Abstract
Immune activation is a major characteristic of human immunodeficiency virus type 1 (HIV-1) infection and a strong prognostic factor for HIV-1 disease progression. The underlying mechanisms leading to immune activation in viremic HIV-1 infection, however, are not fully understood. Here we show that, following the initiation of highly active antiretroviral therapy, the immediate decline of immune activation is closely associated with the reduction of HIV-1 viremia, which suggests a direct contribution of HIV-1 itself to immune activation. To propose a mechanism, we demonstrate that the single-stranded RNA of HIV-1 encodes multiple uridine-rich Toll-like receptor 7/8 (TLR7/8) ligands that induce strong MyD88-dependent plasmacytoid dendritic cell and monocyte activation, as well as accessory cell-dependent T-cell activation. HIV-1-encoded TLR ligands may, therefore, directly contribute to the immune activation observed during viremic HIV-1 infection. These data provide an initial rationale for inhibiting the TLR pathway to directly reduce the chronic immune activation induced by HIV-1 and the associated immune pathogenesis.
Chronic human immunodeficiency virus type 1 (HIV-1) infection is characterized by persistent immune activation, and markers of immune cell activation strongly predict the rate of disease progression and CD4+ T-cell loss in untreated infection (12, 13). Moreover, immune activation decreases rapidly once antiretroviral therapy is initiated (15, 26, 43) and increases within days once HIV-1 viremia rebounds following interruption of antiretroviral therapy (28), suggesting that viral replication directly contributes to the high level of immune cell activation. One widely accepted model of HIV-1 immunopathogenesis postulates that heightened immune activation results in exhaustion of the immune system; accelerated activation, proliferation, and functional impairment and loss of T cells (18, 29, 31); and uncontrolled viral replication in activated T cells (16, 18, 29, 31). The precise mechanisms by which HIV-1 itself induces immune activation, though, are poorly understood.
Toll-like receptors (TLRs) are a family of pattern-recognition receptors involved in the initiation of the immune response, allowing TLR-expressing cells to directly recognize PAMPs (pathogen-associated molecular patterns) of pathogens such as bacteria and viruses (1, 25). TLR ligands have been shown to dramatically modulate dendritic cell function (3, 23, 24, 35), monocyte function (4, 27), and also the function of B and T cells (6, 32), which suggests that stimulation through TLRs can directly activate and modulate effector cells of both the innate and adaptive immune system. TLR ligation initiates complex signaling cascades, ultimately leading to NF-κB activation and subsequent differential gene expression (41), which can vary depending on which TLR has been activated. While bacterial PAMPs, such as lipopolysaccharide (LPS) and flagellin, can be detected by TLRs expressed on the cellular surface (42), the intracellularly located TLRs (TLR3, TLR7, TLR8, and TLR9) have been shown to detect viral RNA and unmethylated DNA (1) in an MyD88-dependent manner (10).
In this study, we demonstrate a significant correlation between the rapid reduction of immune activation and the decline of HIV-1 viremia following the initiation of highly active antiretroviral therapy (HAART), suggesting a direct role of HIV-1 itself, or components of HIV-1, in contributing to immune activation. Furthermore, we describe multiple uridine-rich TLR7/8 ligands encoded by the single-stranded RNA (ssRNA) of HIV-1 that can elicit strong immune activation in vitro, providing a mechanism underlying the induction of immune activation that represents a hallmark of viremic HIV-1 infection.
MATERIALS AND METHODS
Study participants.
Ten HIV-1-infected, treatment-naïve individuals enrolled at several clinics in northern Germany that initiated HAART (stavudine, dideoxyinosine, and nelfinavir) during chronic infection as part of the ARCHY study were studied before the initiation of HAART, as well as after 2, 4, 12, 24, 36, and 48 weeks on HAART. Viral loads were assessed by using the standard and ultrasensitive Roche Amplicor HIV-1 monitor assays. The ARCHY study was approved by the institutional review boards, and all subjects provided written informed consent before enrollment. Furthermore, samples from 12 HIV-1-negative subjects enrolled at Massachusetts General Hospital were included in the studies. The Massachusetts General Hospital Institutional Review Board approved the study, and each subject gave informed consent for participation in the study.
Assessment of markers of immune activation in HIV-1-infected individuals initiating HAART.
Cryopreserved peripheral blood mononuclear cells (PBMC) were used for all study subjects. All follow-up samples for an individual were tested simultaneously to reduce interassay variability. Samples were thawed, stained with CD3, CD4, CD8, HLA-DR, and CD38 antibodies (all BD Biosciences), washed, and fixed in 1% paraformaldehyde. Only CD8+ cells expressing CD38 at a high mean fluorescence intensity were considered to be positive for this marker. Samples using a monoclonal anti-Ki67 antibody (Dako) were fixed with 2% formaldehyde after staining of extracellular markers and then permeabilized in 0.1% saponin-bovine serum albumin before the addition of the antibody against the nuclear antigen Ki67. For all samples, at least 30,000 CD3+ events were acquired on a FACSCalibur system (BD).
Identification of uridine-rich ssRNA sequences within the HIV-1 genome HXB2 and taxonomy of RNA.
Uridine-rich regions containing more than 50% uridines within the ssRNA sequence of HXB2 were identified and synthesized (Invitrogen) and named according to the HIV-1 gene location and the first RNA nucleotide position relative to the coding domain (Table 1).
TABLE 1.
