Abstract
Recently, a paper was published in which it was proposed that the GxxxG motif of the severe acute respiratory syndrome (SARS) coronavirus spike (S) protein transmembrane domain plays a vital role in oligomerization of the protein (E. Arbely, Z. Granot, I. Kass, J. Orly, and I. T. Arkin, Biochemistry 45:11349-11356, 2006). Here, we show that the GxxxG motif is not involved in SARS S oligomerization by trimerization analysis of S GxxxG mutant proteins. In addition, the capability of S to mediate entry of SARS S-pseudotyped particles overall was affected moderately in the mutant proteins, also arguing for a nonvital role for the GxxxG motif in SARS coronavirus entry.
The severe acute respiratory syndrome (SARS) coronavirus (CoV) spike (S) protein is crucial for infectivity of the virus and thus for the onset of the disease. It mediates binding of the virus to the cellular receptor and directs the membrane fusion event between the viral and host membranes (reviewed by Hofmann and Pöhlmann [6]). In addition, it can cause cell-cell fusion once expressed in infected cells (7). Previously, we described the importance of the SARS CoV S protein transmembrane domain (TMD) for both processes. We concluded that the C-terminal TMD might be involved in stabilization of the S oligomer, which would be required for proper function (2).
CoV S proteins are cotranslationally glycosylated (3), followed by a slow oligomerization step. The proteins are subsequently transported to the Golgi, where further processing takes place, resulting in the final, endo-β-N-acetylglucosaminidase H (endo-H)-resistant, mature form (9). Oligomerization of CoV S involves several domains. One domain, a leucine zipper, located in heptad repeat 2 (C-terminal half) (5), has been described as an important determinant of oligomerization (8). Especially for SARS CoV S, also soluble parts of S1 (the N-terminal half) have been reported to form multimers (13). In addition, the SARS CoV S protein TMD contains a putative oligomerization signal. In the TMD, a GxxxG motif is located, a feature that is unique among the CoVs (see Fig. 1A).
FIG. 1.
Basic features of the S proteins mutated in the GxxxG motif uniquely present in the SARS CoV S protein TMD. (A) Alignment of part of the TMDs of CoV S proteins. The transmembrane of SARS CoV S is aligned with representatives of CoV groups 1, 2, and 3. Obviously, the SARS CoV S TMD has a unique GxxxG motif (glycines are boxed). Numbering of amino acids is based on the SARS CoV sequence. Abbreviations: MHV, mouse hepatitis virus; HCoV, human CoV; TGEV, transmissible gastroenteritis virus; IBV, infectious bronchitis virus. (B) Diagram of the GxxxG mutant proteins generated. Glycines in the WT (SWT) sequence are in the gray box. One or both were mutated into leucines as depicted, yielding mutant proteins SG1201L, SG1205L, and SLL. (C) Pulse-chase maturation analysis of mutant S proteins. 293T cells were transfected with plasmids encoding the corresponding S proteins. After a 30-min pulse (lanes P) and a 4-h chase (lanes C), proteins were immunoprecipitated and analyzed on an 8% polyacrylamide gel (− lanes) or subjected to endo-H (E) treatment (+ lanes) and subsequently analyzed on an 8% polyacrylamide gel. The kD lane represents the molecular weight marker. The visible band is the 220-kDa marker band.
GxxxG motifs are well-described, TMD-located oligomerization domains (11). The two glycine residues are separated by three amino acids, which positions them on the same side of the α-helical TMD. This creates a relatively smooth surface on the α-helix that can interact with a similar α-helix, thus allowing the oligomer to form. Normally, these motifs are involved in dimerization of proteins and not trimerization. Recently, however, a paper was published in which it was shown that the GxxxG domain plays a pivotal role in the trimerization of the SARS CoV S protein (1). The authors asserted, by using the TMD of S in the TOXCAT system (10), that trimers are formed through the interaction of the TMDs, in particular through the GxxxG motif. However, it is essential to study this domain in the context of the full-length protein. In addition, the paper by Arbely et al. lacks a functional assay in which the importance of the GxxxG motif-driven oligomerization of the S protein is shown for virus infectivity.
In this paper, we describe the investigation of the role of the GxxxG motif in the oligomerization of the full-length S protein in SARS pseudotyped particles and in cell lysates by site-directed mutagenesis. Additionally, the function of the mutagenized proteins was tested in an infectivity assay.
Construction of S mutant proteins and analysis of expression and maturation.
In Fig. 1A, the alignment of the TMD of CoV S proteins is shown. It is clear that the SARS CoV S TMD contains a unique GxxxG motif. Since GxxxG motifs in TMDs are known to play a role in the oligomerization of proteins, we anticipated a possible role in the oligomerization of the S protein through this motif and created mutant S proteins, designated SG1201L, SG1205L, and SLL, in which either one or both glycine residues were replaced with leucines (Fig. 1B). Leucines were chosen as replacements because these residues are known for their neutral behavior in membranes. Typically, in any of these mutant proteins, disturbance of the GxxxG motif (if it is functional) should result in severe inhibition of oligomerization and thus, most likely, of function.
