Abstract
Immunization against smallpox (variola virus) with Dryvax, a live vaccinia virus (VV), was effective, but now safety is a major concern. To overcome this issue, subunit vaccines composed of VV envelope proteins from both forms of infectious virions, including the extracellular enveloped virion (EV) protein B5, are being developed. However, since B5 has 23 amino acid differences compared with its B6 variola virus homologue, B6 might be a better choice for such a strategy. Therefore, we compared the properties of both proteins using a panel of monoclonal antibodies (MAbs) to B5 that we had previously characterized and grouped according to structural and functional properties. The B6 gene was obtained from the Centers for Disease Control and Prevention, and the ectodomain was cloned and expressed in baculovirus as previously done with B5, allowing us to compare the antigenic properties of the proteins. Polyclonal antibodies to B5 or B6 cross-reacted with the heterologous protein, and 16 of 26 anti-B5 MAbs cross-reacted with B6. Importantly, 10 anti-B5 MAbs did not cross-react with B6. Of these, three have important anti-VV biologic properties, including their ability to neutralize EV infectivity and block comet formation. Here, we found that one of these three MAbs protected mice from a lethal VV challenge by passive immunization. Thus, epitopes that are present on B5 but not on B6 would generate an antibody response that would not recognize B6. Assuming that B6 contains similar variola virus-specific epitopes, our data suggest that a subunit vaccine using the variola virus homologues might exhibit improved protective efficacy against smallpox.
Variola virus is the etiological agent of smallpox. Because no effective animal reservoir existed for the virus, a global immunization program with vaccinia virus (VV) helped eradicate this disease (9, 27).
Concern about the intentional release of variola virus by terrorists has stimulated efforts to develop safer smallpox vaccines (10, 12, 28). Dryvax, the VV vaccine currently licensed in the United States, is an infectious VV. This virus is a member of the orthopoxvirus family and is closely related to variola virus. While it is very effective at producing immunity to smallpox, it has an unacceptable safety profile in today's context of no active smallpox disease (11, 20). Therefore, our approach has been to identify proteins of VV that would constitute an effective subunit vaccine.
VV-infected cells produce two different forms of infectious virions from the cytoplasm. The majority of progeny virus (intracellular mature virions [MV]) remain within necrotic cells and are shed in skin debris or saliva droplets, where they are believed to serve as sources of infection. Some of the MV acquire an additional membrane and are transported to the cell surface. These extracellular enveloped virions (EV) are responsible for cell-to-cell spread and are critical for viral pathogenesis in vivo (3, 5, 19). The outer envelope of each form bears different specific viral proteins (18, 22, 23). Theoretically, antibody responses to MV proteins should reduce the initial infecting inoculum, while antibodies to EV targets would then limit spread of the progeny virus within the infected host. This humoral defense would allow the host immune system to develop and eradicate the infection.
B5 is one of several EV-specific proteins and has highly conserved homologues among all orthopoxviruses, including variola virus (8). It is a glycosylated type I membrane protein of 42 kDa (7, 13). The B5 ectodomain (B5t) encompasses four domains with resemblance to short consensus repeats (SCRs), present in complement regulatory proteins, plus a 51-amino-acid stalk next to the transmembrane region (7, 13). Epitope mapping studies have suggested that the stalk interacts with the first two SCR domains and that these regions are important neutralizing sites (1).
We showed previously that vaccination with a combination of B5t and the ectodomains of two other VV proteins, L1t (MV) and A33t (EV), protects mice against a lethal VV challenge (10, 28). These three proteins differ in sequence to various degrees from their homologues in variola virus, and of these, B5t shows the greatest divergence, with 21 amino acid differences in its ectodomain from its variola virus counterpart, B6. Since a subunit vaccine to protect against smallpox could consist of either VV or variola virus proteins, we focused our attention on the antigenic properties of B5t and the B6 ectodomain (B6t). The question is whether the amino acid differences between B5t and B6t result in proteins with different antigenic properties. To answer this, we produced a soluble recombinant B6t that was comparable in length to the previously described B5t recombinant protein (1) and then used our panel of monoclonal antibodies (MAbs) to B5t (1) to compare its antigenic characteristics with those of B5t. Finding antigenic differences between B5t and B6t would greatly impact the development of subunit vaccines, because unlike live heterologous virus vaccines, such as Dryvax, subunit vaccines rely on a small number of proteins, so that antigenic differences could ultimately affect vaccine efficacy.
Among our panel of 26 MAbs, 10 failed to recognize B6t, and of these, 3 have important anti-VV biologic properties, including their ability to neutralize EV infectivity and block comet formation. We found that one of these MAbs tested by passive immunization protected mice from a lethal VV challenge.
MATERIALS AND METHODS
Cells and viruses.
