 μg/ml). Luciferase assays were performed in triplicate, and results are expressed in relative light units (RLU) per microgram of protein (mean plus standard error of the mean [error bar]). Values that were significantly different (P < 0.05) from the values for the medium (M) and mock control are indicated (*).
TLR2 is involved in EBV-activated MCP-1 gene in human monocytes.
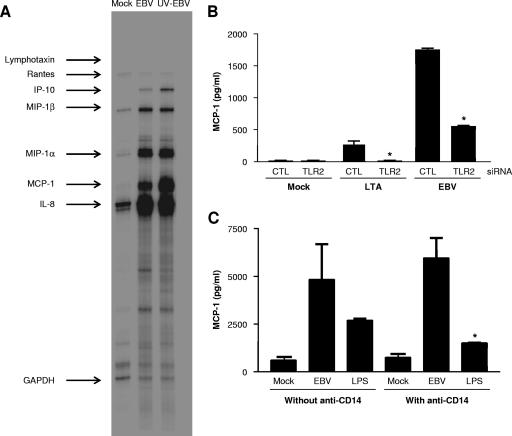
We have previously reported that EBV adsorption to primary human phagocytes leads to the release of proinflammatory cytokines (reviewed in reference 41). Thus, we wanted to verify whether activation of the chemokine gene profile following EBV binding to monocytes could be TLR2 dependent. Human monocytes, which constitutively express TLR2, were isolated from peripheral blood and transfected with TLR2-targeting siRNA to suppress TLR2 expression or with a scrambled sequence as a non-TLR2 siRNA control. Forty-eight hours posttransfection, monocytes were stimulated or not with infectious EBV or with LTA as a positive control. Following stimulation, the expression levels of MCP-1 in cell-free supernatants were measured by ELISAs. Figure 6A shows that different chemokine genes, such as IP-10, MIP-1α, MIP-1β, MCP-1, and IL-8 genes, are activated in monocytes by EBV and by UV-irradiated EBV particles, as reflected by the chemokine mRNA levels evaluated by RPA analysis. Interestingly, we have previously reported that production of the chemotactic factors MIP-1α and IL-8 by EBV-stimulated neutrophils was dependent on viral particle adsorption (34). In experiments using monocytes transfected with scrambled siRNA and stimulated with infectious EBV, appreciable amounts of MCP-1 were measured in culture supernatants (Fig. 6B). While LTA is a modest inducer of MCP-1 (11, 18), significant levels of MCP-1 were measured in supernatants of LTA-treated monocytes. In monocytes transfected with TLR2 siRNA, a complete inhibition of LTA-induced MCP-1 secretion was observed and a decrease of approximately 70% in MCP-1 response to EBV was measured in cell-free supernatants. These results demonstrate that TLR2 is implicated in EBV-induced release of MCP-1 by human monocytes.
FIG. 6.
EBV stimulation leads to TLR2-dependent secretion of MCP-1 by human monocytes. (A) Monocytes were isolated from peripheral blood samples from healthy donors and mock stimulated or stimulated with infectious EBV or UV-irradiated EBV (UV-EBV) (MOI of 0.5) for 4 h before total RNA was extracted. Chemokine mRNA levels were evaluated by RPAs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Monocytes were isolated from peripheral blood samples from healthy donors. Cells (2 × 106 cells) were seeded in 12-well plates and were transfected with either scrambled (control [CTL]) or TLR2 (100 nM) siRNAs. Forty-eight hours posttransfection, cells were mock stimulated or stimulated with infectious EBV (MOI of 0.5) or LTA (20 μg/ml) for 8 h. Cell-free supernatants were harvested, and MCP-1 levels were determined by ELISAs. (C) Monocytes from healthy donors were isolated and pretreated or not with anti-CD14 monoclonal antibodies for 1 h at 37°C before mock stimulation or stimulation with EBV (MOI of 0.5) or LPS (1 μg/ml). Twenty-four hours posttreatment, supernatants were harvested, and MCP-1 concentrations were measured by ELISAs. Assays were performed with monocytes isolated from three different donors (except for panel C in which monocytes from two donors were tested) tested in triplicate. Values that were significantly different (P < 0.05) from the values for the mock-stimulated cells and cells transfected with control (CTL) siRNA are indicated (*).