Uridine-rich regions within HIV-1 HXB2
| Region in HXB2 | Sequence | Name |
|---|---|---|
| Gag 1166-1185 | UUGUUAAGUGUUUCAAUUGU | GagRNA 1166 |
| A variant | AAGAAAAGAGAAACAAAAGA | GagRNA1166(A) |
| Gag 446-465 | UUGGUUGCACUUUAAAUUUU | GagRNA446 |
| A variant | AAGGAAGCACAAAAAAAAAA | GagRNA446(A) |
| Pol 806-825 | CAUAUUUUUCAGUUCCCUUA | PolRNA806 |
| A variant | CAAAAAAAACAGAACCCAAA | PolRNA806(A) |
| Pol2439-2458 | AUAUUUUCUUUUAAAAUUAG | PolRNA2439 |
| A variant | AAAAAAACAAAAAAAAAAAG | PolRNA2439(A) |
| VIF327-346 | GUAUUACUUUGACUGUUUUU | VIFRNA327 |
| A variant | GAAAAACAAAGACAGAAAAA | VIFRNA327(A) |
| VPR203-222 | UGUUUAUCCAUUUUCAGAAU | VPRRNA203 |
| A variant | AGAAAAACCAAAAACAGAAA | VPRRNA203(A) |
| GP160 523-542 | UUUUUUUAUAAACUUGAUAU | GP160RNA523 |
| A variant | AAAAAAAAAAAACAAGAAAA | GP160RNA523(A) |
| GP160 2090-2109 | UAGUUUUUGCUGUACUUUCU | GP160RNA2090 |
| A variant | AAGAAAAAGCAGAACAAACA | GP160RNA 2090(A) |
| GP160 2093-2112 | UUUUUGCUGUACUUUCUAUA | GP160RNA2093 |
| A variant | AAAAAGCAGAACAAACAAAA | GP160RNA 2093(A) |
Stimulation of PBMC by HIV-1-derived ssRNA and cytokine detection.
Fresh PBMC were separated from whole blood by Ficoll-Hypaque (Histopaque 1077; Sigma Aldrich) density gradient centrifugation. One million PBMC/ml were stimulated with 7.5 or 15 μg/ml ssRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP) (Roche), while unstimulated cells and cells treated only with DOTAP served as negative controls. Following 20 h of stimulation, the levels of tumor necrosis factor alpha (TNF-α) in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA) (mabtech). For the detection of intracellular cytokines using flow cytometric analysis, 0.5 μg/ml brefeldin A (Sigma) was added to each tube in order to inhibit cellular cytokine release. Chloroquine (Invivogen) was added as indicated below at a 100 μM concentration to inhibit lysosomal maturation. The TLR7-specific inhibitor IRS954 (5′-TGCTCCTGGAGGGGTTGT-3′) (Integrated DNA Technologies Inc.) was added at the concentrations specified in Fig. 6.
FIG. 6.
Immune activation by HIV-1-derived ssRNA oligonucleotides is MyD88 dependent and can be blocked by using a TLR7/9 antagonist. (A) TNF-α production, as determined by ELISA, of murine BMDM in C57BL/6 (wild type) and MyD88-KO mice after 20 h of stimulation with HIV-1-derived ssRNAs, the corresponding A variants, control ligands RNA40 and RNA41, Gardiquimod, or LPS, all complexed to DOTAP, or DOTAP alone (neg). Data represent the average results ± standard deviations of three independent experiments, each with pooled BMDM of two MyD88-KO and three C57BL/6 mice. (B to E) Normalized percentages of IFN-α+ (B and D) and TNF-α+ (C and E) pDCs after 20 h of stimulation with the HIV-1-derived ssRNA oligonucleotides GagRNA1166 (B and C) and VifRNA327 (D and E) in the absence or presence at increasing concentrations of the described TLR7/9 antagonist IRS954. Cytokine production in the absence of the antagonist was defined as 1. pDCs were defined as CD3neg CD19neg CD56neg CD14neg CD11cneg CD123bright cells. Data represent the average results ± standard deviations from four independent experiments.
Stimulation for all assays was conducted at 37°C and 5% CO2. The intracellular cytokine content of monocytes and plasmacytoid dendritic cells (pDCs) was determined following 20 h of stimulation. Briefly, cells were stained for surface (CD3-Alexa700, CD19-Alexa700, CD56-Alexa700, CD11c-phycoerythrin [PE] CD14-allophycocyanin [APC]-Cy7, and CD123-PE-Cy7) and intracellular markers (alpha interferon [IFN-α]-fluorescein isothiocyanate [PBL Biomedical Laboratories], interleukin 6 [IL-6]-APC, and TNF-α-PE-Cy7 [all from BD unless indicated otherwise]). pDCs were defined as CD3neg CD19neg CD56neg CD14neg CD11cneg CD123bright cells, and monocytes were defined as CD3neg CD19neg CD56neg CD14+ cells. Cells were fixed, permeabilized (Fix perm A and B; Caltag Laboratories, Inc.), and stained intracellularly for IFN-α, TNF-α, and IL-6. All samples were acquired on an LSR II (BD). The frequencies of cytokine-positive pDCs and monocytes, as well as the frequencies of CD69+ CD8+ CD3+ T cells, were determined by subsequent analysis using FlowJo software.
Stimulation of murine C57BL/6 and MyD88-KO BMDM.