Rabbit kidney cell line RK-13 was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1× penicillin-streptomycin. VV strain IHD-J was used for in vitro tests and WR for animal studies. For EV stock preparation, RK-13 cells were infected with IHD-J at a multiplicity of 0.05 PFU/cell as previously described (1). The VV strain WR, used to challenge mice, was grown in BSC-1 cells and pelleted twice through a 36% sucrose cushion.
Construction of recombinant baculovirus expressing soluble B6t EV glycoprotein.
We expressed the extracellular portion of B6 (from residue 20 to 275) (Fig. 1A) as a secreted, soluble protein with an N-terminal His tag in the baculovirus system. The protein was called B6(275t), or B6t for short. (In this paper we also refer to the B5 ectodomain as B5t.) The plasmid harboring the B6 gene from variola major virus (strain Bangladesh) was obtained from the Centers for Disease Control and Prevention and was used as a template. To facilitate the secreted expression of constructs, the expression vector pFastBac (Invitrogen) was modified to add a honeybee melittin secretion signal (HMSS) sequence at the 5′ terminus of the multiple cloning site. Briefly, the HMSS DNA sequence was amplified by PCR from pMelBac (Invitrogen) as a template. The expression vector, containing an HMSS sequence followed by BamHI, EcoRI, and HindIII sites, was named pFBHMH6. B6t (amino acids 20 to 275) fused with His6 at the N terminus was amplified by PCR, digested with BamHI and HindIII, and cloned into vector pFBHMH6 at corresponding sites. The sequences were verified by DNA sequencing. The generation and amplification of recombinant baculovirus followed the manufacturer's protocol (Bac-to-Bac baculovirus expression system, version C). The expressed baculovirus protein was purified using either nickel chromatography as previously described (1) or VMC-14 (a MAb against B5t) as an immunosorbent agent (1). Briefly, the culture supernatant from Bac-B6t-infected Sf9 cells was concentrated and dialyzed against PBS. B6t was then purified through a 20-ml immunosorbent column coated with VMC-14 (8 mg of immunoglobulin G [IgG] per ml of gel). The protein was eluted with 50 mM glycine buffer at pH 2.5 containing 500 mM NaCl. Eluted protein was immediately neutralized to pH 7.2 with 2 M Tris, pH 9.0), concentrated using an Amicon Ultra (molecular weight cutoff, 10,000), dialyzed with phosphate-buffered saline, and finally concentrated to 600 μg/ml. B5t was purified as reported elsewhere (1) except where indicated. We found no difference in the properties of the B5 proteins when purified by nickel or immunosorbent chromatography.
FIG. 1.
Production of soluble recombinant variola virus B6 in baculovirus. (A) Diagram of full-length VV B5, recombinant protein B5t generated in a previous study (1), and the recombinant variola virus B6t generated in this study. Putative transmembrane regions (TM) are shown as dashed rectangles. The signal peptide of B5 is shown as a black rectangle. Consensus N-glycosylation sites are shown as black lollipops. Numbers refer to the residues at the beginning or end of the protein or the feature depicted within the protein (e.g., TM). Additional residues appended to the recombinant protein as a result of cloning are also shown, as well as a six-histidine tag (H6). (B) Sequence alignment of B5 (WR strain; primary accession number, Q01227) and variola virus B6 (Bangladesh-1975 strain; primary accession number, Q85402). The SCRs are underlined. Residues highlighted in gray are the 23 amino acids that differ between B5 and B6, 21 of which are located in the ectodomain. Black boxes indicate putative N-glycosylation sites. The start of the transmembrane domain (TM) and cytoplasmic tail (CT) are indicated. The alignment was made using ClustalW (24).
Monoclonal and PAbs.
Rabbit polyclonal antibodies (PAbs) R195 and R196 were prepared against purified B6t. PAb R182 was prepared against purified B5t (1). IgGs were purified from each serum using a Hi-Trap protein G HP column (Amersham Pharmacia). The MAbs used in this study (also as IgGs) were previously described (1).
SDS-PAGE.
Glycoproteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under native (0.1% SDS, no reducing agent, no boiling [6]) or denaturing (samples boiled 5 min in 2.5% SDS-350 mM β-mercaptoethanol) conditions in precast Tris-glycine gels (Invitrogen). After SDS-PAGE, separated proteins were transferred to nitrocellulose, probed with antibodies, and visualized by enhanced chemiluminescence (Amersham Pharmacia).
EV plaque reduction assay.