Since we observed that CD14 was not required for EBV-mediated TLR2 activation in transfected HEK293 cells, we have considered evaluating whether CD14 was essential for EBV to induce the release of MCP-1 by human primary monocytes. Enriched monocytes were treated or not with anti-CD14 antibodies prior to stimulation with EBV or LPS, and the levels of MCP-1 were measured in 18-hour culture supernatants. As expected (Fig. 6C), inhibition of CD14 had no effect on MCP-1 secretion induced by EBV in human monocyte, while pretreatment with anti-CD14 blocking antibodies significantly abrogated LPS-mediated MCP-1 release.
DISCUSSION
The immune system has evolved over time to recognize a wide variety of molecules expressed by microorganisms in order to exert a successful defense against infection. Intracellular signaling from the activation of TLRs leads to the secretion of different cytokines and antiviral mediators. Structural components of different viruses have been demonstrated to induce inflammatory cytokine production by triggering of TLR2. For example, hemagglutinin protein from measles virus interacts with TLR2 leading to IL-6 secretion by monocytes (6). Core and NS3 proteins from hepatitis C virus are also implicated in TLR2-dependent secretion of proinflammatory cytokines, such as IL-6, tumor necrosis factor alpha, and IL-8, by immune cells (16, 17). Hepatitis B virus capsid particles have the capability to activate TLR2 for cytokine production in macrophage-differentiated THP-1 cell line (14). Different members of the herpesvirus family were also found to activate inflammatory response via TLR2 (13, 30, 45).
In the present study, we show that intact EBV particles induce NF-κB activation in transfected HEK cells and chemokine secretion by primary monocytes in a TLR2-dependent manner. These results are consistent with our previous observations showing the requirement for specific interactions between a protein(s) of the viral envelope of EBV and elements of cytoplasmic membrane in the induction of inflammatory cytokines in human phagocytes (20, 21, 34, 39). Here, we propose that adsorption of EBV particles to the cell membrane TLR2 is one of the mechanisms associated with EBV, leading to the activation of the inflammatory response in monocytes. The EBV envelope is constituted of different glycoproteins that might serve as ligands for TLR2. Indeed, glycoproteins of the viral envelope are implicated in the binding to the cell and also in virus entry into the cells. Interactions of these viral proteins with the cell membrane may thus rapidly initiate detection of viral particles by the host's innate defense during the early stages of infection. EBV infection of target cells involves two main steps, the attachment mediated by the binding of viral envelope gp350/220 with the cellular receptor CD21 and the fusion of viral envelope with cell membrane, a process mediated by at least three viral proteins, gp25, gp42, and gp85. The EBV molecular pattern recognized by TLR2 is probably more complex than we imagine and may involve a combination of different structures of the viral envelope. However, the viral gp350, which is a major envelope glycoprotein that mediates binding to CD21+ cells and CD21− cells could provide an interesting approach to further study the link between TLR2 and cytokine production induced by EBV. We have previously reported that contact between a cell surface molecule and a protein of the viral envelope of EBV was sufficient to induce the release of granulocyte-macrophage colony-stimulating factor or IL-6 by monocytes (21, 35). More recently, we reported that EBV inhibits COX-2 (cyclooxygenase 2) activation in monocytes, a process that could be attributable to the failure of NF-κB activation following EBV entry into the cells (40). Interestingly, such inhibition of COX-2 was found to require the activation of DNA polymerase and the synthesis of a late EBV protein in infected cells. In fact, the use of UV-irradiated virions did not affect expression of COX-2 protein nor the translocation of NF-κB to the nucleus. Furthermore, this process was also prevented by the treatment of viral particles with 72A1 monoclonal antibodies, suggesting that the interaction of EBV gp350/220 (or another viral ligand by antibody steric hindrance) with a molecule expressed on the surfaces of monocytes (such as TLR2) may participate in the activation of NF-κB in monocytes. However, such activation of NF-κB was found to be inhibited following viral entry (40). In B cells, activation of NF-κB was also detected following EBV binding to its cellular receptor CD21 or pretreatment with recombinant gp350/220 (15, 42).