Bone marrow-derived macrophages (BMDM) were prepared from C57BL/6 mice (Charles River Laboratories) and MyD88-knockout (KO) mice on a C57BL/6 background (a gift from W. Chao, Massachusetts General Hospital, Charlestown, MA), as described previously (37). Briefly, stem cells were flushed from femurs and placed in Teflon bags (BioFOLIE; Sartorius). The cells were incubated in L929 supernatant-containing medium for 7 days to allow for proliferation and differentiation and were harvested and stored at −80°C at a concentration of 1 × 107/ml until use. Prior to stimulation, cells were thawed, plated in 96-well plates at a density of 4 × 105 per sq cm of plating surface, and incubated at 37°C and 5% CO2 for 24 h. Adherent BMDM were stimulated with 15 μg/ml ssRNA or 2 μg/ml Gardiquimod, both complexed with DOTAP (Roche), while unstimulated cells and cells treated with DOTAP alone served as negative controls. As a positive control, LPS plus DOTAP was added at 100 ng/ml as indicated below. The cells were subsequently incubated for 20 h at 37°C and 5% CO2, and the TNF-α levels were quantified in supernatants by ELISA (R&D Systems, Minneapolis, MN). The Institutional Animal Care and Use Committee at the Massachusetts General Hospital approved the animal studies.
Purification of human cell subsets.
For CD8+ T-cell purification, blood specimens were drawn in acid citrate dextrose tubes (BD), incubated with a tetrameric antibody complex mediating cell enrichment from whole blood (Rosette Sep human CD8+ T cell enrichment cocktail; Stem Cell Technologies, Inc.), and separated by Ficoll-Hypaque density gradient according to the manufacturer's protocol. An additional positive selection was performed by magnetic cell sorting using MACS CD8+ enrichment MicroBeads and MS Mini MACS columns (Miltenyi Biotec). The purity of the CD8+ T cells was analyzed by staining with anti-CD3 APC-Cy7, anti-CD8 PE and anti-CD14 PE-Cy5 antibodies (BD) and subsequent flow cytometric analysis. The CD8+ T-cell purity was above 98.8% for all experiments performed.
Positive selection of CD14+ cells was performed by using MACS magnetic CD14+ enrichment MicroBeads and MS Mini MACS columns (Miltenyi Biotec). The purity of the CD14+ cells was assessed by staining with anti-CD3 APC-Cy7 and anti-CD14 PE-Cy5 antibodies (BD) and was subsequently evaluated by flow cytometric analysis (range, 76% to 85%).
Add-back and transwell experiments.
Highly purified (>98.8%) fresh CD8+ T cells were resuspended at 106 cells/ml and added to a 24-well plate. For the add-back experiments, purified CD14+ cells were added directly to the CD8+ T cells at a ratio of 1:5. For the transwell assay, CD8+ T cells were layered in the outer chamber of the well, while CD14+ cells, separated by a semipermeable membrane as described previously (44), were placed in the top compartment of the corresponding well at a ratio of 1:5. The respective stimulant added to the medium was able to diffuse freely between the compartments, although direct cell-to-cell contact was prevented.
Statistical analysis.
Other than in Fig. 1, all data shown represent the average results and standard deviations from at least three independent experiments. Unpaired two-tailed Student t tests were employed to assess the statistical significance of differences. P values of less than 0.05 were considered significant.
FIG. 1.
Correlation of viral load and activation markers upon initiation of HAART in chronic HIV infection. Shown are the simultaneous decay of the plasma viral load (VL) (open squares) and the percentage of activated CD8+ T cells (closed triangles) in 10 chronically HIV-infected subjects before and up to 1 year after the initiation of HAART. The dotted line represents the limit of detection for the viral load assay (50 RNA copies/ml). In panel A, the percentage of proliferating Ki67+ CD8+ cells closely follows the decay in viral loads (r = 0.6391, P < 0.0001, Spearman rank correlation). Similarly, panels B and C show the decline in the percentage of CD8+ HLA-DR+ cells (r = 0.6466, P < 0.0001) and CD8+ CD38+ cells (r = 0.6532, P < 0.0001), respectively, in relation to the viral load. All values are presented as median values, and bars represent the interquartile ranges.
RESULTS
Rapid decline in immune activation following initiation of HAART is directly associated with reduction of HIV-1 viremia.
Treatment of HIV-1-infected individuals with HAART has been shown to significantly and rapidly reduce immune activation (5, 11, 15, 39). In a study of 10 treatment-naïve, chronically HIV-1-infected individuals who initiated HAART, we quantified markers of immune activation in relation to viral load over the course of 1 year, including very early assessments of viral load and immune activation after 2 and 4 weeks of HAART. As shown in Fig. 1, markers of immune activation on CD8+ T cells, including Ki67, CD38, and HLA-DR, declined rapidly after initiation of HAART, closely following the decline of HIV-1 RNA plasma levels, in line with the results of previous studies (5, 11, 15, 39). The levels of HIV-1 viremia correlated significantly with the individual activation markers assessed (HIV-1 RNA versus the percentage of Ki67+ CD8+ T cells, r = 0.6391; HIV-1 RNA versus the percentage of HLA-DR+ CD8+ cells, r = 0.6466; HIV-1 RNA versus the percentage of CD38+ CD8+ cells, r = 0.6532; P < 0.0001 for all three associations). While the activation markers after 48 weeks of treatment remained higher than in HIV-1-negative controls (percentages of Ki67+ CD8+ T cells, 3.6 versus 1.1 [P = 0.0004], respectively, and percentages of HLA-DR+ CD8+ T cells, 18.76 versus 14.9 [P > 0.05], respectively), the dramatic decline of immune activation following the suppression of HIV-1 viremia by HAART strongly suggests a direct causal effect of HIV-1 itself, or components of the virus, on immune activation.