Fresh EV from VV strain IHDJ (1,000 PFU/ml in heat-inactivated Dulbecco's modified Eagle's medium) were incubated for 1 h at 37°C with the PAbs as previously described (1). In addition, the neutralizing anti-L1R MAb VMC-2 (at 50 μg/ml) (2) and the anti-A27L rabbit PAb R194 (at 100 μg/ml) (unpublished data) were added to neutralize any contaminating MV or damaged EV (17). The virus-antibody mixture (100 PFU/well) was added to confluent BSC-1 cells in 48-well plates, and plates were incubated for 18 h at 37°C. The cells were fixed with formaldehyde, and the plaques were counted after the monolayer was stained with 0.2% crystal violet in 50% ethanol. This experiment was performed twice.
Comet tail inhibition assay.
Monolayers of BSC-1 cells grown in 12-well plates were infected with VV strain IHDJ at ∼30 PFU/well for 2 h at 37°C. The inoculum was removed, and fresh modified Eagle's medium containing heat-inactivated fetal calf serum and the corresponding PAb was added. The cells were incubated for 36 h at 37°C and then stained with crystal violet. This experiment was performed twice.
Optical biosensor analysis.
Experiments were carried out on a BIAcore X optical biosensor (BIAcore AB) at 25°C as previously described (1, 15, 26).
(i) Properties of binding of IgG to B6t protein.
To test the binding properties of any Ig, anti-His MAb (QIAGEN Inc.) was covalently coupled to a CM5 BIAcore chip. Then, 250 resonance units (RU) of purified B6t protein was captured by the antibody via its N-terminal His tag (15). Purified antibody (20 μg/ml) was injected, except for VMC-21, which had to be injected at 400 μg/ml, and the association of the antigen-antibody complex was monitored for 3 min.
(ii) Competition studies: IgG blocking of binding.
In the blocking assay, a primary antibody was allowed to bind for 3 min to the captured B6t on the BIAcore chip. The second antibody was then injected, and its association was monitored for another 3 min (1, 15). The formula used to calculate the percentage of blocking is 100 − {[(RUIgn − RUself) × 100]/(RUcontrol − RUself)}, where RUcontrol represents binding in the absence of primary MAb and RUself represents residual binding after self-blocking. This formula considers RUcontrol to represent 100% binding and RUself to represent background normalized to zero.
Passive antibody transfer studies.
Groups of female 6-week-old BALB/c mice, purchased from Charles River Labs, were inoculated intraperitoneally with 200 μg of murine MAb VMC-11, VMC-25, or VMC-29 approximately 24 h prior to challenge with VV strain WR. Control mice were not treated. Mice were challenged intranasally with 5 × 104 to 1 × 105 PFU, and each mouse was monitored for weight loss for 18 days. Animals that exhibited weight loss of >30% were euthanized. This experiment was performed three times.
RESULTS
Production and characterization of B6t recombinant protein.
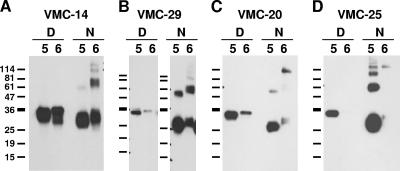
The ectodomain of B6 (amino acids 20 to 275) was expressed by the recombinant baculovirus Bac-B6t in essentially the same manner as that of recombinant B5(275t) (B5t for short) (Fig. 1) (1). Initial purification attempts of B6(275t) (B6t for short) using a combination of nickel-chelate chromatography followed by ion-exchange chromatography gave a low yield. To enhance recovery of the secreted protein, we purified it from an immunosorbent column containing the B5t MAb VMC-14. This MAb recognizes residues 56 to 75 of B5t and reacts strongly against B5t by Western blotting (1). Its epitope is conserved in B6t. We also purified B5t in this manner for biosensor experiments. The purified proteins were analyzed under denaturing or native conditions by SDS-PAGE and Western blotting (Fig. 2). B6t and B5t migrated as broad bands around 30 to 36 kDa under denaturing conditions. Under native SDS-PAGE conditions, B6t migrated as two separate bands: a 30- to 32-kDa band, which represents the protein monomer, and a 60- to 70-kDa band, which is likely a dimer. The larger B5t band migrated somewhat faster (at 60 kDa).
FIG. 2.
Western blot of purified B5t and B6t. Purified baculovirus-expressed B5t (indicated by a 5) and B6t (indicated by a 6) were electrophoresed on a 12% Tris-glycine polyacrylamide gel under denaturing (D) or nonreducing (N) conditions, transferred to nitrocellulose, and probed with polyclonal IgG R182 to B5t (1 μg/ml). The film was exposed longer to show the dimer. The sizes of molecular mass markers are shown in kDa.
Anti-B6 PAbs neutralize VV entry, inhibit VV comet tail formation, and confer protection to VV-challenged mice.
We prepared PAbs against B6t in rabbits to see if they neutralized VV EV. As expected, these anti-B6t PAbs (R195 and R196) recognized both B6t and B5t under denaturing and native conditions (data not shown), indicating the similarities of the two proteins.