The results of our blocking experiments using F(ab′)2 fragments of anti-gp350 antibodies may suggest two different hypotheses concerning the viral epitope recognized by TLR2 following EBV binding on epithelial or monocyte cell surface. First, our results could suggest that EBV gp350/220 could be a TLR2 ligand. This was indicated by experiments using increasing concentrations of F(ab′)2 fragments generated from 72A1 antibodies raised against gp350/220. Prototypical ligands for TLR2 include lipoteichoic acids and lipoproteins, but this TLR seems to have a broad spectrum of ligands. As examples, nonconventional ligands, such as proteins, e.g., porin from Neisseria species (33), toxins such as seeligeriolysin O of Listeria seeligeri (25) and even amphotericin B, a polyene antifungal agent (38), were reported to activate TLR2 signaling. Second, and this is a more probable explanation, interaction between EBV and TLR2 could imply binding of a viral epitope that was hidden by the presence of antibodies to gp350/220. The fact that the neutralizing effect of 72A1 antibodies at higher concentrations did not totally abrogate TLR2 activation induced by EBV could indicate that a ligand(s) of the viral envelope might be sterically hindered by the presence of 72A1 and therefore inaccessible for recognition by TLR2. Other potential candidate ligands for TLR2 could be EBV gB and gH proteins. It has been reported that human CMV gB and gH protein interaction with TLR2 is essential for cytokine release (7). Authors have also demonstrated that gB coimmunoprecipitates with TLR2 and TLR1, showing direct interaction between these receptors and the viral protein. This might also be the case for EBV interaction with TLR2. The possible involvement of gB in the interaction between EBV and cell membrane might be suggested by the surprising observation made by Turk et al. using antibodies directed against EBV gp350/220 (44). Antibodies to gp350/220 were found to enhance infection of epithelial cells independently of CD21. The authors suggest that blocking of gp350/220 could facilitate access to other glycoproteins, such as gB of the viral envelope, that are more relevant to epithelial cell infection. Moreover, it has been documented that gp350/220-null virus is still able to infect epithelial cells (26). As mentioned earlier, the use of 72A1 antibodies is not irrefutable proof that gp350/220 is the ligand for TLR2, since antibodies could mask other viral epitopes by steric hindrance. Further experimentation is therefore necessary in order to determine the exact EBV epitope recognized by TLR2.
CD14 is recognized as a component of the TLR2 complex which participates in TLR2 signaling (reviewed in references 5 and 32). VZV and human CMV are two examples of herpesviruses that activate the production of inflammatory cytokines by human cells via TLR2 and CD14 (13, 45). In an attempt to evaluate whether CD14 is involved in TLR2 activation by EBV, we observed that CD14 seems to be dispensable for EBV-mediated TLR2 activation. In fact, NF-κB activation by EBV in TLR2-transfected HEK cells was not enhanced after transfection of the CD14 plasmid. We have previously demonstrated that CD14 is not involved in EBV signaling. Our previous studies showed that EBV does not modulate the expression level of CD14 on primary monocytes and also that treatment of monocytes with antibodies directed against CD14 does not prevent viral adsorption to the cell surface (40; also unpublished observations). Taken together, these results suggest that CD14 is not essential for EBV interactions with the target cell and that the composition of the TLR2 complex recognizing EBV particles is different from the complex involved in VZV and CMV recognition. TLR2 is known to use accessory molecules for microbial recognition. In fact, TLR2 forms a heterodimer with TLR1 or TLR6 for detection of different ligands, and in some cases, neither TLR1 nor TLR6 is required for molecular recognition (12). TLR2 may also signal as a homodimer to recognize different species of ligands. Moreover, depending on the nature of the ligand, the presence of CD14 is not absolutely required by all TLR2 complexes for signaling activity (28). In this regard, LTA requires CD36 rather than CD14 as a component of the TLR2/TLR6 complex for a full signaling efficacy (22). Identification of the TLR heterodimer (TLR2/TLRx) and the coreceptors participating in the recognition of EBV remain to be clarified.
Our results also suggest that EBV may also engage mechanisms other than TLR2 to induce the secretion of MCP-1 by human monocytes. While the cytokine response to LTA was totally suppressed in monocytes transfected with TLR2 siRNA, a 70% decrease in MCP-1 response to EBV was observed. Innate recognition of herpesviruses is now known to involve the detection of viral genomic double-stranded DNA by TLR9. It is then likely that following EBV entry into monocytes, viral DNA is recognized by TLR9, a process leading to the activation of cytokine gene expression. Therefore, EBV particles could first be detected by TLR2 at the surfaces of monocytes, and subsequently viral DNA could be recognized by intracellular TLR9. Such dual action of TLR2 and TLR9 was recently proposed by Sato et al. for recognition of herpes simplex viruses by dendritic cells (37).