HIV-1 encodes multiple uridine-rich oligonucleotides that can elicit strong cytokine release in human PBMCs.
Recent studies have demonstrated that pDCs can sense HIV-1 through TLR7 (3) and that a uridine-rich region within the HIV-1 long terminal repeat encodes a TLR7/8 ligand termed RNA40 (17). We hypothesized that numerous additional uridine-rich ssRNA oligonucleotides are encoded by the HIV-1 ssRNA of structural and accessory genes and that these HIV-1-derived TLR ligands are responsible for the observed HIV-1-induced immune activation. We therefore screened the RNA sequence of HIV-1 HXB2 for regions that contained more than 50% uridines and synthesized nine 20-nucleotide-long ssRNA oligonucleotides spanning these regions (Table 1). As control sequences, the corresponding uridine to adenosine (U-to-A) variants (Table 1) that have been previously shown to lack the ability to activate TLRs in the context of RNA40 and its U-to-A variant RNA41 (17) were synthesized.
Similar to the effect of RNA40, as well as the described TLR7/8 ligands 3M002 and 3M011, these novel HIV-1-derived U-rich ssRNA oligonucleotides resulted in strong TNF-α secretion by PBMC (Fig. 2) in the presence of a cationic transfection agent, DOTAP, which allows for the stabilization of RNA oligonucleotides, thereby enabling them to reach the intracellular compartment. In contrast, no TNF-α secretion was observed either after incubation with the U-to-A variants of these oligonucleotides or with DOTAP alone, as shown for five representative examples in Fig. 2. This U-rich, oligonucleotide-specific cytokine secretion was completely blocked by the addition of chloroquine (Fig. 2), an inhibitor of lysosomal acidification known to abolish signaling of the intracellularly located TLRs (36). However, chloroquine did not significantly block the activation mediated by phorbol myristate acetate (PMA)-ionomycin (P > 0.05). Taken together, these data demonstrate that HIV-1 encodes several U-rich ssRNA oligonucleotides that can induce significant cytokine release.
FIG. 2.
TNF-α production of human PBMCs in response to HIV-1-derived ssRNA. Shows the production of TNF-α by PBMCs unstimulated (neg), stimulated with different RNA oligonucleotides in the absence or presence of chloroquine, or stimulated with PMA or DOTAP alone. Numbers on the y axis indicate the order of appearance in Table 1 of the RNA oligonucleotides. Strong induction of TNF-α release in human PBMCs was observed following stimulation with HIV-1-derived ssRNA (U-Variants) and described TLR7/8 ligands (3M011 and 3M002) as measured by ELISA. No secretion of TNF-α was induced in response to the U-to-A control oligonucleotides, in which the uridines were replaced by adenosines (A-Variants). The activation of PBMCs induced by U-rich HIV-1 ssRNA oligonucleotides was blocked by chloroquine, a known inhibitor of intracellular TLR signaling (U-Variants + chloroquine). Data represent the results ± standard deviations from four independent experiments.
HIV-1-derived U-rich oligonucleotides promote survival and induce cytokine secretion of monocytes and pDCs.
Previous studies have demonstrated that monocytes and pDCs express TLR8 and TLR7, respectively, and that these cells can be activated by TLR ligands (3, 20, 27). To further assess the ability of the novel HIV-1-derived oligonucleotides to activate monocytes and pDCs, multiparameter flow cytometric analysis was employed to characterize monocyte and pDC numbers and cytokine production following incubation with these U-rich oligonucleotides or their U-to-A variants. We observed a significantly higher frequency of pDCs and monocytes when they were incubated for 20 h with the HIV-1-derived U-rich oligonucleotides than with the U-to-A variants (Fig. 3). These results were comparable to the increased frequency of pDCs and monocytes observed after incubation with the described TLR7/8 agonist 3M011, as well as the TLR8 agonist 3M002 that only has limited TLR7 activity (14, 27) (Fig. 3). Furthermore, pDCs upregulated costimulatory molecules, including CD80 and CD86, in response to HIV-1-derived TLR ligands, indicating the maturation of these cells (data not shown). These data suggest that the tested oligonucleotides promoted the maturation and survival of monocytes and pDCs during this short-term culture in vitro.
FIG. 3.
Survival of pDCs and monocytes after 20 h of in vitro culture in the presence or absence of HIV-1-derived ssRNA oligonucleotides. The frequencies of pDCs (CD3neg CD14neg CD123bright+) and monocytes (CD3neg CD14+) in PBMC stimulated with HIV-1-derived ssRNA oligonucleotides (U-Variants) or described TLR7/8 ligands 3M011 and 3M002 after 20 h of culture were significantly higher than those in unstimulated PBMC (neg), PBMC stimulated with the transfection reagent DOTAP alone, or PBMC stimulated with the control U-to-A variants (A-Variants). Data represent the averages ± standard deviations of the results of four independent experiments. *, P = <0.05.