We performed in vitro assays with VV to measure the anti-VV activity of the anti-B6t PAbs (1). By EV plaque reduction, 100 μg/ml of R195 or R196 IgG inhibited EV infectivity by 50% (Fig. 3). R182, a PAb against B5t, was somewhat more effective at neutralizing VV, though the greatest difference was seen only at high antibody concentrations. The anti-B6t PAbs were also tested for their ability to inhibit comet tail formation by VV strain IHDJ, which releases large amounts of EV that form comet-shaped plaques (Fig. 4, no-IgG control) (25). Both anti-B6t PAbs were also able to reduce comet tail formation, but higher concentrations of IgG were needed to achieve the level of inhibition seen with anti-B5t PAb R182. These results are consistent with those in the EV plaque reduction assay. Thus, PAbs against B6t cross-reacted with B5t and had significant biological activity against VV.
FIG. 3.
Inhibition of plaque formation by anti-B6 PAbs. BSC-1 cell monolayers were infected with EVs that were previously incubated for 1 h with the indicated antibody (along with anti-MV neutralizing antibodies). After 18 h of incubation at 37°C, the cells were fixed and stained with crystal violet, and plaques were counted. Data are expressed as the percentage of plaque reduction relative to the control with no IgG. Each plotted point represents an average of two wells. NRS, normal rabbit serum IgG. R195 and R196 are rabbit PAbs to B6t; PAb R182 is a rabbit PAb to B5. This experiment was done twice with the same results.
FIG. 4.
Inhibition of comet tail formation by anti-B6 PAbs. BSC-1 cell monolayers were infected with VV strain IHDJ. Following adsorption, a liquid overlay containing the indicated amount of antibody was added. After 36 h of incubation at 37°C, the cultures were fixed and stained with crystal violet and wells photographed. NRS, normal rabbit serum IgG used as a negative control; R182, a rabbit PAb against B5t used as a positive control. R195 and R196 are rabbit PAbs against B6t.
We next examined if anti-B6 PAbs could protect mice from a VV challenge. We found that the anti-B6 polyclonal IgG protected against significant illness after intranasal VV challenge. Untreated control mice had a maximum weight loss of ∼26% on day 7, while mice treated 24 h prior to challenge with a single 200-μg dose of anti-B6 polyclonal rabbit IgG had a maximum weight loss of only ∼12% on day 7. This level of protection is similar to what we had obtained in the past using similarly prepared anti-B5 polyclonal rabbit IgG. This experiment is additional evidence for cross protection afforded by B5 and B6 against VV. However, since the PAb by definition contains antibodies against many epitopes, the absence of any one B5 epitope would not necessarily be highlighted in a passive protection experiment of this type.
Western blot analysis of B6t using anti-B5t MAbs.
We used our panel of anti-B5t MAbs to study possible antigenic differences between B6t and B5t by Western blot and by biosensor analysis (Fig. 5 and Table 1). Equal amounts of B6t and B5t were electrophoresed. Sixteen B5t MAbs cross-reacted with B6t by Western blotting, while ten anti-B5t MAbs did not (Table 1). For example, VMC-14 reacted equally well with both proteins when they were electrophoresed under denaturing and native conditions (Fig. 5A). VMC-29 reacted equally well against both proteins under native conditions but was less reactive against B6t under denaturing conditions (Fig. 5B). Under native conditions, both VMC-14 and VMC-29 reacted against higher-molecular-weight bands on the B6t lane but not on the B5t lane. A third antibody, VMC-20, recognized B6t weakly compared to its reactivity to B5t (Fig. 5C). Finally, VMC-25 failed to react with B6t under denaturing or native conditions (Fig. 5D). Nine other MAbs had similar properties to VMC-25 (Table 1). Of these 10 MAbs nonreactive to B6t, three (VMC-10, VMC-25, and VMC-26) have significant neutralizing activity against VV (1). Although we do not know the location of the VMC-10 epitope on B5t (1), we mapped VMC-25 and VMC-26 to different regions of B5t: VMC-25 binds to a B5t truncation comprising both SCR1 and the SCR2 (data not shown) and VMC-26 binds to residues 256 to 275 (corresponding to the stalk [1]). It is possible that VMC-25 distinguishes a primary sequence difference between B5t and B6t in this region, but its epitope is not fully mapped at present. In contrast, the sequence of the VMC-26 epitope is the same in both proteins (Fig. 1B), which suggests that this antigenic site is presented differently in B6t. These results indicate that at least two major functional antigenic epitopes on B5t that are involved in neutralization of VV are different on B6t.
FIG. 5.