Many pieces of evidence indicate that cytokines may play an important role in the pathogenesis of EBV infection. In this regard, elevated levels of IL-1, IL-6, and IL-8 were detected in sera from EBV patients. In tonsils, expression of several inflammatory cytokines, such as IL-8 and IP-10, was detected in EBV-infected cells and in the neighboring EBV-negative cells of the interfollicular region. Monocytes are a key cell type linking innate and adaptive immunity, and they have the capacity to migrate to lymphatic tissues. We can speculate that monocytes are involved in the production of chemokines in lymphoid organs through the activation of TLR2 and such a mechanism may participate in the recruitment of leukocytes, including B cells, during the early phase of infection.
The results presented in this study suggest that monocytes could contribute to the production of inflammatory mediators induced by EBV and also provide a mechanism linking the innate immune response to EBV pathogenesis. Further studies aimed at characterizing the interactions of EBV with the TLR system will certainly improve our knowledge on events of the inflammatory response activated during the early stages of EBV infection.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli, R. N., S. Hu, T. N. Rowen, J. M. Palmquist, and J. R. Lokensgard. 2005. TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189-4193. [DOI] [PubMed] [Google Scholar]
- 3.Bangham, C. R. M., J. J. Cannon, D. T. Karson, and B. A. Askomas. 1985. Cytotoxic T-cell response to respiratory syncytial virus in mice. J. Virol. 56:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu, A. D., R. Paquin, and J. Gosselin. 1995. Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood 86:2789-2798. [PubMed] [Google Scholar]
- 5.Beutler, B., Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, and K. Hoebe. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353-389. [DOI] [PubMed] [Google Scholar]
- 6.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehme, K. W., M. Guerrero, and T. Compton. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094-7102. [DOI] [PubMed] [Google Scholar]
- 8.Borovec, S., C. Broumis, W. Adcock, R. Fang, and E. Uren. 1998. Inactivation kinetics of model and relevant blood-borne viruses by treatment with sodium hydroxide and heat. Biologicals 26:237-244. [DOI] [PubMed] [Google Scholar]
- 9.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 10.Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. [DOI] [PubMed] [Google Scholar]
- 11.Brun, P., I. Castagliuolo, M. Pinzani, G. Palù, and D. Martines. 2005. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 289:571-578. [DOI] [PubMed] [Google Scholar]
- 12.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2005. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 35:282-289. [DOI] [PubMed] [Google Scholar]
- 13.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper, A., G. Tal, O. Lider, and Y. Shaul. 2005. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J. Immunol. 175:3165-3176. [DOI] [PubMed] [Google Scholar]
- 15.D'Addario, M., T. A. Libermann, J. Xu, A. Ahmad, and J. Menezes. 2001. Epstein-Barr virus and its glycoprotein-350 upregulate IL-6 in human B-lymphocytes via CD21, involving activation of NF-κB and different signaling pathways. J. Mol. Biol. 308:501-514. [DOI] [PubMed] [Google Scholar]
- 16.Dolganiuc, A., S. Oak, K. Kodys, D. T. Golenbock, R. W. Finberg, E. Kurt-Jones, and G. Szabo. 2004. Hepatitis C core and nonstructural 3 proteins trigger Toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127:1513-1524. [DOI] [PubMed] [Google Scholar]
- 17.Duesberg, U., A. von dem Bussche, C. Kirschning, K. Miyake, T. Sauerbruch, and U. Spengler. 2002. Cell activation by synthetic lipopeptides of the hepatitis C virus (HCV)-core protein is mediated by Toll like receptors (TLRs) 2 and 4. Immunol. Lett. 84:89-95. [DOI] [PubMed] [Google Scholar]
- 18.Ellaban, E., G. Bolgos, and D. Remick. 2004. Selective macrophage suppression during sepsis. Cell. Immunol. 231:103-111. [DOI] [PubMed] [Google Scholar]
- 19.Fingeroth, J. D., M. E. Diamond, D. R. Sage, J. Hayman, and J. L. Yates. 1999. CD21-dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 73:2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselin, J., L. Flamand, M. D'Addario, J. Hiscott, and J. Menezes. 1992. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses: differential induction of interleukin-6 and tumor necrosis factor alpha. J. Clin. Investig. 89:1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselin, J., L. Flamand, M. D'Addario, I. Stefanescu, D. V. Ablashi, R. C. Gallo, and J. Menezes. 1992. Modulatory effects of Epstein-Barr, herpes simplex and human herpes-6 viral infections and co-infections on cytokine synthesis: a comparative study. J. Immunol. 149:181-187. [PubMed] [Google Scholar]