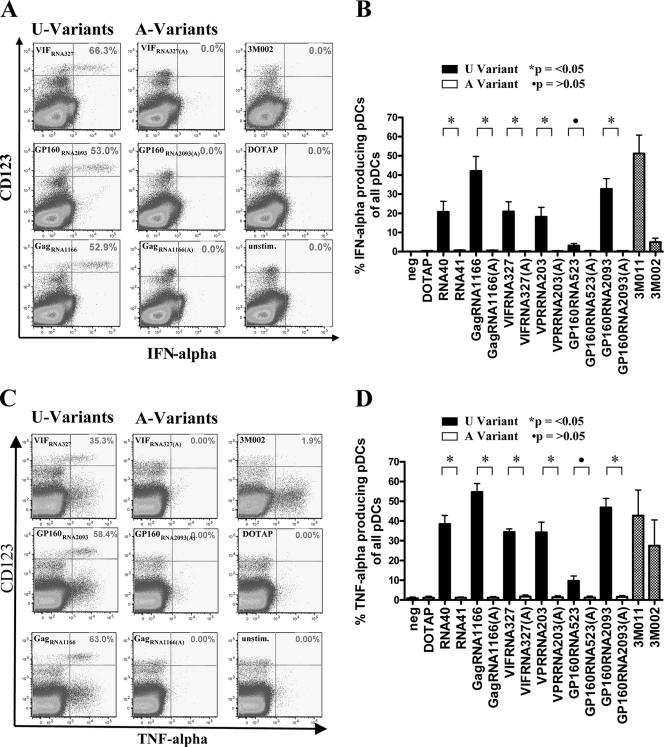
In addition, monocytes and pDCs were assessed for their ability to produce IL-6, IFN-α, and TNF-α in response to these oligonucleotides by using a multiparameter flow cytometric assay. After stimulation with the U-rich HIV-1-derived oligonucleotides, the monocytes produced significant amounts of TNF-α and IL-6 (Fig. 4), whereas the pDCs produced significant amounts of IFN-α and TNF-α (Fig. 5). In contrast, CD8+ T cells did not produce TNF-α, IFN-γ, or IL-2 in response to these ligands, as assessed by intracellular cytokine staining (data not shown). Again, this activating effect was observed only for the U-rich variants, including RNA40, and not for the respective U-to-A variants (P < 0.05 for all comparisons; Fig. 4B and D and 5B and D). The only exception was GP160523, which induced strong activation in monocytes but little activation of pDCs, similar to the effect of 3M002, a described ligand with preferential TLR8 specificity, which is in line with the lack of TLR8 expression on pDCs. Concurrently, the TLR7/8 agonist 3M011 induced strong TNF-α and IFN-α production in pDCs, as well as TNF-α and IL-6 production in monocytes. Taken together, these data demonstrate that HIV-1-derived ssRNA can induce significant cytokine secretion in pDCs and monocytes, but not T cells, as well as promote their survival during short-term culture in vitro.
FIG. 4.
Cytokine production by monocytes in response to HIV-1-derived ssRNA oligonucleotides and control ligands 3M011 and 3M002. Flow plots show PBMC gated on CD3neg CD14+ monocytes stained intracellularly for IL-6 (A) and TNF-α (C). HIV-1-derived ssRNA oligonucleotides (U-Variants) induced strong IL-6 and TNF-α production, whereas the control U-to-A sequences (A-Variants) and DOTAP alone induced responses similar to the background activity of unstimulated controls (neg). The graphs show the mean percentages ± standard deviations of IL-6+ (B) and TNF-α+ (D) monocytes for all tested sequences. Data represent the results from four independent experiments.
FIG. 5.
Cytokine production by pDCs in response to HIV-1-derived ssRNA oligonucleotides and control ligands 3M011 and 3M002. Flow plots show PBMC gated on CD3neg CD14neg CD123bright pDCs stained intracellularly for IFN-α (A) and TNF-α (C). HIV-1-derived ssRNA oligonucleotides (U-Variants) induced strong IFN-α and TNF-α production, whereas the control U-to-A sequences (A-Variants) and DOTAP alone induced responses similar to the background activity of unstimulated controls (neg). The graphs show the mean percentages ± standard deviations of IFN-α+ (B) and TNF-α+ (D) pDCs for all tested sequences. Data represent the results from four independent experiments.
The activaton of murine macrophages by HIV-1-derived ssRNA oligonucleotides is MyD88 dependent and inhibited by a TLR7/TLR9-specific antagonist.
The abolition of the ssRNA activity once uridines were replaced by adenines, their intracellular activity, and the ability of chloroquine, a described inhibitor of intracellular TLR activity (36), to block activation all strongly suggest that the newly identified oligonucleotides mediate their activity through the ssRNA-sensing TLRs TLR7 and TLR8. In order to further validate that the immune activation induced by these HIV-1-derived oligonucleotides was mediated by TLR7/8, we used murine macrophages from MyD88-KO mice. MyD88 is essentially involved in the signaling pathway of TLR7, -8, and -9 but is not essential for TLR4 signaling, which can also be mediated by TRIF (41). Significant cytokine secretion in response to HIV-1 RNA sequences, but not the corresponding A variants, was observed in murine macrophages derived from wild-type C57BL/6 mice, similar to the results obtained by using human PBMC (Fig. 6A). Interestingly, RNA GP160523, the TLR ligand with preferential TLR8 activity in humans (Fig. 4 and 5), did not induce significant TNF-α in murine macrophages (Fig. 6A). These data are in line with previous data demonstrating differences between murine and human TLR8 in the responsiveness to ligands (21, 42). In contrast, macrophages derived from MyD88-KO mice were not able to respond to any of the HIV-1-derived ssRNA ligands, while still being responsive to LPS, although, as expected, to a lesser degree than the wild type (Fig. 6A). These findings demonstrate that the response to the newly identified ssRNAs from HIV-1 depends on the MyD88 adaptor protein, which is essential for signaling of the ssRNA-sensing receptors TLR7 and TLR8.