We analyzed the expression and maturation of the mutant proteins to ensure proper processing. A pulse-chase experiment was carried out, and immunoprecipitated protein was subjected to endo-H treatment to monitor the maturation of the protein as described before (2). Figure 1C shows that WT S and the mutant proteins had matured after 4 h of chase, as evidenced by the appearance of the endo-H-resistant band (+ lanes).
GxxxG motif is not important for oligomerization of SARS CoV S protein.
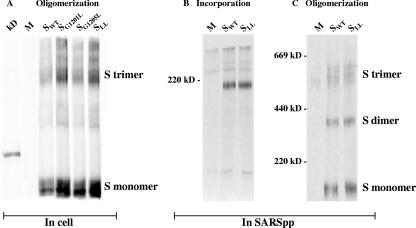
To test the importance of the GxxxG motif for oligomerization of the S protein, 293T cells were transfected with a plasmid encoding the WT S protein (2) or one of the three mutant proteins. Twenty-four hours after transfection, proteins were radiolabeled and the S proteins were immunoprecipitated with anti-S serum. Precipitates were incubated at 80°C and subsequently analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This is a validated method by which to visualize the S protein's oligomeric forms (2, 12) (see Fig. 3). Evidently, mutagenesis of the GxxxG motif did not influence the stability of the S trimers, since for all three mutant proteins the ratio between S monomer and trimer was comparable to that of WT S (Fig. 2A).
FIG. 3.
Capacity of S mutant proteins to mediate entry of SARSpp (A) and cell-cell fusion (B). (A). Infectivity of SARSpp was assayed as described before (2), by fluorescence-activated cell sorting. The infectivity of the mutant SARSpp is relative to WT SARSpp infectivity (set to 100%). Two experiments were carried out. (B) Fusion of cells expressing S (red [DsRed, Discosoma red]) and ACE-2 (green [eGFP, enhanced green fluorescent protein]), resulting in double fluorescence (yellow). Representative samples are presented, showing cell fusion activity of all S mutant proteins. Magnification, ×40.
FIG. 2.
Analysis of S oligomerization in cells and in SARSpp. (A) Immunoprecipitated S proteins from cell lysates were analyzed on a 4% polyacrylamide gel under nonreducing conditions. The kD lane contains the molecular weight marker. The visible marker band represents 220 kDa. M, mock. S monomers and S trimers are indicated. (B) Radiolabeled SARSpp containing SWT or SLL were lysed, and S proteins were immunoprecipitated and analyzed on an 8% polyacrylamide gel under reducing conditions. M, mock. (C) Same as panel B, but precipitates were analyzed on a 4% polyacrylamide gel under nonreducing conditions. M, mock. S oligomers are indicated.
Subsequently, radiolabeled SARS pseudotyped retrovirus particles (SARSpp) were used to further test the oligomerization behavior of the mutant S proteins. Incorporated wild-type (WT) S or SLL was immunoprecipitated, and subsequently sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis on a 8% polyacrylamide gel was performed. Equal amounts of particles, based on reverse transcriptase activity, were used for the immunoprecipitation, as described previously (2). It is obvious that the SLL trimers were incorporated as efficiently as WT S, indicating that the GxxxG motif is not important for S incorporation in SARSpp (Fig. 2B). In addition, the same immunoprecipitations were analyzed under nonreducing conditions on a 4% polyacrylamide gel. In Fig. 2C, SLL and SWT show the same relative amount of trimers.
In a recent paper by Arbely et al. (1), the GxxxG motif is described as an amino acid stretch that would be vital for the process of SARS CoV S oligomerization. The authors base this conclusion on the observations that (i) the TMD of SARS CoV S mediates oligomerization in a TOXCAT assay (11) and (ii) the high-molecular-weight band they see on a Western blot is 30% less pronounced in a GxxxG mutant than in WT S protein. In contrast, we show unambiguously by phosphorimaging of radiolabeled S proteins, on a 4% polyacrylamide gel, that the trimer of GxxxG mutant proteins is as prominently present as the WT S trimer in both cell lysates and pseudotyped particles. A possible explanation for the discrepancy between our results and the data in the paper by Arbely et al. could be the fact that we replaced the glycines in the GxxxG motif with leucine residues whereas Arbely et al. used isoleucines. We realize, however, that this explanation is not very likely.
G1205, but not the GxxxG motif, is important for S-mediated entry.
To investigate the importance of the GxxxG motif for the function of the S protein, in particular the entry of the virus, the efficiency of the SARSpp to transduce VeroE6 cells was determined as described earlier (2). The same amount of SARSpp, based on reverse transcriptase activity, was used for each mutant. Figure 3A shows the infectivity of the mutant S-containing SARSpp, relative to the WT S-containing SARSpp. SG1201L-containing SARSpp were as efficient at infection as WT S SARSpp (Fig. 3A). In contrast, SG1205L SARSpp were severely hampered in their entry efficiency, resulting in approximately 20% relative infectivity. SARSpp containing the double mutant SLL displayed an intermediate phenotype, 35% relative infectivity. Typically, if GxxxG-mediated oligomerization were important for the function of the S protein, one would expect a low entry efficiency for the single mutant proteins and a synergistic effect, hence no activity, for the double mutant. Our results show that although there is an effect on infectivity, the mutations that should disturb the GxxxG motif do not interfere in a manner that suggests an important role for the GxxxG motif.