Patterns of MAb recognition of B5t and B6t by Western blotting. Purified baculovirus-expressed B5t (indicated by a 5) or B6t (indicated by a 6) was electrophoresed on a 12% Tris-glycine polyacrylamide gel under denaturing (D) or nonreducing (N) conditions, transferred to nitrocellulose and probed with each of the purified MAbs. Representative recognition patterns are shown. (A) Strong recognition of both proteins; (B) strong recognition of B6t under nonreducing conditions; (C) weak recognition of B6t under both conditions; (D) no recognition of B6t. The sizes of molecular mass markers are shown in kDa.
TABLE 1.
Properties of MAbs to B5t and B6t
| Group | MAb | Neutralizationa | Epitopeb | B5t
|
B6t
|
|||
|---|---|---|---|---|---|---|---|---|
| Western blot assay | Biosensor
|
Western blot assay | Biosensor binding | |||||
| Binding | Competing antibody | |||||||
| 1A | VMC-20 | P, C | 56-75 | + (low) | + | 21, 24 | + (low) | + |
| VMC-33 | P, C | 65-75 | ++ | − | ++ | + | ||
| VMC-26 | P, C | 256-275 | + | − | − | NTc | ||
| VMC-29 | P, C | 256-275 | + | + | Rat MAb 19C2 | + | + | |
| VMC-25 | P, C | + | + | Rat MAb 19C2 | − | − | ||
| 1B | VMC-21 | P | 65-84 | + | + | 20, 24 | + (low) | + |
| VMC-22 | P | 65-84 | + | + | + (low) | − | ||
| 1C | VMC-14 | C | 56-75 | + | − | + | + | |
| VMC-31 | C | 65-75 | + | − | + | + | ||
| VMC-32 | C | 65-75 | + | + | 19 | + | + | |
| VMC-24 | C | 256-275 | + (low) | + | 20, 21 | + (low) | + | |
| VMC-10 | C | + | − | − | NT | |||
| 2A | VMC-19 | 65-84 | + | + | 32 | + | + | |
| VMC-30 | 56-75 | + | − | + | −/+ | |||
| 2B | VMC-11 | 256-275 | + | + | 23 | + | + | |
| VMC-23 | 256-275 | + | + | 11 | + | + | ||
| VMC-27 | 256-275 | + | − | − | NT | |||
| 2C | VMC-15 | 56-75; 254-264 | + | + | + | + | ||
| VMC-16 | 56-75; 254-264 | + | − | + | NT | |||
| VMC-18 | 65-75; 245-264 | + | − | + | NT | |||
| 2D | VMC-7 | + | − | − | NT | |||
| VMC-8 | + | − | − | NT | ||||
| VMC-9 | + | − | − | NT | ||||
| VMC-12 | + | − | − | NT | ||||
| VMC-13 | + | − | − | NT | ||||
| VMC-28 | + | − | − | NT | ||||
P, plaque reduction assay; C, comet tail inhibition.
Amino acid residues that form the minimal region recognized by the MAb.
NT, not tested.
dNumbers correspond to competing antibodies.
BIAcore studies with B6t.
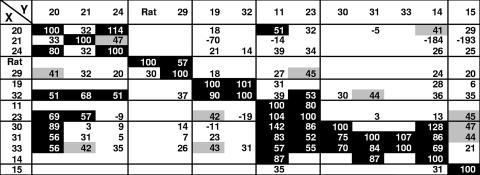
Earlier, we described seven groups of MAbs for B5t based on competition of binding as well as on other biological properties (1) (Table 1). A similar biosensor study was carried out here with B6t using the 16 cross-reactive MAbs prepared against B5t (1). In this assay, anti-His antibody was covalently coupled to the biosensor chip and the protein was captured by its His tag. Next, the primary antibody was bound to the captured B6t. The secondary (test) antibody was then injected, and its binding pattern was monitored. The rationale is that if the second MAb fails to bind to B6t, its epitope overlaps that recognized by the first MAb. If the second MAb binds, its epitope is independent of the epitope bound by the first MAb (1, 15). Reciprocal blocking assays were carried out here with each of the 16 cross-reactive MAbs using B6t, and the results were compared with previous results for B5t (Fig. 6 and 7).
FIG. 6.
Percentage of blocking when each MAb is injected after initial binding of the primary antibody. X, second test antibody; Y, primary antibody. The MAbs bound to B6t with values around 200 to 300 RU, which are lower than those for the same MAbs on B5t. The MAbs are arranged according to the competition groups of B5 (1). Reciprocal blocking was done only for competing pairs. Black background, high blocking (50 to 100%); gray background, low blocking (40 to 50%); white background, no blocking. A negative value indicates increased binding of the antibody to B6t in the presence of the primary antibody.
FIG. 7.