To further assess if the activation in human pDCs induced by these HIV-1-derived oligonucleotides was mediated by TLR7, pDCs were coincubated with the U-rich oligonucleotides in the presence and absence of the previously described TLR7/9 antagonist IRS954 (2). As exemplified in the results shown in Fig. 6B for GagRNA1166 and VifRNA327, the addition of this TLR7/9 antagonist resulted in a concentration-dependent reduction of cytokine production (IFN-α and TNF-α) by TLR7-expressing pDCs (Fig. 6), whereas no significant inhibition of cytokine secretion (TNF-α and IL-6) by IRS954 was observed in the TLR8-expressing human monocytes (data not shown), in line with the results of previous reports (2). Taken together, these data strongly suggest that the newly identified HIV-1-derived ssRNA oligonucleotides mediate their activity through TLR7/8 and that pDC activation by these oligonucleotides can be specifically blocked by a TLR7 antagonist.
HIV-1-derived oligonucleotides activate CD8+ T cells in a monocyte-dependent manner.
The above-described data demonstrate that the newly identified U-rich oligonucleotides derived from HIV-1 ssRNA induce strong activation of monocytes and pDCs. We subsequently studied the impact of this activation on T cells by assessing the cytokine secretion and CD69 expression of T cells following stimulation of PBMC with the HIV-1-derived oligonucleotides. CD4+ and CD8+ T cells did not produce cytokines (TNF-α, IFN-γ, and IL-2) after a 6 h, 12 h, or 24 h incubation period (data not shown) but significantly upregulated CD69 following stimulation with the U-rich oligonucleotides, as shown for two representative examples in Fig. 7A. Again, no activation was observed when PBMC were stimulated with the U-to-A variants, the transfection reagent alone, or in the presence of chloroquine (Fig. 7). Furthermore, the activation of CD8+ T cells by HIV-1-derived oligonucleotides depended on the presence of accessory cells, as highly purified (> 98.9%) CD8+ T cells alone were not activated by the oligonucleotides, as exemplified for RNA40 in Fig. 8. CD8+ T-cell activation, however, was reconstituted when purified CD8+ T cells were stimulated with the HIV-1-derived TLR7/8 ligands in the presence of CD14+ monocytes (Fig. 8). This activation was dependent on direct cell-to-cell contact, as the separation of monocytes and CD8+ T cells by a semipermeable membrane again abolished CD8+ T-cell activation (Fig. 8). Taken together, these data demonstrate that HIV-1-derived TLR7/8 ligands can induce strong accessory cell-dependent activation of T cells, but no cytokine production, in this in vitro system.
FIG. 7.
Activation of CD8 T cells in response to HIV-1-derived TLR ligands. Upregulation of CD69 on CD8+ T cells after 6 h of stimulation with HIV-1-derived oligonucleotides. No activation was observed after incubation with the corresponding U-to-A variants or after coincubation with chloroquine. Chloroquine did not suppress the activation induced by PMA-ionomycin (PMA+I). (A) Stimulation of PBMC with two representative HIV-1-derived oligonucleotides, as well as their corresponding U-to-A variants. (B) Mean percentages ± standard deviations of CD69+ CD8+ T cells following incubation with these nine novel HIV-1-derived oligonucleotides and their corresponding U-to-A variants and after coincubation of the oligonucleotides with chloroquine in five independent experiments. RNA40, GCCCGUCUGUUGUGUCACUC; Poly U, UUUUUUUUUUUUUUUUUUUU.
FIG. 8.
Stimulation of CD8+ T cells by HIV-1-derived TLR ligands is dependent on direct cell-to-cell contact with CD14+ cells. (A) CD69 expression on highly purified (>98.8%) CD8+ T cells after stimulation with RNA40 plus DOTAP, RNA41 plus DOTAP, or 3M002. No upregulation of CD69 was observed on purified CD8+ T cells (upper panels). After the adding back of purified CD14+ cells, upregulation of CD69 on CD8+ T cells was observed following stimulation with RNA40 plus DOTAP and 3M002 (middle panels). When purified CD14+ cells were separated from CD8+ T cells by a semipermeable membrane in transwell experiments (lower panels), no activation of CD8+ T cells was observed. PMA+I, PMA-ionomycin. (B) Average results ± standard deviations from three independent experiments are shown.
DISCUSSION
Persistent immune activation is a characteristic and prognostic factor of HIV-1 infection and absent in nonpathogenic models of SIV infection occurring in natural hosts of SIV, such as sooty mangabeys, African green monkeys, and mandrills (38, 40). These observations suggest a crucial role for immune activation in HIV-1 pathogenesis. The underlying mechanisms, however, are not fully understood. Here we show that immune activation in HIV-1-infected individuals declines rapidly after initiation of HAART and that immune activation is directly associated with the level of HIV-1 viremia, as demonstrated previously (5, 15, 39). These findings suggest a direct effect of HIV-1 on the activation of the immune system. In support of this model, we demonstrate that HIV-1 encodes multiple uridine-rich ssRNA TLR7/8 ligands that induce significant MyD88-dependent immune activation of pDCs and monocytes in vitro. This immune activation was suppressed by chloroquine, an inhibitor of lysosomal acidification known to abolish signaling of the intracellularly located TLRs (36) and, in pDCs, in a dose-dependent manner, by a TLR7 antagonist. Furthermore, the activation of pDCs and monocytes resulted in a strong secondary activation of T cells. Taken together, these data demonstrate that components of HIV-1 itself can induce strong immune activation via TLR7 and TLR8, which can be blocked by interfering with TLR signaling.