To further investigate the role of the G residues in SARSpp entry, we measured the cell-cell fusion-mediating capacity of the SARS GxxxG mutant S proteins and compared this to WT S activity. Cells (293T) were cotransfected with either S-expressing plasmid phCMV-S (2) and pDsRed1-N1 (Clontech) or an ACE-2-expressing plasmid (2) and phCMV-GFP. Twenty-four hours after transfection, the cells were trypsinized, mixed at a 2:1 ratio (S to ACE-2), and replated on coverslips. After 24 h of incubation at 37°C, cells were analyzed for fluorescence and double-fluorescing cells were counted. Results are shown in Table 1. It is clear that all of the mutant proteins display cell-cell fusion activity comparable to the WT level and thus that the GxxxG mutant proteins are not impaired in cell-cell fusion. Apparently, the cell-cell fusion has less stringent requirements than S-mediated entry of SARSpp. Representative examples showing fusion of cells (yellow) expressing S (red) and ACE-2 (green) are shown in Fig. 3B.
TABLE 1.
Cell-cell fusion activity of SARS S GxxxG mutant proteinsa
| Protein | Avg no. of syncytia |
|---|---|
| WT | 139 ± 30 |
| SG1201L | 138 ± 33 |
| SG1205L | 110 ± 14 |
| SLL | 103 ± 37 |
| None (mock transfection) | 15 ± 8 |
Cell-cell fusion was determined by counting the double-fluorescing cells in a defined view of the microscope. The number of syncytia is an average of two experiments.
Although the results show that the GxxxG motif is not important for the entry-mediating capacity of S, they also show that somehow the G1205 residue is involved in this function. Previously, glycines have been shown to be of crucial importance during vesicular stomatitis virus (VSV) G-mediated fusion, without influencing G trimerization (4). Although the effect on VSV G-mediated fusion was more drastic than we observed with SARS CoV S, the function of glycines in the SARS CoV S TMD might be similar to the function of the glycines in the VSV G TMD. These glycines have been implicated in destabilization of the TMD helical structure, thereby mediating possible bends in the TMD needed for membrane fusion pore formation (4). We are currently further investigating the requirements for the formation of the SARS CoV S-mediated membrane fusion pore.
Acknowledgments
We thank Peter Bredenbeek and François Penin for helpful and motivating discussions.
This work is part of the activities of the Euro-Asian SARS-DTV Network (SP22-CT-2004-511064).
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Arbely, E., Z. Granot, I. Kass, J. Orly, and I. T. Arkin. 2006. A trimerizing GxxxG motif is uniquely inserted in the severe acute respiratory syndrome (SARS) coronavirus spike protein transmembrane domain. Biochemistry 45:11349-11356. [DOI] [PubMed] [Google Scholar]
- 2.Broer, R., B. Boson, W. Spaan, F. L. Cosset, and J. Corver. 2006. Important role for the transmembrane domain of severe acute respiratory syndrome coronavirus spike protein during entry. J. Virol. 80:1302-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh, D. 1995. The coronavirus surface glycoprotein, p. 73-113. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, NY.
- 4.Cleverley, D. Z., and J. Lenard. 1998. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 95:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot, R. J., W. Luytjes, M. C. Horzinek, B. A. van der Zeijst, W. J. Spaan, and J. A. Lenstra. 1987. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J. Mol. Biol. 196:963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann, H., and S. Pöhlmann. 2004. Cellular entry of the SARS coronavirus. Trends Microbiol. 12:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. X. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. Leduc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 8.Luo, Z., A. M. Matthews, and S. R. Weiss. 1999. Amino acid substitutions within the leucine zipper domain of the murine coronavirus spike protein cause defects in oligomerization and the ability to induce cell-to-cell fusion. J. Virol. 73:8152-8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemann, H., B. Boschek, D. Evans, M. Rosing, T. Tamura, and H. D. Klenk. 1982. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1:1499-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ, W. P., and D. M. Engelman. 1999. TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc. Natl. Acad. Sci. USA 96:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russ, W. P., and D. M. Engelman. 2000. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296:911-919. [DOI] [PubMed] [Google Scholar]
- 12.Song, H. C., M. Y. Seo, K. Stadler, B. J. Yoo, Q. L. Choo, S. R. Coates, Y. Uematsu, T. Harada, C. E. Greer, J. M. Polo, P. Pileri, M. Eickmann, R. Rappuoli, S. Abrignani, M. Houghton, and J. H. Han. 2004. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J. Virol. 78:10328-10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao, X., Y. Feng, S. Chakraborti, and D. S. Dimitrov. 2004. Oligomerization of the SARS-CoV S glycoprotein: dimerization of the N-terminus and trimerization of the ectodomain. Biochem. Biophys. Res. Commun. 322:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]