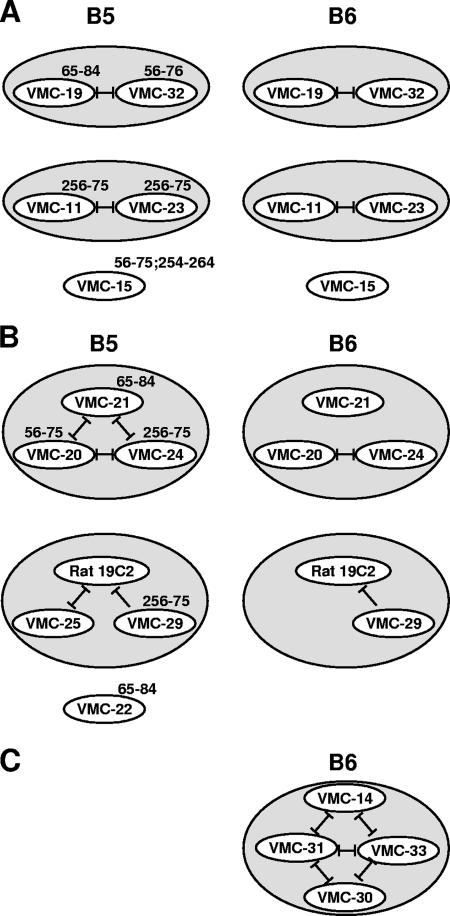
Groupings of MAbs based on blocking interactions with B5t and B6t by biosensor. Diagrammatic representation of blocking interactions between antibodies on B5t (left) or on B6t between antibodies from the same B5t competition group (right). Blocking is represented by a black line. (A) Antibodies that react with B6t as they did on B5t; (B) antibodies that bound to B5t but behaved differently on B6t; (C) antibodies that did not bind to B5t but bound to B6t.
From these studies the MAbs were classified as follows (summarized in Fig. 7): (i) antibodies that behaved on B6t as they did on B5t and were grouped in the manner previously described for B5t (1); (ii) antibodies that had been grouped together for B5t (1) but bound differently to B6t; and (iii) antibodies that did not bind to B5t but bound to B6t.
(i) Antibodies that react with B6t as they did on B5t.
Five nonneutralizing MAbs (VMC-11, VMC-15, VMC-19, VMC-23, and VMC-32) were in this category and formed two groups plus a single MAb (VMC-15) that was outside these groups (Fig. 7A). Thus, the epitopes for these MAbs are presented the same way on both proteins.
(ii) Antibodies that bound to B5t but behaved differently on B6t.
One set of MAbs in this category corresponds to a competition group composed of VMC-20, VMC-21, and VMC-24, where each competed with the others in a reciprocal manner on B5t (Fig. 7B). However, although VMC-20 and VMC-24 competed in a reciprocal manner for binding to B6t, neither of these MAbs competed with VMC-21 (Fig. 7B). This result is surprising, since both VMC-20 and VMC-21 bound to peptides located in the SCR1-SCR2 border (amino acids 56 to 84) of B5t. Thus, these results suggest that these epitopes are presented differently on B6t than on B5t.
Another set of MAbs in this category comprised VMC-25, VMC-29, and 19C2. Here, VMC-25 and 19C2, an anti-B5 rat MAb (21), competed reciprocally for binding to B5t as previously described (1). However, VMC-25 failed to bind B6t (Fig. 7B and 8A), confirming the Western blot data in Fig. 5. Since the previously reported biosensor data for the MAbs' reactivity with B5t (1) were obtained using B5t purified in a different manner than we purified B6t, we wanted to make sure that the way the proteins were purified did not cause the differences we found. Thus, biosensor data for VMC-25 on both B5t (purified by nickel [B5(N)] or by the immunosorbent agent VMC-14 [B5(I)]) and B6t are shown in Fig. 8A. VMC-25 still bound to B5(I), indicating that its inability to bind to B6t was not due to the purification method. The behavior of 19C2 was the same for both proteins, in that binding to B5t and B6t was blocked by prior binding to VMC-29. The behavior of VMC-29 was also the same for both proteins. Another MAb in this category was VMC-22. VMC-22 bound to B5t but failed to bind to B6t. These data emphasize the fact that a neutralizing epitope on B5t is missing on B6t.
FIG. 8.
(A) Example of an antibody that bound to B5t but not to B6t (overlaid sensorgram)—in this case, VMC-25. (B) Example of an antibody that did not bind to B5t but bound to B6t (VMC-31).
(iii) Antibodies that did not bind to B5t but bound to B6t.