TLRs play a crucial role in the recognition of foreign material, including viruses and bacteria (42). More recently, it has been demonstrated that a uridine-rich sequence within the HIV-1 long terminal repeat, termed RNA40, can serve as a TLR7/8 ligand (17) and that endosomally derived HIV-1 RNA can modulate pDC function via TLR7 in humans (3). Here we demonstrate that multiple uridine-rich oligonucleotides are encoded by the ssRNA of HIV-1 gag, vpr, vif, pol, and the gp160 gene. These ssRNA sequences, as well as synthetic ligands for TLR7 and TLR8, strongly activated pDCs and monocytes, resulting in a secondary activation of T cells. Although other receptors, such as RIG-1, have been shown to detect ssRNA (19, 33), the activation induced by the described HIV-1-derived ssRNA was dependent on the adaptor protein MyD88, which is essential in the signaling of TLRs, and was blocked by chloroquine. Activation was, furthermore, specifically suppressed in human pDCs in a dose-dependent manner by a previously described TLR7/TLR9-specific antagonist (IRS954). In accordance with the specificity of the TLR antagonist IRS954 for TLR7 and TLR9 (2), the activation of TLR8+ monocytes by the HIV-1-derived oligonucleotides was not inhibited by IRS954 (17). Taken together, these data demonstrate that the HIV-1-derived ssRNA oligonucleotides signaled through TLR7/8 and induced strong immune activation in vitro.
A recent study has demonstrated elevated levels of circulating LPS, an important component of the cell walls of gram-negative bacteria, in the plasma of chronically HIV-1-infected individuals (8). LPS is a strong immunostimulatory ligand for TLR4 (34). The level of LPS in chronic HIV-1 infection, which was significantly associated with increased immune activation, decreased following 48 weeks of antiretroviral therapy. These data suggest that intestinal microbial translocation, resulting from the breakdown of the mucosal barrier during HIV-1 infection (9, 30), can serve as a cause of immune activation in chronic HIV-1 infection (8, 16). In addition to these bacterium-derived TLR4 ligands, the rapid initial decline of immune activation associated with the suppression of HIV-1 replication following the initiation of HAART, described here and previously in other cohorts (15, 26, 43), and the rapid increase in T-cell activation—within days—associated with the rebound of HIV-1 viremia following interruption of HAART (28), indicate a direct effect of HIV-1 itself on immune activation. Interestingly, no elevation of plasma LPS levels is observed during acute HIV-1 infection (8, 16), while very strong immune activation is characteristic for this earliest phase of HIV-1 infection, resulting in the development of “flu-like” symptoms in the majority of infected individuals (22), further suggesting a direct impact of the virus itself on the initial activation of the immune system.
These rapid changes in immune activation that follow HIV-1-viremia observed during HIV-1 infection and the identification of several HIV-1-encoded TLR ligands with significant immunostimulatory activity described here support a model that proposes a direct role of the virus in the activation of the immune system during HIV-1 infection. These data agree with the results of studies in other viral models demonstrating that TLRs play a crucial role in sensing PAMPs encoded by these viruses early in infection and in initiating the activation of the innate and subsequent adaptive immune response (1, 42). Furthermore, the persistent circulation of high levels of HIV-1 in chronic infection may directly contribute to the maintenance of increased immune activation, in conjunction with additional PAMPs resulting from pathogens that enter the host as a result of the weakening of the host's immune barriers, including opportunistic pathogens and the described intestinal microbial translocation through a compromised mucosal barrier (7, 8, 16). Further studies will be needed to dissect the contributions of these different components to the immune activation observed in acute and chronic HIV-1 infection.
In conclusion, our data demonstrate that HIV-1 encodes multiple TLR ligands that can induce strong MyD88-dependent pDC and monocyte activation, as well as accessory cell-dependent T-cell activation. These data support a model in which HIV-1 directly contributes to the persistent immune activation observed during viremic HIV-1 infection and provide a rationale for the development of interventions aiming to reduce the immune activation induced by pathogen-encoded PAMPs.
Acknowledgments
We do not have a commercial or other association that might pose a conflict of interest.
This study was supported by the NIH (RO1 AI50429 and RO1 AI 067031).