- 22.Hoebe, K., P. Georgel, S. Rutschmann, X. Du, S. Mudd, K. Crozat, S. Sovath, L. Shamel, T. Hartung, U. Zahringer, and B. Beutler. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523-527. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman, G. J., S. G. Lazarowitz, and S. D. Hayward. 1980. Monoclonal antibody against a 250,000 Da glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc. Natl. Acad. Sci. USA 77:2979-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutt-Fletcher, L., and S. M. Turk. 2001. Virus isolation. Methods Mol. Biol. 174:119-123. [DOI] [PubMed] [Google Scholar]
- 25.Ito, Y., I. Kawamura, C. Kohda, K. Tsuchiya, T. Nomura, and M. Mitsuyama. 2005. Seeligeriolysin O, a protein toxin of Listeria seeligeri, stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int. Immunol. 17:1597-1606. [DOI] [PubMed] [Google Scholar]
- 26.Janz, A., M. Oezel, C. Kurzeder, J. Mautner, D. Pich, M. Kost, W. Hammerschmidt, and H. J. Delecluse. 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74:10142-10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaramillo, M., M. Godbout, and M. Olivier. 2005. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J. Immunol. 174:475-484. [DOI] [PubMed] [Google Scholar]
- 28.Jiang, Z., P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, M. Huber, C. Kalis, S. Keck, C. Galanos, M. Freudenberg, and B. Beutler. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6:565-570. [DOI] [PubMed] [Google Scholar]
- 29.Ku, C. L., K. Yang, J. Bustamante, A. Puel, H. von Bernuth, O. F. Santos, T. Lawrence, H. H. Chang, H. Al-Mousa, C. Picard, and J. L. Casanova. 2005. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol. Rev. 203:10-20. [DOI] [PubMed] [Google Scholar]
- 30.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutok, J. L., and F. Wang. 2006. Spectrum of Epstein-Barr virus-associated diseases. Annu. Rev. Pathol. Mech. Dis. 1:375-404. [DOI] [PubMed] [Google Scholar]
- 32.Manukyan, M., K. Triantafilou, M. Triantafilou, A. Mackie, N. Nilsen, T. Espevik, K. H. Wiesmuller, A. J. Ulmer, and H. Heine. 2005. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur. J. Immunol. 35:911-921. [DOI] [PubMed] [Google Scholar]
- 33.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 34.McColl, S. R., C. J. Roberge, B. Larochelle, and J. Gosselin. 1997. EBV induces the production and release of IL-8 and macrophage inflammatory protein-1 alpha in human neutrophils. J. Immunol. 159:6164-6168. [PubMed] [Google Scholar]
- 35.Roberge, C. J., B. Larochelle, M. Rola-Pleszczynski, and J. Gosselin. 1997. Epstein-Barr virus induces GM-CSF synthesis by monocytes: effect on EBV-induced IL-1 and IL-1 receptor antagonist production in neutrophils. Virology 238:344-352. [DOI] [PubMed] [Google Scholar]
- 36.Salek-Ardakani, S., S. A. Lyons, and J. R. Arrand. 2004. Epstein-Barr virus promotes human monocyte survival and maturation through a paracrine induction of IFN-α. J. Immunol. 173:321-331. [DOI] [PubMed] [Google Scholar]
- 37.Sato, A., M. M. Linehan, and A. Iwasaki. 2006. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. USA 103:17343-17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 39.Savard, M., C. Belanger, M. Tardif, P. Gourde, L. Flamand, and J. Gosselin. 2000. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 74:2612-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savard, M., C. Belanger, M. J. Tremblay, N. Dumais, L. Flamand, P. Borgeat, and J. Gosselin. 2000. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J. Immunol. 164:6467-6473. [DOI] [PubMed] [Google Scholar]
- 41.Savard, M., and J. Gosselin. 2006. Epstein-Barr virus immunosuppression of innate immunity mediated by phagocytes. Virus Res. 119:134-145. [DOI] [PubMed] [Google Scholar]
- 42.Sugano, K., W. Chen, M. L. Roberts, and N. R. Cooper. 1997. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-κB induction. J. Exp. Med. 186:731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda, K., and S. Akira. 2004. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34:73-82. [DOI] [PubMed] [Google Scholar]
- 44.Turk, S. M., R. Jiang, L. S. Chesnokova, and L. M. Hutt-Fletcher. 2006. Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. J. Virol. 80:9628-9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, J. P., E. A. Kurt-Jones, O. S. Shin, M. D. Manchak, M. J. Levin, and R. W. Finberg. 2005. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J. Virol. 79:12658-12666. [DOI] [PMC free article] [PubMed] [Google Scholar]