We tested four MAbs that reacted to B5t by Western blotting or enzyme-linked immunosorbent assay but did not bind to B5t on the biosensor. Surprisingly, these MAbs did bind to B6t (Fig. 7C). As an example, the biosensor binding data for VMC-31 are shown (Fig. 8B). Three MAbs with this property are neutralizing MAbs (VMC-14, VMC-31, and VMC-33), all of which competed reciprocally with each other and with the nonneutralizing MAb VMC-30. VMC-30 bound weakly to B6t. Peptide mapping showed that VMC-14, VMC-31, and VMC-33 bound to amino acids 56 to 75 at the SCR1-SCR2 border of B5t. These results suggest that this region is altered on B6t such that the epitopes are exposed on the folded B6t protein presented by biosensors but hidden on B5t in its native state. Since these MAbs were derived from B5t immunization, it is likely that this portion was presented to the immune system due to some unfolding of the protein.
Passive-protection studies with B5t MAbs.
We examined the ability of our two most potent VV-neutralizing MAbs (VMC-25 and VMC-29) to protect mice against a VV challenge in BALB/c mice. In these experiments, VMC-11 was used as a nonneutralizing control MAb. Mice (five per group) were inoculated intraperitoneally with 200 μg of either VMC-11, VMC-25, or VMC-29. The control group was untreated. Twenty-four hours later, mice were challenged intranasally with approximately 1 50% lethal dose (∼5 × 104 PFU) of VV (Fig. 9). Since it is known that combinations of MAbs to various VV targets provide optimal protection against a higher-dose challenge (16), we used this sublethal dose so that the efficiency of a single MAb could be assessed. The control untreated mice became ill and lost approximately 25% of their original body weight during the first 10 days of infection. Recipients of VMC-11 behaved the same as control mice. Importantly, animals given VMC-25 or VMC-29 exhibited significantly altered pathogenesis, as measured by weight loss (∼5% of original body weight lost by day 7 postinfection). These mice recovered their original weight by day 10 postinfection and continued to gain weight thereafter. These data showed that pretreatment with either VMC-25 or VMC-29 protects mice from VV disease. From these studies, we hypothesize that the epitopes for VMC-25 and VMC-29 are important triggers of a protective antibody response to VV, and thus, the finding that VMC-25 does not recognize B6t suggests that such an antibody generated by B5 would not be active against variola virus.
FIG. 9.
Passive-protection studies on mice with B5t MAbs. Different anti-B5t MAbs (200 μg) were inoculated intraperitoneally into groups of five mice 24 h before intranasal challenge with approximately 1 50% lethal dose (∼5 × 104 PFU) of VV. The weight of the mice was monitored, and the mean weight change ± standard error for each group is plotted. Antibodies were tested three times with similar results.
DISCUSSION
Previously we showed that a combination of three VV proteins, L1t (MV), B5t (EV), and A33t (EV) protected mice against a lethal VV challenge (10, 28). In another report, we showed that the EV-neutralizing activity of vaccinia virus immune globulin is directed mainly against B5t (4), suggesting that this protein is an important component of the VV subunit vaccine. While these results are encouraging, the ability of these proteins to protect against variola virus is not known, since L1, B5, and A33 proteins differ in sequence to various degrees from their homologues in variola virus. Since B5 shows the greatest number of amino acid differences compared with its B6 variola virus homologue (Fig. 1B), in this study we examined the antigenic properties of the two proteins. We previously reported on a panel of MAbs to B5t which were grouped according to structural and functional properties (1). We produced soluble B6t protein and analyzed its antigenic characteristics with this panel of anti-B5t MAbs. We believe that our panel of B5t MAbs is representative of the antibodies that would be stimulated during vaccination with the subunit vaccine. Our most important finding was that at least two epitopes of B5 that stimulate neutralizing antibodies are either missing or altered in B6.
B5t has VV-specific epitopes.
We found that the presentation of antigenic sites of B6t differs from that of B5t. This is not surprising, since there are 21 amino acid differences between the ectodomains of the two proteins. A previous study using MAbs to compare D4R, the variola virus counterpart of the VV growth factor precursor, to VGF, the VV growth factor, showed that a three-amino-acid difference is enough to significantly alter or abolish antibody cross-reactivity (14). In addition to the amino acid difference, B6t is missing a potential N-glycosylation site that is present on B5t (Fig. 1A). This difference could indirectly affect exposure of the antigenic sites of the two proteins.