We thank all patients participating in this study.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Barrat, F. J., T. Meeker, J. Gregorio, J. H. Chan, S. Uematsu, S. Akira, B. Chang, O. Duramad, and R. L. Coffman. 2005. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202:1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. Dasilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 115:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekeredjian-Ding, I., S. I. Roth, S. Gilles, T. Giese, A. Ablasser, V. Hornung, S. Endres, and G. Hartmann. 2006. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J. Immunol. 176:7438-7446. [DOI] [PubMed] [Google Scholar]
- 5.Bisset, L. R., R. W. Cone, W. Huber, M. Battegay, P. L. Vernazza, R. Weber, P. J. Grob, and M. Opravil. 1998. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort Study. AIDS 12:2115-2123. [DOI] [PubMed] [Google Scholar]
- 6.Bourquin, C., L. Schmidt, V. Hornung, C. Wurzenberger, D. Anz, N. Sandholzer, S. Schreiber, A. Voelkl, G. Hartmann, and S. Endres. 2007. Immunostimulatory RNA oligonucleotides trigger an antigen-specific cytotoxic T cell and IgG2a response. Blood 109:2953-2960. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7:235-239. [DOI] [PubMed] [Google Scholar]
- 8.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 11.Evans, T. G., W. Bonnez, H. R. Soucier, T. Fitzgerald, D. C. Gibbons, and R. C. Reichman. 1998. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res. 39:163-173. [DOI] [PubMed] [Google Scholar]
- 12.Fahey, J. L., J. M. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J. V. Giorgi. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166-172. [DOI] [PubMed] [Google Scholar]
- 13.Fahey, J. L., J. M. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 14.Gorski, K. S., E. L. Waller, J. Bjornton-Severson, J. A. Hanten, C. L. Riter, W. C. Kieper, K. B. Gorden, J. S. Miller, J. P. Vasilakos, M. A. Tomai, and S. S. Alkan. 2006. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int. Immunol. 18:1115-1126. [DOI] [PubMed] [Google Scholar]
- 15.Gray, C. M., J. M. Schapiro, M. A. Winters, and T. C. Merigan. 1998. Changes in CD4+ and CD8+ T cell subsets in response to highly active antiretroviral therapy in HIV type 1-infected patients with prior protease inhibitor experience. AIDS Res. Hum. Retrovir. 14:561-569. [DOI] [PubMed] [Google Scholar]
- 16.Haynes, B. F. 2006. Gut microbes out of control in HIV infection. Nat. Med. 12:1351-1352. [DOI] [PubMed] [Google Scholar]
- 17.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 18.Hellerstein, M. K., R. A. Hoh, M. B. Hanley, D. Cesar, D. Lee, R. A. Neese, and J. M. McCune. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Investig. 112:956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 20.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A. M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 22.Kahn, J. O., and B. D. Walker. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339:33-39. [DOI] [PubMed] [Google Scholar]
- 23.Kaisho, T., and S. Akira. 2004. Pleiotropic function of Toll-like receptors. Microbes Infect. 6:1388-1394. [DOI] [PubMed] [Google Scholar]
- 24.Kaisho, T., and S. Akira. 2003. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 3:373-385. [DOI] [PubMed] [Google Scholar]
- 25.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 26.Lempicki, R. A., J. A. Kovacs, M. W. Baseler, J. W. Adelsberger, R. L. Dewar, V. Natarajan, M. C. Bosche, J. A. Metcalf, R. A. Stevens, L. A. Lambert, W. G. Alvord, M. A. Polis, R. T. Davey, D. S. Dimitrov, and H. C. Lane. 2000. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc. Natl. Acad. Sci. USA 97:13778-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy, O., E. E. Suter, R. L. Miller, and M. R. Wessels. 2006. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libois, A., A. Lopez, F. Garcia, P. Castro, M. J. Maleno, A. Garcia, N. Climent, M. Arnedo, T. Gallart, J. M. Gatell, and M. Plana. 2006. Dynamics of T cells subsets and lymphoproliferative responses during structured treatment interruption cycles and after definitive interruption of HAART in early chronic HIV type-1-infected patients. AIDS Res. Hum. Retrovir. 22:657-666. [DOI] [PubMed] [Google Scholar]
- 29.McCune, J. M., M. B. Hanley, D. Cesar, R. Halvorsen, R. Hoh, D. Schmidt, E. Wieder, S. Deeks, S. Siler, R. Neese, and M. Hellerstein. 2000. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Investig. 105:R1-R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 194:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364-368. [DOI] [PubMed] [Google Scholar]
- 33.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 34.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 35.Romagnani, C., M. Della Chiesa, S. Kohler, B. Moewes, A. Radbruch, L. Moretta, A. Moretta, and A. Thiel. 2005. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur. J. Immunol. 35:2452-2458. [DOI] [PubMed] [Google Scholar]
- 36.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G. B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 34:2541-2550. [DOI] [PubMed] [Google Scholar]
- 37.Schilling, D., K. Thomas, K. Nixdorff, S. N. Vogel, and M. J. Fenton. 2002. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J. Immunol. 169:5874-5880. [DOI] [PubMed] [Google Scholar]
- 38.Silvestri, G., A. Fedanov, S. Germon, N. Kozyr, W. J. Kaiser, D. A. Garber, H. McClure, M. B. Feinberg, and S. I. Staprans. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 79:4043-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvestri, G., C. Munoz, L. Butini, P. Bagnarelli, and M. Montroni. 1997. Changes in CD8 cell subpopulations induced by antiretroviral therapy in human immunodeficiency virus infected patients. Viral Immunol. 10:207-212. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 41.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 42.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 43.Tilling, R., S. Kinloch, L. E. Goh, D. Cooper, L. Perrin, F. Lampe, J. Zaunders, B. Hoen, C. Tsoukas, J. Andersson, and G. Janossy. 2002. Parallel decline of CD8+/CD38++ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS 16:589-596. [DOI] [PubMed] [Google Scholar]
- 44.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]