In our prior study of the MAb reactivity with B5t we developed a model of how B5 might fold, where the N terminus and C terminus interact. The results described in this paper indicate that B6t forms a similar structure. This is based on the reactivity of MAbs VMC-20 and VMC-24, which recognize distal sites of B5t (1) and compete for binding on B6t, and MAb VMC-15, which recognized both ends of B5t (1) and which recognized B6t in a similar fashion. While this overall structure is similar, the antigenic sites are different enough that 10 anti-B5t MAbs are unable to cross-react with B6t on a Western blot. Among the 10 MAbs that do not cross-react with B6t, one (VMC-25) has important biologic properties, including the ability to neutralize EV infectivity, block comet formation, and protect mice from VV challenge. Our biosensor results also showed that some of the competition groups that we defined for B5t were the same on B6t. However, in contrast to B5t, where MAbs in one group did not compete with MAbs of different groups, on B6t some MAbs of one competition group did affect the binding of MAbs of a different competition group (Fig. 6). In addition, four MAbs that did not bind to B5t on the biosensor bound to B6t. Thus, while the overall structure is similar, B6 differs by folding in such a manner that new interactions are revealed.
To be certain that the inability of VMC-25 to bind B6t was not due to the method of purification, we purified B5t through the same type of immunosorbent column as B6t and named it B5(I). In biosensor experiments, VMC-25 still bound to B5(I) (Fig. 8A). VMC-22 (Table 1) also recognized both B5(I) and B5(N) but not B6t. Hence, the inability of VMC-22 and VMC-25 to bind to B6t is specific to the B6t protein. Moreover, MAbs that, using biosensors, did not bind to B5(N) but bound to B6t (VMC-14, VMC-30, VMC-31, and VMC-33) (Fig. 7C) did not bind to B5(I) either, indicating that these antibodies bind specifically to B6t in its folded state. Since the protein on the biosensor is presented in a folded state, we hypothesize that these epitopes are hidden below the surface on B5t but are exposed on the surface of B6t. We further suggest that a comparable variola virus-specific epitope will also contribute to virus neutralization.
Our work clearly shows antigenic differences between B5t and B6t, with the obvious caveat that these proteins were truncated, N-terminally modified, and examined by in vitro methods. We have purified B5 from VV-infected cell extracts, and it reacts with the full panel of anti-B5 MAbs (data not shown). Similar experiments are under way to examine the antigenic properties of full-length B6 from extracts of transfected mammalian cells.
Implications for antibody therapeutics and subunit vaccine development.
Our results show cross-protection conferred by anti-B6 PAb in mice challenged with VV. However, the absence of the epitope for VMC-25 points out that therapeutic MAbs should only be ones against epitopes known to be common to VV and its variola virus homologue. Our data further suggest that a subunit smallpox vaccine, which relies on a protective response against only a few selected proteins, would likely be improved by using the variola virus homologue B6t instead of B5t. To test this, we plan to compare the remaining VV components of the proposed subunit vaccine with their variola virus counterparts in the same manner as that used here.
Acknowledgments
This work was supported by National Institutes of Health grants R21-AI53404, AI48487, NIH 1 UC1 AI062486, and RCE-U54-AI57168 from the National Institute of Allergy and Infectious Diseases and by a block grant from the state of Pennsylvania to the University of Pennsylvania. E. L. Reinherz had funding from the National Institutes of Health grant AI57330 from NIAID.
We thank Inger K. Damon from the Centers for Disease Control and Prevention for her kind gift of the plasmid harboring the B6 gene from variola major virus (strain Bangladesh). We are grateful to Claude Krummenacher for BIAcore assistance and useful comments. We thank Florent Bender for helpful discussions.
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. L. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 341:59-71. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 13:9-17. [DOI] [PubMed] [Google Scholar]
- 4.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 5.Boulter, E. A., and G. Appleyard. 1973. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog. Med. Virol. 16:86-108. [PubMed] [Google Scholar]
- 6.Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelstad, M., S. T. Howard, and G. L. Smith. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801-810. [DOI] [PubMed] [Google Scholar]
- 8.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194:627-637. [DOI] [PubMed] [Google Scholar]
- 9.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 10.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 66:7217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, M., H. Yang, S. K. Kim, P. A. Reche, R. S. Tirabassi, R. E. Hussey, Y. Chishti, J. G. Rheinwald, T. J. Morehead, T. Zech, I. K. Damon, R. M. Welsh, and E. L. Reinherz. 2004. Biochemical and functional analysis of smallpox growth factor (SPGF) and anti-SPGF monoclonal antibodies. J. Biol. Chem. 279:25838-25848. [DOI] [PubMed] [Google Scholar]
- 15.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 18.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 19.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 20.Poland, G. A., J. D. Grabenstein, and J. M. Neff. 2005. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine 23:2078-2081. [DOI] [PubMed] [Google Scholar]
- 21.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, G. L., and A. Vanderplasschen. 1998. Extracellular enveloped vaccinia virus. Entry, egress, and evasion. Adv. Exp. Med. Biol. 440:395-414. [PubMed] [Google Scholar]
- 23.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 24.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J. Gen. Virol. 78:2041-2048. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 1980. Declaration of global eradication of smallpox. Wkly. Epidemiol. Rec. 55:145-152. [Google Scholar]
- 26.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]